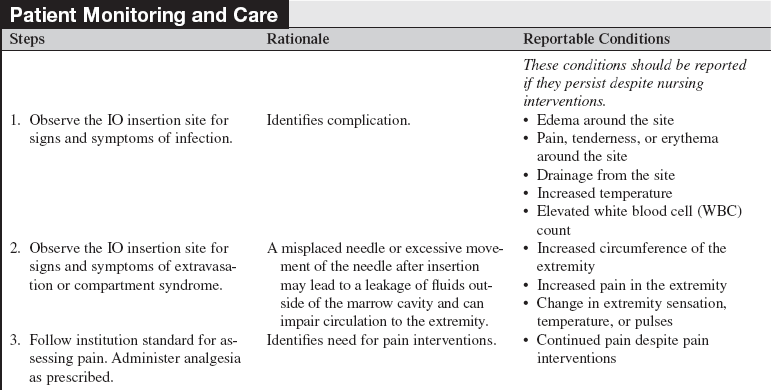
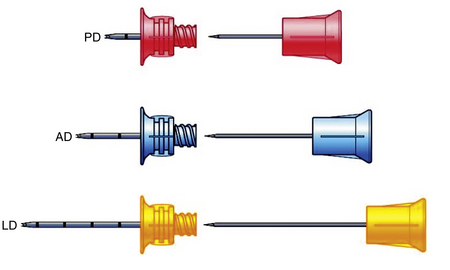
Intraosseous Devices
Intraosseous access is indicated when intravenous access cannot be obtained or cannot be obtained in a timely manner (within 90 seconds10) and access to venous circulation is needed for the administration of medications or fluids.
PREREQUISITE NURSING KNOWLEDGE
• Intraosseous (IO) access is a safe and reliable access point into the noncollapsible marrow cavity that allows direct access to the venous circulation.
• The Volkmann’s canals that are located throughout the bone connect with the medullary canal and the blood vessels of the periosteum (Fig. 84-1). When medications and fluids are introduced into the medullary canal, they flow through the vascular plexi directly into the vascular system.6
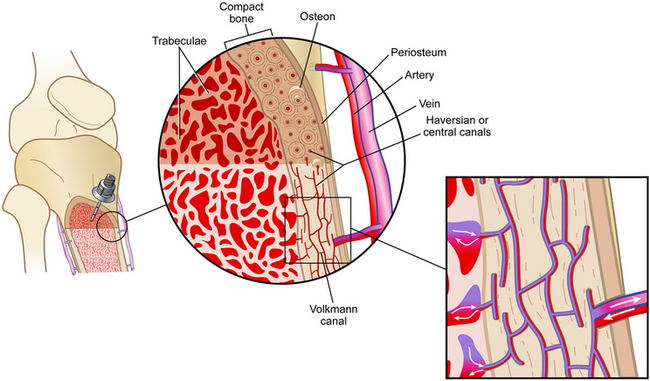
Figure 84-1 IO Circulation. (From Day MW: Intraossesous devices in the adult trauma patient,Crit Care Nurs. In press.)
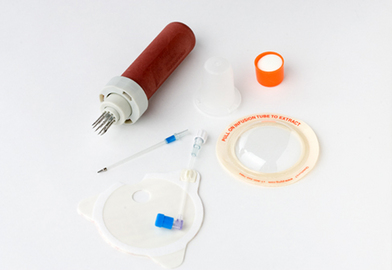
• Insertion devices are available for insertion of IO needles.6 These devices include the bone injection gun (BIG; Waismed, a Persys Medical Co, Houston, TX; Fig. 84-2), the FAST1 adult intraosseous infusion system (Pyng Medical Corp, Vancouver, BC, Canada, Fig. 84-3), and the EZ-IO (Vidacare Corp, Shavano Park, TX; Fig. 84-4). These three devices are approved by the US Food and Drug Administration (FDA) for IO access in adult patients. Two of the devices (BIG, EZ-IO) use a specially designed needle with a stylet or trocar. The third device (FAST1) uses a metal-tipped plastic catheter.
• In adults, the available IO access sites, depending on the specific device and following each manufacturer’s guidelines, include:
 Tibial plateau: 1 to 2 cm distal to the tibial tuberosity3,5,8
Tibial plateau: 1 to 2 cm distal to the tibial tuberosity3,5,8
 Distal tibia: 1 to 2 cm above the medial malleolus7
Distal tibia: 1 to 2 cm above the medial malleolus7
• IO blood can be used for many laboratory tests, including typing and screening, electrolyte values, chemistries, blood gas values, drug levels, and hemoglobin levels.2 However, specimen samples from the marrow have a lower correlation to serum levels after 30 minutes of resuscitation.3 In addition, drawing of blood from an IO device may not be recommended by specific manufacturers and has the potential of occluding the device.
• The onset of action for medications is similar to that of intravenous (IV) medications.13 However, administration via the IO route may result in lower serum concentrations versus the IV route for the following medications: ceftriaxone, chloramphenicol, phenytoin, tobramycin, and vancomycin.5
• Marrow-toxic medications should not be infused via the IO route.8
• All resuscitation medications, isotonic fluids, and blood products may be given via the IO route2,3,5,14; however, myonecrosis has been reported with the infusion of hypertonic saline solution via the IO route.10,12
• Medications administered via the IO route should be followed by a 5- to 10-mL flush of normal saline solution.4
• Fluids running into an IO line should be administered with a pressure bag because the pressure needed to push the fluid into the bone marrow may exceed that of volumetric IV pumps.
• Complications of IO access include compartment syndrome, osteomyelitis, fracture, extravasation,5 necrosis,5 and infection.7
• A syringe should not be attached directly to the hub of the IO needle because it could cause dislodgment, increase the size of the hole, and cause extravasation or loss of the IO site. To extend access to the IO needle, attach extension tubing to the hub of the IO needle and secure it to the skin.11 Some device insertion kits come with extension tubing.
• Absolute contraindications to attempting an IO access include previous attempts or fractures of the targeted bone.
• Relative contraindications to IO access include infection at the access site, previous bone surgery at the insertion site, fractures above the insertion site,9 and bone disorders, such as osteoporosis and osteogenesis imperfecta.3 Another relative contraindication to the FAST1 is skin damage at the insertion site, which may preclude the adherence of the target patch used to secure the device.
• IO access in obese patients may be more difficult. The EZ-IO has a needle set specifically designed for the patient with “excessive tissue” at the insertion site.
• IO access is meant to be a temporary venous access; IO lines should be removed as soon as other venous access is obtained or within 24 hours of insertion.
EQUIPMENT
PATIENT AND FAMILY EDUCATION
• Explain to the patient and family the reason for the IO access.  Rationale: Clarification of information is an expressed patient need and helps to diminish anxiety, enhance acceptance, and encourage questions.
Rationale: Clarification of information is an expressed patient need and helps to diminish anxiety, enhance acceptance, and encourage questions.
• Describe the major steps of the procedure, including the patient’s role in the procedure.  Rationale: Explanation decreases patient anxiety, enhances cooperation, provides an opportunity for the patient to voice concerns, and prevents accidental contamination of the sterile field and equipment.
Rationale: Explanation decreases patient anxiety, enhances cooperation, provides an opportunity for the patient to voice concerns, and prevents accidental contamination of the sterile field and equipment.
• Explain the expected outcomes of the procedure.  Rationale: Explanation reduces anxiety and clarifies the duration and goals of IO access.
Rationale: Explanation reduces anxiety and clarifies the duration and goals of IO access.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess the patient for fractures or infections at the insertion site, for previous bone surgeries at the site, and for a history of osteoporosis or fractures of the target bone.  Rationale: An alternate site should be accessed to avoid possible complications associated with the previous conditions.
Rationale: An alternate site should be accessed to avoid possible complications associated with the previous conditions.
• Obtain the patient’s baseline vital signs and cardiac rhythm.  Rationale: Baseline data facilitate the identification of clinical problems and identify the urgency of obtaining IO access.
Rationale: Baseline data facilitate the identification of clinical problems and identify the urgency of obtaining IO access.
• If possible, determine the patient’s allergy history (e.g., lidocaine, antiseptic solutions).  Rationale: This assessment decreases the risk for allergic reactions by avoiding known allergenic products.
Rationale: This assessment decreases the risk for allergic reactions by avoiding known allergenic products.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that the patient and family understand preprocedural teaching. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Perform a pre-procedure verification and time out, if non-emergent.  Rationale: Ensures patient safety.
Rationale: Ensures patient safety.
References
1. American Heart Association, Access for medication, part 3 . intraosseous access. 2006. In Advanced cardiac life support provider manual, student CD: AHA.
2. Bailey, P, Intraosseous cannulation, UpToDate database. www.uptodate.com/online/content/topic.do?topicKey=ped_proc/4947&view=print# retrieved July 15, 2009. Waltham, MA, 2008.
3. Blumberg, SM, Gorn, M, Crain, EF, Intraosseous infusion. a review of methods and novel devices. Pediatr Emerg Care 2008; 24:50–59.
4. Bosomworth, NJ. The occasional intraosseous infusion. Can J Rural Med. 2008; 13:80–83.
5. Buck, ML, Wiggins, BS, Sesler, JM. Intraosseous drug administration in children and adults during cardiopulmonary -resuscitation. Ann Pharmacother. 2007; 41:1679–1686.
6. Day MWIntraosseous devices in the adult trauma patient Crit Care Nurs. In press.
![]() 7. Gluckman, W, Forti, RJ. Intraosseous cannulation, emedicine. http://emedicine.medscape.com/article/908610-overview . 2003. [retrieved June 29, 2009].
7. Gluckman, W, Forti, RJ. Intraosseous cannulation, emedicine. http://emedicine.medscape.com/article/908610-overview . 2003. [retrieved June 29, 2009].
8. Holleran, RS. Procedure 67 intraosseous access. In Proehl J, ed. : Emergency nursing procedures, ed 4, St Louis: Elsevier, 2009.
9. Infusion Nurses Society. The role of the registered nurse in the insertion of intraosseous (IO) access devices, INS Position Statement. INS, Norwood, MA: Infusion Nurses Society; 2009.
10. Langley, DM, Moran, M, Intraosseous needles. not just for kids anymore. J Emerg Nurs 2007; 34:318–319.
11. MacKinnon, KA. Intraosseous vascular use at Signature Healthcare Brockton Hospital Department of Emergency Services. J Emerg Nurs: In press; 2009.
12. Ong, MEH, Chan, YH, Oh, JJ, et al. An observational, prospective study comparing tibial and humeral intraosseous access using the EZ-IO. Am J Emerg Med. 2009; 27:8–15.
13. Von Hoff DD, Kuhn, JG, Burris, HA, et al. Does intraosseous equal intravenous? A pharmacokinetic study. Am J Emerg Med. 2008; 26:31–38.
![]() 14. Vreede, E, Bulatovic, A, Rosseel, P, et al. Article 10 intraosseous infusion, update in anesthesia, 12. www.nda.ox.ac.uk/wfsa/html/u12/u1210_01.htm, June 29, 2009.
14. Vreede, E, Bulatovic, A, Rosseel, P, et al. Article 10 intraosseous infusion, update in anesthesia, 12. www.nda.ox.ac.uk/wfsa/html/u12/u1210_01.htm, June 29, 2009.
Brenner, T, Bernhard, M, Helm, M, et al. Comparison of two intraosseous infusion systems for adult emergency medical use. Resuscitation. 2008; 78:314–319.
Gunal, I, Kose, N, Gurer, D, Compartment syndrome after -intraosseous infusion. an experimental study in dogs. J Pediatr Surg. 1996; 31(11):1491–1493.
Rosetti, VA, Thompson, BM, Miller, J, et al, Intraosseous infusion. an alternative route of pediatric intravascular access. Ann Emerg Med 1985; 14:885–888.