Combination Intraventricular/Fiberoptic Catheter Insertion (Assist), Monitoring, Nursing Care, Troubleshooting, and Removal
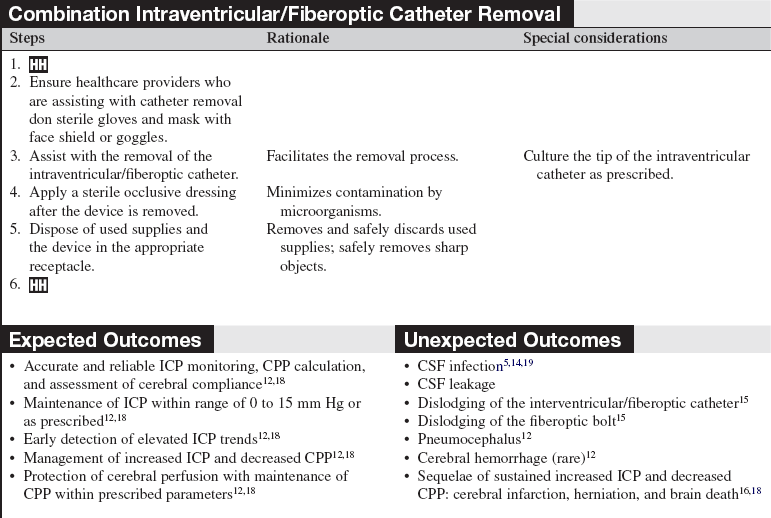
The combination intraventricular/fiberoptic catheter combines the capability of external ventricular drainage of cerebrospinal fluid with monitoring of intracranial pressure. This hybrid device can be used to monitor intracranial pressure intermittently or continuously and to drain cerebrospinal fluid intermittently or continuously.16
PREREQUISITE NURSING KNOWLEDGE
• A fundamental understanding of neuroanatomy and physiology is needed.
• Knowledge of aseptic and sterile technique is necessary.
• Proper equipment assembly and setup specific to fiberoptic intracranial pressure monitoring device should be understood.
• Intracranial pressure (ICP) is the pressure exerted by the intracranial contents, brain tissue, blood, and cerebrospinal fluid (CSF) within the cranium. Increased ICP occurs when the intracranial volume exceeds the brain’s ability to compensate for increased volume.16,18 Increased ICP contributes to secondary neuronal injury.
• The ventricular catheter with external strain gauge transducer is considered the gold standard for ICP monitoring.3,4 The external ventricular drain (EVD) is considered the most accurate and reliable method of monitoring ICP and ICP waveform and allows for CSF drainage.3 However, the fluid-filled system of the external ventricular catheter has the greatest infection rate1,2 and hemorrhage rate and requires repeated zeroing and leveling with the anatomic reference point for the foramen of Monro.3
• The parenchymal fiberoptic catheter provides quality ICP monitoring but cannot be rezeroed once inserted, cannot be used for CSF drainage, and is subject to drift, particularly after 5 days.3,14
• The combination catheter has some of the advantages and disadvantages of both the ventricular catheter with an external strain gauge transducer and the fiberoptic transducer tipped catheter. The combination catheter can only be zeroed before insertion. However, because the transducer is in the tip of the fiberoptic catheter, there is no external strain gauge transducer and therefore no repetitive zeroing and leveling of a transducer with the anatomic reference point for the foramen of Monro. In addition, the combination catheter allows for CSF drainage but still requires attention to the level of the reference point of the drip chamber to the anatomic reference point for the foramen of Monro and setting of the pressure level at the top of the graduated burette (drip chamber) to prevent underdrainage or overdrainage of CSF.12
• The anatomic reference point for the foramen of Monro is the external auditory canal.11
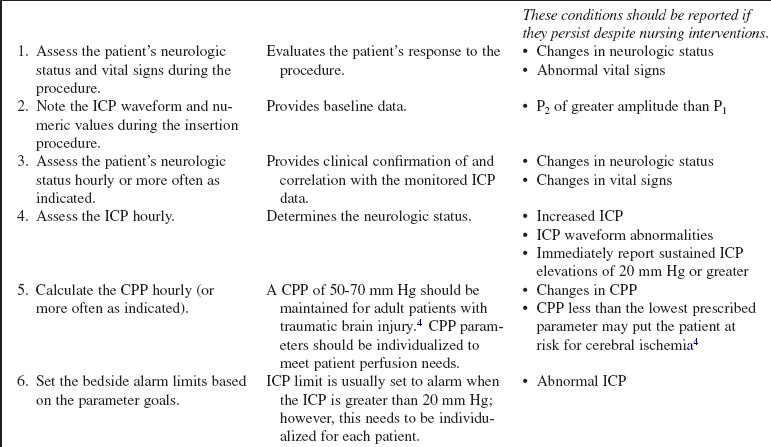
• Normal ICP ranges from 0 to 15 mm Hg; sustained ICPs of greater than 20 mm Hg are generally considered neurologic emergencies.17
• The normal ICP waveform has three or four peaks, with P1 being of greater amplitude than P2 and P2 of greater amplitude than P3. P1 is thought to reflect arterial pressure; P2, P3, and P4 (if present) have been described as choroid plexus or venous in origin (see Fig. 88-2).18 The amplitude of P2 may exceed P1 with increased ICP or decreased intracranial compliance (see Fig. 88-3).
• ICP waveform trends include a, b, and c waves. The a waves, also referred to as plateau waves, are associated with ICP values of 50 to 100 mm Hg and last 5 to 20 minutes. The a waves (see Fig. 88-4) are associated with abrupt neurologic deterioration and herniation. The b waves (see Fig. 88-5) with ICP values of 20 to 50 mm Hg, lasting 30 seconds to 2 minutes, may become a waves. The c waves (see Fig. 88-6) may coincide with ICPs as high as 20 mm Hg but are short lasting and without clinical significance.3,10,18
• Cerebral perfusion pressure (CPP) is a derived mathematic calculation that indirectly reflects the adequacy of cerebral blood flow. The CPP is calculated by subtracting the ICP from the mean arterial pressure (MAP); thus CPP = MAP – ICP. The normal CPP range for adults is approximately 60 to 100 mm Hg, or a mean of 80 mm Hg. The optimal CPP for a given patient and clinical condition is not entirely known. ICP and CPP should be managed concomitantly. According to the Brain Trauma Foundation Guides, an acceptable CPP for an adult with a severe traumatic brain injury (Glasgow Coma Scale [GCS] score of equal to or less than 8) lies between 50 and 70 mm Hg.4 Patients with aneurysmal subarachnoid hemorrhage vasospasm may need higher CPPs to maintain adequate perfusion through vasospastic cerebral blood vessels. Patients with strokes, aneurysmal subarachnoid hemorrhage, or other neurologic injuries may require higher or individualized CPP parameters reflective of the neuropathology and brain perfusion needs. Research continues regarding the relationship between cerebral blood flow and CPP.
• ICP and CPP must be considered together in management of the patient.
• Cerebral autoregulation is the intrinsic ability of the cerebral vessels to constrict and dilate as needed to maintain adequate cerebral perfusion. Cerebral autoregulation is impaired with brain injury, and the cerebral blood flow becomes passively dependent on the systemic blood flow. The cerebral blood vessels are no longer able to react to maintain CPP in response to a change in blood pressure.
• Sustained ICP elevations of 20 mm Hg or greater necessitate immediate reporting and intervention. ICP waveform changes that indicate loss of cerebral compliance or cerebral autoregulation should be reported immediately.11,16
• ICP monitoring is indicated for the following:
 Traumatic brain injury with a GCS score less than 8 and abnormal computed tomographic (CT) scan or normal CT scan with two of the following: hypotension, age more than 40 years, and posturing4
Traumatic brain injury with a GCS score less than 8 and abnormal computed tomographic (CT) scan or normal CT scan with two of the following: hypotension, age more than 40 years, and posturing4
 Aneurysmal subarachnoid hemorrhage18
Aneurysmal subarachnoid hemorrhage18
 Fulminant hepatic failure with encephalopathy18
Fulminant hepatic failure with encephalopathy18
• CSF drainage is indicated for the following12:
• Consequences of CSF underdrainage include headache, neurologic deterioration, hydrocephalus, increased intracranial pressure, secondary neuronal injury, herniation, and death.
• Consequences of CSF overdrainage include headache, subdural hematoma, pnemocephalus, ventricular collapse, herniation, and death.
• A contraindication for ICP monitoring is coagulopathies.
• Issues regarding accuracy primarily relate to displacement, misplacement, or breakage of the fiberoptic catheter and drift (especially after 5 days).3,16
• Management of the patient with increased ICP and decreased CPP is a multi-tiered approach that includes nursing interventions (e.g., positioning, maintaining normothermia) and the administration of pharmacologic agents and surgical procedures.4,10,16
EQUIPMENT
• Sterile gloves, surgical caps, masks, goggles or face shields, and sterile surgical gowns
• Sterile towels, half-sheets, and drapes
• Local anesthetic (lidocaine 1% or 2% without epinephrine), 5- or 10-mL Luer-Lok syringe with 18-gauge needle (for drawing up of lidocaine), and 23-gauge or 25-gauge needle (for administration of lidocaine)
PATIENT AND FAMILY EDUCATION
• Assess patient and family understanding of the purpose of the intraventricular and fiberoptic catheter.  Rationale: Explaining the purpose of the procedure may decrease patient and family anxiety.
Rationale: Explaining the purpose of the procedure may decrease patient and family anxiety.
• Explain the intraventricular and fiberoptic catheter insertion procedure. Review normal parameters and patient care after insertion. Review the family’s role in maintenance of an optimal ICP with limitation of patient stimulation.  Rationale: Explanation of expected interventions may allay patient and family anxieties, encourage questions, and promote therapeutic family interaction.
Rationale: Explanation of expected interventions may allay patient and family anxieties, encourage questions, and promote therapeutic family interaction.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess the patient’s neurologic status.  Rationale: A baseline neurologic assessment enables the nurse to identify changes that may occur during or as a result of the intraventricular/fiberoptic catheter placement.
Rationale: A baseline neurologic assessment enables the nurse to identify changes that may occur during or as a result of the intraventricular/fiberoptic catheter placement.
• Assess the patient’s current laboratory profile, including complete blood count (CBC), platelet count, prothrombin time (PT), international normalized ratio (INR), and partial thromboplastin time (PTT).  Rationale: Baseline coagulation studies determine the risk for bleeding during intraventricular catheter insertion.
Rationale: Baseline coagulation studies determine the risk for bleeding during intraventricular catheter insertion.
• Assess for allergies.  Rationale: Insertion of the intraventricular fiberoptic catheter may necessitate local anesthetic, an antiseptic to clean the site, and analgesia and sedation. Assessment minimizes the risk of allergic reaction.
Rationale: Insertion of the intraventricular fiberoptic catheter may necessitate local anesthetic, an antiseptic to clean the site, and analgesia and sedation. Assessment minimizes the risk of allergic reaction.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that informed consent has been obtained.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient; however, in emergency circumstances, time may not allow for the consent form to be signed.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient; however, in emergency circumstances, time may not allow for the consent form to be signed.
• Perform a pre-procedure verification and time out, if non-emergent.  Rationale: Ensures patient safety.
Rationale: Ensures patient safety.
• Administer preprocedural analgesia or sedation as prescribed.  Rationale: The patient needs to remain still during catheter insertion. In an emergency situation, the patient may already be receiving continuous analgesia and sedation.
Rationale: The patient needs to remain still during catheter insertion. In an emergency situation, the patient may already be receiving continuous analgesia and sedation.
• Assist the patient to a supine position with the head of the bed at 30 to 45 degrees and the neck in a midline, neutral position.  Rationale: This position provides access for intraventricular/fiberoptic catheter insertion and enhances jugular venous outflow, contributing to possible reduction in intracranial pressure.
Rationale: This position provides access for intraventricular/fiberoptic catheter insertion and enhances jugular venous outflow, contributing to possible reduction in intracranial pressure.
References
![]() 1. Arabi, Y, Memish, ZA, Balkhy, HH, et al, Ventriculostomy-associated infections. incidence and risk factors. Am J Infect Control 2005; 33:137–143.
1. Arabi, Y, Memish, ZA, Balkhy, HH, et al, Ventriculostomy-associated infections. incidence and risk factors. Am J Infect Control 2005; 33:137–143.
![]() 2. Arbour, R, Intracranial hypertension. monitoring and nursing assessment. Crit Care Nurs 2004; 24:19–32.
2. Arbour, R, Intracranial hypertension. monitoring and nursing assessment. Crit Care Nurs 2004; 24:19–32.
3. Bershad, EM, Humphreis, WE, Suarez, JI. Intracranial hypertension. Semin Neurol. 2008; 28:690–702.
4. Bratton, SL, Chesnut, RM, Ghajar, J, et al, Guidelines for the management of severe traumatic brain injury. a joint project of the Brain Trauma Foundation, American Association of Neurological Surgeons (AANS), Congress of Neurological Surgeons (CNS), AANS/CNS Joint Section on Neurotrauma and Critical Care. J Neurotrauma. 2007; 24(Suppl 1):S1–S106.
5. Lo, CH, Spelman, D, Bailey, M, et al, External ventricular drain infections are dependent of drain duration. an argument against elective revision. J Neurosurg 2007; 106:378–383.
![]() 6. Davis, JW, Davis, IC, Bennink, LO, et al, Placement of intracranial pressure monitors. are “normal” coagulation parameters necessary. J Trauma 2004; 57:1173–1176.
6. Davis, JW, Davis, IC, Bennink, LO, et al, Placement of intracranial pressure monitors. are “normal” coagulation parameters necessary. J Trauma 2004; 57:1173–1176.
7. Fan, JY, Kirkness, C, Vicini, P, et al. Intracranial pressure waveform morphology and intracranial adaptive capacity. Am J Crit Care. 2008; 17:545–554.
8. Hebl, JR. The importance and implications of aseptic techniques during regional anesthesia. Reg Anesth Pain Med. 2006; 31:311–323.
![]() 9. Hetherington, NJ, Dooley, MJ. Potential for patient harm from intrathecal administration of preserved solutions. Med J Aust. 2000; 173:141–143.
9. Hetherington, NJ, Dooley, MJ. Potential for patient harm from intrathecal administration of preserved solutions. Med J Aust. 2000; 173:141–143.
10. Hickey, JV, Olson, DM, Intracranial hypertension. theory and management of increased intracranial pressureHickey JV, ed.. The clinical practice of neurological and neurosurgical nursing. ed 6. Lippincott Williams & Wilkins, Philadelphia, 2009:270–307.
![]() 11. Kirkness, CJ, Mitchell, PH, Burr, RL, et al, Intracranial pressure waveform analysis. clinical and research implications. J Neurosci Nurs 2000; 32:271–277.
11. Kirkness, CJ, Mitchell, PH, Burr, RL, et al, Intracranial pressure waveform analysis. clinical and research implications. J Neurosci Nurs 2000; 32:271–277.
12. Leeper, B, Lovasik, D, Cerebrospinal drainage systems. external ventricular and lumbar drainsLittlejohns LR, Bader MK, eds.. AACN-AACN protocols for practice: monitoring technologies in critically ill neuroscience patients. Jones and Bartlett: Sudbury, MA, 2009 :71–82.
![]() 13. Littlejohns, LR, Trimble, B. Our policy on external ventricular drainage systems includes the procedure for priming the system. Does it really have to be primed. Crit Care Nurse. 2005; 25:57–59.
13. Littlejohns, LR, Trimble, B. Our policy on external ventricular drainage systems includes the procedure for priming the system. Does it really have to be primed. Crit Care Nurse. 2005; 25:57–59.
![]() 14. Lozier, AP, Sciacea, RR, Romagnoli, MF, et al, Ventriculostomy-related infections. a critical review of the literature. Neurosurgery 2002; 51:170–181.
14. Lozier, AP, Sciacea, RR, Romagnoli, MF, et al, Ventriculostomy-related infections. a critical review of the literature. Neurosurgery 2002; 51:170–181.
![]() 15. March, K. Application of technology in the treatment of traumatic brain injury. Crit Care Nurs Q. 2000; 23:26–37.
15. March, K. Application of technology in the treatment of traumatic brain injury. Crit Care Nurs Q. 2000; 23:26–37.
![]() 16. March, K. Intracranial pressure monitoring and assessing intracranial compliance in brain injury. Crit Care Nurs Clin North Am. 2000; 12:429–436.
16. March, K. Intracranial pressure monitoring and assessing intracranial compliance in brain injury. Crit Care Nurs Clin North Am. 2000; 12:429–436.
![]() 17. March, K. Technology. In: Bader MK, Littlejohns LR, eds. Core curriculum for neuroscience nursing. ed 4. Chicago, American Association of Neuroscience Nurses, St Louis: Saunders; 2004:199–226.
17. March, K. Technology. In: Bader MK, Littlejohns LR, eds. Core curriculum for neuroscience nursing. ed 4. Chicago, American Association of Neuroscience Nurses, St Louis: Saunders; 2004:199–226.
18. March, K, Madden, L. Intracranial pressure management. In: Littlejohns LR, Bader MK, eds. AACN-AANN protocols for practice: monitoring technologies in critically ill neuroscience patients. Sudbury, MA: Jones and Bartlett; 2009:35–69.
![]() 19. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30:476–489.
19. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30:476–489.
20. Reynolds, F. Neurologic infections after neuraxial anesthesia. Anesthesiol Clin. 2008; 26:23–52.
![]() 21. Wilkinson, HA, et al, Erroneous measurement of intracranial pressure caused by simultaneous ventricular drainage. a hydrodynamic model study. Neurosurgery 1989; 24:348–354.
21. Wilkinson, HA, et al, Erroneous measurement of intracranial pressure caused by simultaneous ventricular drainage. a hydrodynamic model study. Neurosurgery 1989; 24:348–354.
![]() 22. Woodward, S, Addison, C, Shah, S, et al. Benchmarking best practice for external ventricular drainage. Br J Nurs. 2002; 11:47–53.
22. Woodward, S, Addison, C, Shah, S, et al. Benchmarking best practice for external ventricular drainage. Br J Nurs. 2002; 11:47–53.