Brain Tissue Oxygen Monitoring: Insertion (Assist), Care, and Troubleshooting
PREREQUISITE NURSING KNOWLEDGE
• Incorporated as an adjunct monitor of trends in concert with concurrent neurologic multimodality monitoring parameters (intracranial pressure [ICP], cerebral perfusion pressure [CPP], systemic jugular venous oxygen [SjVO2]), brain tissue oxygen saturation (also abbreviated as PbtO2, PbrO2, PtiO2, tiO2) monitoring reflects the oxygenation of cerebral tissue local to the sensor placement.4,22,30
• In institutions where SjVO2 is used as a monitoring parameter, the difference between SjVO2 measurements and PbtO2 values must be noted. SjVO2 is a measure of the oxygen contained in the blood draining from the cerebral venous sinuses into the jugular bulb (a measure of global brain oxygenation), whereas PbtO2 measures regional (local to the catheter placement in the cerebral white matter) brain tissue oxygenation.22,24,26
• Understanding of neuroanatomy and physiology, specifically intracranial dynamics, is needed.
• Knowledge of sterile and aseptic technique is necessary.
• Currently, only one brain tissue oxygen monitoring system is available.24
• A brain tissue oxygen probe may be inserted through an intracranial bolt or tunneled.2,30
• PbtO2 monitoring provides information that reflects brain tissue oxygen levels associated with cerebral oxygen demand and systemic oxygen delivery.
• PbtO2 values are relative within an individual. Establishing and following the patient’s cerebral oxygen trends provides the healthcare providers with information that will aid in the assessment and treatment of cerebral hypoxia.
• Indications for PbtO2 monitoring include patients at risk for secondary injury from cerebral edema. Conditions most likely to cause cerebral edema include severe traumatic brain injury, aneurysmal and traumatic subarachnoid hemorrhage, brain tumor, stroke, and any condition that increases ICP.
• Contraindications for PbtO2 monitoring include patients with a coagulopathy, those receiving anticoagulation therapy, and those with an insertion site infection.
• PbtO2 probes are safe with a 1.5-T magnetic resonance imaging (MRI) system as long as the fiberoptic ICP catheter is not in place.11
• PbtO2 probes are safe with computed tomography (CT).
• Cerebral oxygen data are accurate and reliable when the PbtO2 probe is located in the deep white matter of the brain, the location where oxygen availability is most stable.
• Parameters such as ICP and brain tissue temperature can be measured immediately at the time of probe placement.
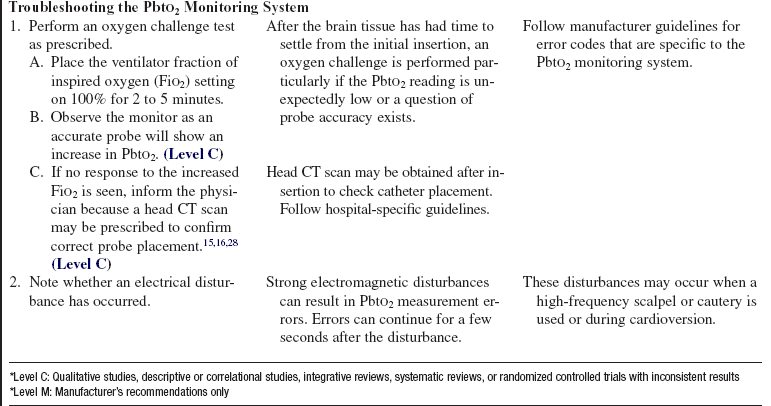
• Monitoring of PbtO2 values may be delayed as long as 2 hours as time is needed for the brain tissue to settle after the microtrauma caused by probe placement.5–7,12,28
• The normal range for brain tissue oxygen values is between 20 and 35 mm Hg.12,13,17,18,24 Treatment goals usually aim to keep the PbtO2 equal to or greater than 20 mm Hg.30
• A PbtO2 of less than 15 mm Hg is a critical threshold associated with a greater chance of functional disability and mortality related to cerebral ischemia.1,3,12,25
• A PbtO2 of less than 10 mm Hg is directly associated with severe disability, poor outcome at discharge, and death.1,3,28
• A PbtO2 of less than 5 mm Hg is indicative of cerebral cell death and an approximately 90% mortality rate.1,3,14,23,25
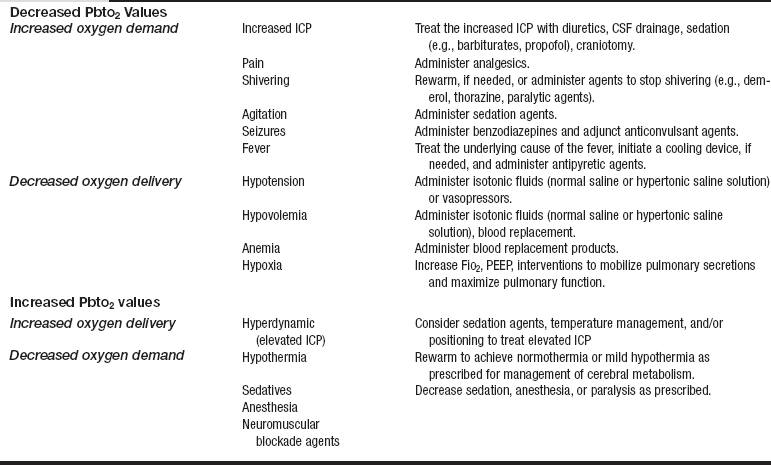
• Brain tissue oxygen values can be used to manage potential cerebral hypoxia. Clinical interventions can be aimed at increasing oxygen delivery or decreasing cerebral oxygen demand.
• Decreases in PbtO2 values occur when cerebral blood flow or cerebral oxygen delivery is inadequate or states of increased metabolic demands exist, indicating the potential for secondary brain injury.
• Increases in PbtO2 values denote decreased oxygen uptake by cerebral cells that may be caused by states of increased oxygen delivery or decreased oxygen utilization.
• Table 87-1 outlines treatment options for patients with a decrease or increase in PbtO2 values.
• PbtO2 probe placement: The physician placing the probe device determines the catheter placement location after review of the CT scan and after consideration of the most appropriate monitoring area based on diagnosis and pathology, avoiding areas of infarct or hematoma.4,17 Placement of the probe may be ipsilateral or contralateral to the pathology.26
 The probe may be placed in the nondominant hemisphere (e.g., right frontal region) to minimize risk of injury from catheter insertion.6,12,29 The right hemisphere is a safer location for probe placement than the left hemisphere because speech function is located in the left hemisphere in most individuals.
The probe may be placed in the nondominant hemisphere (e.g., right frontal region) to minimize risk of injury from catheter insertion.6,12,29 The right hemisphere is a safer location for probe placement than the left hemisphere because speech function is located in the left hemisphere in most individuals.
 Placement may be near a lesion when the clinical goal is to monitor oxygen availability to damaged but salvageable tissue.
Placement may be near a lesion when the clinical goal is to monitor oxygen availability to damaged but salvageable tissue.
 If a patient has a subarachnoid hemorrhage, the probe may be placed in the area of the brain expected to develop vasospasm. Placement is determined by the distribution of subarachnoid blood on CT scan and by aneurysm location.16,21
If a patient has a subarachnoid hemorrhage, the probe may be placed in the area of the brain expected to develop vasospasm. Placement is determined by the distribution of subarachnoid blood on CT scan and by aneurysm location.16,21
 Manufacturer’s recommended guidelines for probe device removal manufacturer’s guidelines or replacement is 5 to 7 continuous days per device. Drift may affect accuracy after 5 days.9 Practice may vary based on institutional guidelines.
Manufacturer’s recommended guidelines for probe device removal manufacturer’s guidelines or replacement is 5 to 7 continuous days per device. Drift may affect accuracy after 5 days.9 Practice may vary based on institutional guidelines.
EQUIPMENT
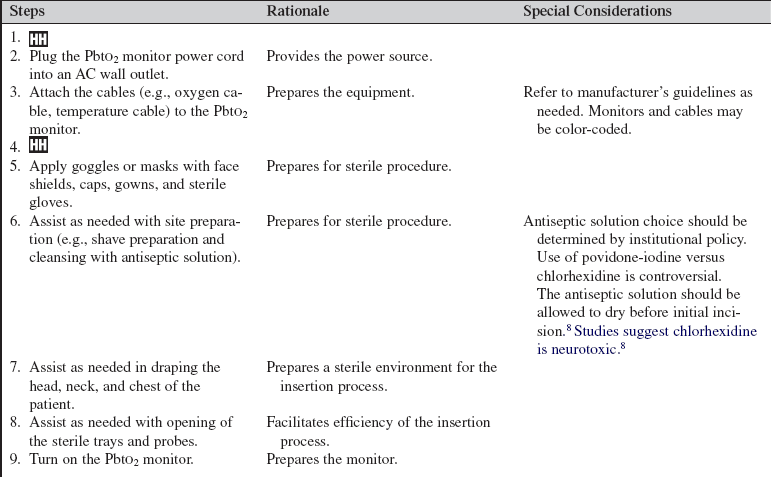
• Sterile gowns, sterile drapes, sterile gloves, nonsterile gloves, caps, goggles, and face masks
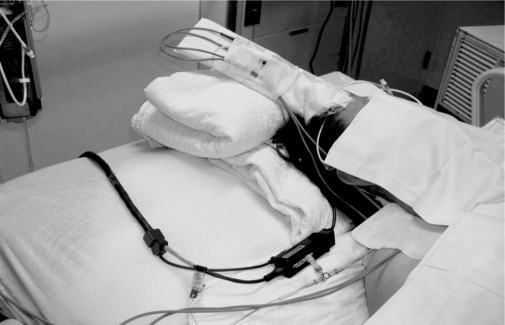
• PbtO2 monitor (Fig. 87-1) or module
Additional equipment to have available as needed includes the following:
• An intravenous (IV) arm board may be used to stabilize the monitor probe and cable
• Extra transparent and soft cloth adhesive dressing or any appropriate dry, sterile occlusive dressing
• A compatible fiberoptic ICP catheter may be inserted through the intracranial bolt system as well and will require a separate monitor to measure ICP
PATIENT AND FAMILY EDUCATION
• Assess patient or family understanding of the purpose of PbtO2 monitoring. Most patients who need brain tissue oxygen monitoring are in an altered level of consciousness with a score of 8 or less on the Glasgow Coma Scale; education is directed toward the family.3  Rationale: Understanding may reduce anxiety and stress, stimulates requests for clarification or additional information, and increases awareness of the goals, duration, and expectations of the monitoring system.
Rationale: Understanding may reduce anxiety and stress, stimulates requests for clarification or additional information, and increases awareness of the goals, duration, and expectations of the monitoring system.
• Explain the insertion process, patient monitoring, and care involving the PbtO2 monitoring system.  Rationale: Explanation may alleviate anxiety and stress and stimulates requests for clarification or additional information.
Rationale: Explanation may alleviate anxiety and stress and stimulates requests for clarification or additional information.
• Explain the expected outcomes of the PbtO2 system.  Rationale: Explanation may decrease patient and family anxiety and stress by increasing awareness of PbtO2 monitoring duration and therapy goals.
Rationale: Explanation may decrease patient and family anxiety and stress by increasing awareness of PbtO2 monitoring duration and therapy goals.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess the patient’s neurologic status.  Rationale: Performing a baseline neurologic assessment enables the nurse to identify changes that may occur as a result of the PbtO2 probe insertion.
Rationale: Performing a baseline neurologic assessment enables the nurse to identify changes that may occur as a result of the PbtO2 probe insertion.
• Assess the patient for signs or symptoms of local infection at the intended insertion location.  Rationale: Evidence of local infection is a contraindication to brain tissue oxygen catheter placement.
Rationale: Evidence of local infection is a contraindication to brain tissue oxygen catheter placement.
• Obtain and review coagulation laboratory results (e.g., complete blood count, platelet count, prothrombin time, partial thromboplastin time, bleeding time, and international normalized ratio).  Rationale: Assessment identifies the patient’s risk for bleeding.
Rationale: Assessment identifies the patient’s risk for bleeding.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that the patient and family understand the procedure teaching. Answer questions as they arise, and reinforce information as needed. Most patients who need brain tissue oxygen monitoring are in an altered level of consciousness with a Glasgow Coma Scale score of eight or less.  Rationale: Information previously taught is evaluated and reinforced.
Rationale: Information previously taught is evaluated and reinforced.
• Ensure that informed consent has been obtained.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient; however, in emergency circumstances, time may not allow for the consent form to be signed.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient; however, in emergency circumstances, time may not allow for the consent form to be signed.
• Perform a pre-procedure verification and time out, if non-emergent.  Rationale: Ensures patient safety.
Rationale: Ensures patient safety.
• Administer sedation or analgesia as prescribed before beginning the insertion procedure.  Rationale: Sedation or analgesia facilitates the insertion process.
Rationale: Sedation or analgesia facilitates the insertion process.
• Assist the patient to the semi-Fowler’s position with the head in the neutral position and the head of bed elevated 30 to 45 degrees.  Rationale: Patients who are candidates for brain tissue oxygen monitoring may have increased ICP. Elevating the head of the bed and placing the head in the neutral position act to decrease intracranial pressure by enhancing jugular venous outflow and provides for optimal insertion accessibility.
Rationale: Patients who are candidates for brain tissue oxygen monitoring may have increased ICP. Elevating the head of the bed and placing the head in the neutral position act to decrease intracranial pressure by enhancing jugular venous outflow and provides for optimal insertion accessibility.
References
![]() 1. Bardt, TF, et al, Monitoring of brain tissue Po2 in traumatic brain injury. effect of cerebral hypoxia on outcome. Acta Neurochir (Wien). 1998; 71(Suppl):153–156.
1. Bardt, TF, et al, Monitoring of brain tissue Po2 in traumatic brain injury. effect of cerebral hypoxia on outcome. Acta Neurochir (Wien). 1998; 71(Suppl):153–156.
2. Bhatia, A, Gupta, AK, Neuromonitoring in the intensive care unit. IIcerebral oxygenation monitoring and microdialysis. Intens Care Med 2007; 33:1322–1328.
3. Bratton, SL, et al, Guidelines for the management of severe traumatic brain injury. Xbrain oxygen monitoring and thresholds. J Neurotrauma 2007; 24:S65–S70.
![]() 4. De Georgia, MA, Deogaonkar, A. Multimodal monitoring in the neurological intensive care unit. Neurologist. 2005; 11:45–54.
4. De Georgia, MA, Deogaonkar, A. Multimodal monitoring in the neurological intensive care unit. Neurologist. 2005; 11:45–54.
![]() 5. Dings, J, Meixenberger, J, Roosen, K, Brain tissue -PO2 monitoring. catheter stability and complications. Neurol Res 1997; 19:241–245.
5. Dings, J, Meixenberger, J, Roosen, K, Brain tissue -PO2 monitoring. catheter stability and complications. Neurol Res 1997; 19:241–245.
![]() 6. Dings, J, et al. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery. 1998; 43:1082–1095.
6. Dings, J, et al. Clinical experience with 118 brain tissue oxygen partial pressure catheter probes. Neurosurgery. 1998; 43:1082–1095.
![]() 7. Haitsma, IK, Maas, AIR, Advanced monitoring in the -intensive care unit. brain tissue oxygen tension. Curr Opin Crit Care 2002; 8:115–120.
7. Haitsma, IK, Maas, AIR, Advanced monitoring in the -intensive care unit. brain tissue oxygen tension. Curr Opin Crit Care 2002; 8:115–120.
8. Hebl, JR. The importance and implications of aseptic -techniques during regional anesthesia. Reg Anesth Pain Med. 2006; 31:311–323.
9. Integra, NeuroSciences. LICOX®IMC complete neuromonitoring directions for use. model IP2. P* complete brain IMC-PROBE KIT,. Plainsboro, NJ: Integra NeuroSciences; 2004.
10. Integra, NeuroSciences. LICOX CMP brain oxygen monitoring system operations manual,. Plainsboro, NJ: Integra Neurosciences; 2004.
11. Integra, NeuroSciences. MRI safety of the Licox IT2 complete brain tunneling probe kit, including the model CC1. P1 oxygen and temperature probe and model VK5. 2 parenteral probe guide at 1. 5 Tesla,. Plainsboro, NJ: Integra Neurosciences; 2006.
12. Lang, EW, et al, Direct cerebral oxygenation monitoring. a systematic review of recent publications. Neurosurg Rev 2007; 30:99–106.
![]() 13. Maas, AIR, et al, Monitoring cerebral oxygenation. experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir. 1993; 59(Suppl):50–57.
13. Maas, AIR, et al, Monitoring cerebral oxygenation. experimental studies and preliminary clinical results of continuous monitoring of cerebrospinal fluid and brain tissue oxygen tension. Acta Neurochir. 1993; 59(Suppl):50–57.
14. Mazzeo, AT, Bullock, R, Monitoring brain tissue oximetry. will it change management of critically ill neurologic patients. J Neurol Sci 2007; 261:1–9.
![]() 15. Meixensberger, J, et al. Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2003; 74:760–764.
15. Meixensberger, J, et al. Brain tissue oxygen guided treatment supplementing ICP/CPP therapy after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2003; 74:760–764.
![]() 16. Meixensberger, J, et al. Monitoring of brain tissue oxygenation following severe subarachnoid hemorrhage. Neurol Res. 2003; 25:445–450.
16. Meixensberger, J, et al. Monitoring of brain tissue oxygenation following severe subarachnoid hemorrhage. Neurol Res. 2003; 25:445–450.
![]() 17. Mulvey, JM, et al, Multimodality monitoring in severe traumatic brain injury. the role of brain tissue oxygenation monitoring. Neurocrit Care 2004; 1:391–402.
17. Mulvey, JM, et al, Multimodality monitoring in severe traumatic brain injury. the role of brain tissue oxygenation monitoring. Neurocrit Care 2004; 1:391–402.
![]() 18. Sarrafzadeh, AS, et al, Cerebral oxygenation in contusioned vs. nonlesioned brain tissue. monitoring of Ptio2 with Licox and Paratrend. Acta Neurochir (Wien). 1998; 71(Suppl):186–189.
18. Sarrafzadeh, AS, et al, Cerebral oxygenation in contusioned vs. nonlesioned brain tissue. monitoring of Ptio2 with Licox and Paratrend. Acta Neurochir (Wien). 1998; 71(Suppl):186–189.
19. Stewart, C, et al, The new licox combined brain tissue oxygen and brain temperature monitor. assessment of in vitro accuracy and clinical experience in severe traumatic brain injury. Neurosurgery 2008; 63:1159–1165.
![]() 20. Stiefel, MF, et al. Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg. 2004; 101(2):241–247.
20. Stiefel, MF, et al. Cerebral oxygenation following decompressive hemicraniectomy for the treatment of refractory intracranial hypertension. J Neurosurg. 2004; 101(2):241–247.
![]() 21. Stiefel, MF. The effect of nimodipine of cerebral oxygenation following subarachnoid hemorrhage. J Neurosurg. 2004; 101(4):594–599.
21. Stiefel, MF. The effect of nimodipine of cerebral oxygenation following subarachnoid hemorrhage. J Neurosurg. 2004; 101(4):594–599.
![]() 22. Stiefel, MF, et al, Multi-modality monitoring in the management of refractory intracranial hypertension. a case report. J Trauma. 2005; 59(3):757–761.
22. Stiefel, MF, et al, Multi-modality monitoring in the management of refractory intracranial hypertension. a case report. J Trauma. 2005; 59(3):757–761.
![]() 23. Stiefel, MF, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005; 103(5):805–811.
23. Stiefel, MF, et al. Reduced mortality rate in patients with severe traumatic brain injury treated with brain tissue oxygen monitoring. J Neurosurg. 2005; 103(5):805–811.
24. Tisdall, MM, Smith, M, Mulimodal monitoring in traumatic brain injury. current status and future directions. Br J Anaesth 2007; 99:61–67.
![]() 25. Valadka, AB, et al. Relationship of brain tissue Po2 to outcome after severe head injury. Crit Care Med. 1998; 26:1576–1581.
25. Valadka, AB, et al. Relationship of brain tissue Po2 to outcome after severe head injury. Crit Care Med. 1998; 26:1576–1581.
![]() 26. Valadka, AB, et al, Brain tissue Po2. correlation with cerebral blood flow. Acta Neurochirurgica. 2002; 81(Suppl):299–301.
26. Valadka, AB, et al, Brain tissue Po2. correlation with cerebral blood flow. Acta Neurochirurgica. 2002; 81(Suppl):299–301.
![]() 27. van den Brink WA, et al, Monitoring brain oxygen tension in severe head injury. the Rotterdam experience. Acta Neurochir. 1998; 71(Suppl):190–194.
27. van den Brink WA, et al, Monitoring brain oxygen tension in severe head injury. the Rotterdam experience. Acta Neurochir. 1998; 71(Suppl):190–194.
![]() 28. van den Brink WA, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000; 46:868–878.
28. van den Brink WA, et al. Brain oxygen tension in severe head injury. Neurosurgery. 2000; 46:868–878.
![]() 29. van Santbrink, H, et al. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996; 38:21–31.
29. van Santbrink, H, et al. Continuous monitoring of partial pressure of brain tissue oxygen in patients with severe head injury. Neurosurgery. 1996; 38:21–31.
30. Wartenberg, KE, Schmidt, JM, Mayer, SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007; 23:507–538.
Bader, MK, Littlejohns, LR, March, K, Brain tissue oxygen monitoring II. implications for critical care teams and case study. Crit Care Nurse 2003; 23:29–44.
Blissitt, PA. Brain oxygen monitoring. In: Bader MK, Littlejohns LR, eds. AACN AANN protocols for practice: monitoring technologies in critically ill neuroscience -patients. Boston: Jones and Bartlett; 2009:103–144.
Gracias, VH, et al, Cerebral cortical oxygenation. a pilot study. J Trauma Injury Infect Crit Care. 2004; 56(3):469–474.
Maloney Wilensky, E, et al. Brain tissue oxygen practice guidelines using the LICOX CMP monitoring system. J Neurosci Nurs. 2005; 37(5):278–288.
Littlejohns, LR, Bader, MK, Guidelines for the management of severe head injury. clinical application and changes in practice. Crit Care Nurse 2001; 21:48–65.
Littlejohns, LR, Bader, MK, March, K, Brain tissue -oxygen monitoring in severe brain injury. Iresearch -and usefulness in critical care. Crit Care Nurse 2003; 23:17–25.
Patterson, J, et al, Successful outcome in severe traumatic brain injury. a case study. J Neurosci Nurs. 2005; 37(5):236–242.
Smith, MJ, et al. Packed red blood cell transfusion increases local cerebral oxygenation. Crit Care Med. 2005; 33:1104–1108.
Stiefel, MF, et al. Conventional neurocritical care does not ensure cerebral oxygenation after traumatic brain injury. J Neurosurg. 2006; 105:568–575.