CHAPTER 81 Traumatic Brain Injury
OVERVIEW
The human brain can be injured in a variety of ways (e.g., from acquired brain injuries and from developmental or congenital abnormalities of the brain). Head trauma, vascular disorders, degenerative disorders (such as Pick’s disease, Huntington’s chorea, or amyotrophic lateral sclerosis [ALS]), toxic exposure, infectious processes (e.g., acquired immunodeficiency syndrome [AIDS]), neoplasms, anoxia, metabolic or endocrine disorders, and nutritional deficiencies can each damage neuroanatomical structures and alter neurological function. Closed head injury, or traumatic brain injury (TBI), is the most common acquired brain injury.1
TBI, referred to as a “silent epidemic,”2 is one of the leading causes of death and disability in the United States.3 Nearly 1.4 million head injuries occur each year,2 and of these, approximately 50,000 people die from their injuries, 235,000 require hospitalization, and 1.1 million are treated and released from the emergency department (ED).2,4 An unknown number of additional patients with TBI are never seen at the hospital.5 Injury to the brain disrupts cognitive, physical, emotional, and behavioral function. These deficits may resolve within a few months or be permanent. While the majority of individuals who sustain a TBI recover, 80,000 to 90,000 Americans sustain permanent neuropsychiatric disabilities each year; most of those return home either directly from the ED or after a relatively brief hospitalization.2 Even among these individuals, physical, cognitive, behavioral, and emotional impairments result in substantial disability and cause significant stress within families.3 Complications (e.g., suicide, divorce, chronic unemployment, economic strain, and substance abuse) increase after TBI.5 The consulting psychiatrist plays an important role in the evaluation and treatment of these patients at all stages of recovery.
EPIDEMIOLOGY AND RISK FACTORS
Among the most common causes of TBI are falls (28%) and motor vehicle accidents (20%).2 Having the head struck by, or against, an object (19%), being assaulted (11%), and experiencing other (13%) or unknown (9%) problems make up the remaining cases.2 Men sustain TBI at a rate 1.5 times higher than women and are hospitalized almost twice as frequently.2 TBI occurs most frequently in children ages 0 to 4 years, followed by older adolescents (15 to 19 years of age).2 The highest rates of hospitalization and death following TBI are found in adults over age 75.2 Falls produce the most injuries for children younger than 15 years and for adults over age 55.2 Motor vehicle accidents account for the most injuries among adolescents ages 15 to 19 and adults ages 20 to 55.2 Earlier research has found that 56% of adults identified as having brain injuries had an elevated blood alcohol level (BAL) at the time of injury; 49% of them had a BAL at or above the legal level. Recurrent brain injury is common; the risk of a second injury is three times higher than it is for those in the general (noninjured) population.4 Following a second injury, the risk for a third injury becomes nearly 10 times higher than the risk for an initial injury.4 Finally, review of data from the United States National Health Interview, a national database used to estimate the incidence and features of persons with brain injury, found that the highest rates of injury occurred in families with the lowest income levels (Table 81-1).4
Table 81-1 Risk Factors for Traumatic Brain Injury in Adults
PATHOPHYSIOLOGY
TBI is a spectrum disorder; injury can be focal, diffuse, or both.6 Damage can occur as a result of forces exerted on the brain at the time of injury, known as the primary injury,1,7,8 and from subsequent physiological processes (such as swelling or hypoxia) triggered by the initial insult; these are classified as secondary injuries.7,8 Focal damage is typically the result of contusions or mass lesions.6 Most often they arise from contact injuries (such as falls or blows to the head)6 and result in skull fractures and hematomas (extradural, subarachnoid, subdural, or intracerebral hematomas).6 Hematomas may develop at the point of contact and at a point contralateral to the point of contact (known as coup-contrecoup contusions).1 Contusions are seen more frequently in the poles of the frontal lobes, the inferior aspects of the frontal lobes, the cortex above and below the operculum of the Sylvian fissures, the temporal poles, and the lateral and inferior aspects of the temporal lobes.6 They may develop quickly within minutes of the injury or evolve slowly over several hours or days. The presence of these contusions contributes to neuronal necrosis and to elevated intracranial pressure (ICP).5,6 In addition to contusions, contact forces can result in small or complete tears at the pontomedullary junction, damage to any of the cranial nerves, damage to the hypothalamus or pituitary gland, and damage to blood vessels.6 Moreover, there can be multiple areas of focal damage.
Diffuse damage involves multiple neurological structures. It is seen more frequently in injuries that involve rapid acceleration, deceleration, or rotational forces.1 Diffuse damage can also result from disruption of vascular function and from hypoxia.6 Diffuse axonal injury (DAI) occurs at the level of the axons and disrupts cellular function and structures.5 Several structures (subcortical frontal and temporal white matter, the corpus callosum, and brainstem) are most frequently involved.6 It appears that the permeability of the axolemma is altered and results in impaired axonal transport and in subsequent swelling before the axon detaches from its downstream segment. While this process appears to be triggered by the mechanical forces of the original injury, it is one that evolves over several hours to days.5,6 Because this damage occurs at a microscopic level, DAI is often missed on computed tomography (CT) scans.1 It is more easily identified with magnetic resonance imaging (MRI), where the signature axonal swelling and axonal bulbs can be seen more readily, particularly if the brain is imaged several days after the injury.1 Even with MRI, however, the absence of significant findings on radiological imaging does not mean that damage has not taken place.7
In addition to the primary injuries, further damage can occur as a result of complications associated with TBI (known as secondary injury).1 Hematomas develop as a result of the hemorrhaging from torn blood vessels. Edema develops when there is an increase in intercellular or intracellular water concentration (or both), due to direct mechanical forces or changes in cell permeability.8,9 Since the skull is fixed and unable to expand, hematomas and edema raise the ICP, which leads to further neurological damage as the surrounding (softer) structures become deformed. The pressure can push the brain through the base of the skull, causing damage to the brainstem.1 The vascular system that supplies the brain is compressed, restricting the flow of blood, which results in ischemic damage.8 Compromised cardiopulmonary function, as either a direct result of brain damage or the structural damage sustained in the initial trauma, can lead to hypoxic injury. Hyper-release of catecholamines following TBI can produce transient hypertension, as well as changes in glucose, cortisol, and thyroid hormones, which further disrupts neurological function.9 Focal contusions can produce seizures (which convey the severity of the injury in that they are seen more frequently following a severe injury than with a moderate or mild injury).9
The brain’s plasticity, or neuroplasticity, produces structural and organizational changes that result in recovery of function.10 The hippocampus retains the ability to generate new neurons from dividing progenitor cells in the dentate gyrus.10 Surviving cortical structures take over the function of damaged areas.11 Other adaptive and restorative processes include changes in the amount of neurotransmitters released, the number and distribution of postsynaptic receptors, the size and complexity of the dendritic trees of spared neurons, and their collateral sprouting of spared axons to innervate deafferented neurons.11 These processes are dependent on purposeful and active interactions with the environment.10 Unfortunately, they are not well regulated, and can lead to maladaptive, as well as to beneficial, change.11 While they are responsible for restoring function, they can result in maladaptive behavior and psychiatric disorders. In the case of mild TBI, symptoms will usually resolve within 6 months of injury.12 In the case of moderate to severe TBI, the greatest amount of recovery typically takes place in the first 1 to 2 years following injury.1 Recovery can continue, however, at an increasingly slower pace for many years following the injury.5,9
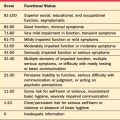
TBI is typically classified as mild, moderate, or severe (Table 81-2) based primarily on the duration of altered mental status (including the degree of responsiveness, as measured by the Glasgow Coma Scale [GCS], and the duration of disrupted memory). These terms can be misleading as they reflect the degree of damage the brain has sustained; they do not necessarily reflect the severity of the disruption in the patient’s daily function. Individuals with a severe injury can make essentially full recovery, whereas others with mild to moderate injuries can remain significantly disabled for many years. The GCS, developed by Teasdale and Jennett, assigns points for increasingly complex levels of response to three dimensions (verbal and motor response and eye opening); the ratings in each domain are totaled to produce an overall score that can range from 3 to 15 (Table 81-3). Ratings can also be done serially to provide a measure of recovery. GCS scores have been predictive of ultimate outcome, with lower initial scores being associated with more severe injury and worse recovery.1,4
| Category | Score |
|---|---|
| Eye Opening | |
| Spontaneous | 4 |
| To voice | 3 |
| To painful stimulus | 2 |
| None | 1 |
| Verbal Response | |
| Oriented | 5 |
| Confused | 4 |
| Inappropriate words | 3 |
| Unintelligible sounds | 2 |
| None | 1 |
| Motor Response | |
| Follows commands | 6 |
| Localizes pain | 5 |
| Withdraws from pain | 4 |
| Flexor response | 3 |
| Extensor response | 2 |
| None | 1 |
From Teasdale G, Jennett B: Assessment and prognosis of coma after head injury, Acta Neurochir (Wien) 34(1-4):45-55, 1976.
Mild TBI (MTBI) may not show up on a CT scan, an MRI, or an electroencephalogram (EEG). Where there are positive radiological findings, the injury is classified as a complicated MTBI. Performance on a routine neurological examination (see Chapter 72), which tends to focus on sensorimotor function, may be essentially normal, although performance may represent a decline relative to preinjury performance.12 Acute symptoms may persist for varying lengths of time. Physical symptoms often include nausea, vomiting, dizziness, headaches, blurred vision, an increased sensitivity to noise and light, diminished libido, sleep disturbance, quickness to fatigue, lethargy, or sensory loss (Table 81-4).7 Cognitive deficits typically involve attention, concentration, perception, memory, speech/language, or executive functions.7,12,13 These cognitive deficits are best identified through an in-depth neuropsychological evaluation. Behavioral changes (such as irritability, quickness to anger, disinhibition, or emotional lability) may follow.7,12,13 Symptoms generally resolve within 6 months of the injury. Physical, cognitive, emotional, and behavioral symptoms frequently persist, and cannot be accounted for by other peripheral injuries, or one’s emotional state or psychological reaction to physical or emotional stressors.12
Table 81-4 Symptoms of Mild Traumatic Brain Injury
| Physical | Cognitive | Behavior |
|---|---|---|
| Nausea | Decreased attention | Irritability |
| Vomiting | Decreased concentration | Quick to anger |
| Dizziness | Problems with perception | Disinhibition |
| Headaches | Problems with memory | Emotional lability |
| Blurred vision | Problems with speech production | |
| Increased sensitivity to noise and light | Problems with speech comprehension | |
| Diminished libido | Executive dysfunction | |
| Sleep disturbance | ||
| Reduced stamina | ||
| Lethargy | ||
| Sensory loss |
While neurological damage can occur without formal loss of consciousness (LOC), LOC is considered a hallmark of most TBIs. The depth and duration of lost consciousness generally reflect the severity of injury. The longer the duration, the more severe the injury and more guarded the prognosis for recovery.9 No single path or pattern of recovery follows a brain injury,5 because there are so many variables involved (e.g., the location and extent of injury, the patient’s age and overall health, the presence of alcohol, the medical and psychological history, concurrent processes [such as infections or seizures], and availability of appropriate rehabilitation services and supports).
The Rancho Los Amigos Levels of Cognitive Functioning Scale (RLAS) is widely used in delineating the stages of recovery (Table 81-5).1,5 It is an eight-point scale describing stages of cognitive and behavioral change used to track improvement following a TBI. While the scale provides a useful way of identifying a patient’s level of recovery, it has not been able to predict the ultimate rate or level of recovery. It has less relevance beyond level IV because patients show increasingly varied patterns of recovery beyond this level. Patients do not progress through the levels at a uniform or predictable rate. Individuals may progress through different levels at different rates. Progress in various domains is not universal; some levels of function (such as motor function) progress more rapidly and recover more fully than do language or memory.
Table 81-5 Ranchos Los Amigos Levels of Cognitive Functioning Scale (RLAS)
| Level | Behavior |
|---|---|
| I | No response |
| II | Generalized response |
| III | Localized response |
| IV | Confused—agitated |
| V | Confused—inappropriate |
| VI | Confused—appropriate |
| VII | Automatic—appropriate |
| VIII | Purposeful and appropriate |
CLINICAL FEATURES
No single profile characterizes the presentation of TBI (Table 81-6). A patient’s profile is the result of the location, depth, and volume of focal lesions and the extent of diffuse axonal injury. Age, previous injury, use of alcohol, and co-morbid conditions (such as hypoxia or hypertension) further contribute to the specific collection of deficits observed.14 Generally speaking, cognitive deficits, personality and behavioral changes, and psychiatric disorders follow TBI. The domains of attention, memory, language, and executive function are typically affected. Since they are somewhat hierarchical, deficits in more fundamental areas (such as attention) can limit performance in higher-level tasks of executive function. Day-to-day and within-day performance can vary considerably.15
Cognitive Impairments
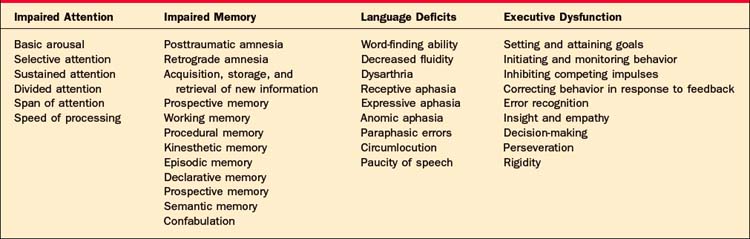
Impaired attention is one of the most common deficits associated with TBI involving the reticular activating system and the prefrontal or connecting white matter.14 Individuals with attentional difficulties report decreased concentration, being unable to follow conversations in a group setting, losing track of what they are reading, being distractible, being unable to do more than one thing at a time, and being unable to sustain attention.1,14 Reduced speed of processing, while not strictly an attention deficit, limits the amount of information that can be processed, the ability to respond quickly, and the ability to complete tasks within traditional time frames.
Impaired memory is also common following TBI.16 The duration of posttraumatic amnesia (PTA), the inability to recall information presented after the accident, correlates with the severity of one’s injury. While some patients have a period of retrograde amnesia (i.e., the inability to recall information acquired before the trauma), problems acquiring, storing, and retrieving new information are more common.16 Memory is not a unitary construct; there are different forms of memory that may be affected to differing degrees depending on the nature and the location of injury.1,16 Because different neuroanatomical structures are involved with these various forms, there is typically sparing of some forms of memory. Procedural memory (i.e., memory for motor sequences that occur outside of conscious awareness) is typically less affected than is memory for more language-based or visual information.1,16 This also means that there is not a specific profile of memory deficit associated with TBI. Declarative memory (i.e., the ability to recall events [episodic memory] and specific facts [semantic memory]) is more vulnerable to damage because of the active processes and neural structures involved. Encoding, consolidating, and retrieving new information involves a degree of effortful, controlled, and generally conscious processing.14 Much of this activity appears to involve the hippocampus, as well as the prefrontal, temporal, and frontal structures. The hippocampus is particularly vulnerable to diffuse axonal injury. The prefrontal and frontal areas, due to their anatomic location and their proximity to orbital cranial structures, are also vulnerable to contusions and hematoma formation.14 The ability to retrieve old or previously learned information typically returns before the ability to acquire new information. For a period of time amnestic individuals may confabulate,1 or generate false memories, which can be problematic because they can be indistinguishable from “real memories.” False memories typically have elements of truth embedded within them, as people involved may actually exist or events recalled may really have happened. The memories, however, contain significant distortions. The affected individual may recall a visit the day before from a friend who has been dead for many years, or may report returning from a trip the day before that had taken place a number of years ago. Confabulation is distinguished from being delusional in that the confabulation is often more isolated, less organized, and often more transient than are delusions. Confabulation typically resolves as the patient’s overall memory improves. The ability to recall new information typically takes more time to resolve and may be a persistent or permanent deficit.14 Acquisition, storage, and retrieval of new information are procedures that involve attention, sensory function, language, and executive function. Deficits in any of these areas limit the ability to participate in new learning. Even in individuals with mild TBI, new learning is less efficient, thus requiring more effort than was required before their injury.14 This inefficiency and increased effort makes it harder for an individual to sustain performance relative to his or her preinjury levels.
Impaired language results from damage to frontal and temporal areas. The nature of the deficits will depend on the location and extent of the injury.17 Disruption of language (both receptive and expressive) occurs more frequently after moderate to severe injuries than after mild TBI. In mild TBI, disturbances tend to be limited to difficulties with word finding and with decreased fluidity when speaking. Global aphasia (total loss of both receptive and expressive language) is relatively rare. More commonly, specific aphasic syndromes include anomic aphasia (in which an individual has difficulty naming specific objects and proper names), paraphasic errors, and circumlocution. In addition to these primary language impairments, patients with TBI tend to generate less speech, are less efficient in their discourse, and have more trouble managing the interpersonal pragmatics of speech (such as taking turns, maintaining a topic of conversation, taking a listener’s perspective, and interpreting the nonverbal elements of communication).14
Finally, impaired executive function can be seen at all levels of TBI. These skills are the functions of the frontal lobes and their projections, which are particularly prone to injury.18 Executive function encompass those skills needed to operate independently in the world (i.e., to identify goals, to plan, and to organize behavior to meet those goals). They involve initiating and monitoring behavior, inhibiting competing impulses or behaviors, and correcting behavior in response to feedback. They are essential for self-determination, self-direction, and self-regulation. Problems with regulation of attention, working memory, insight and empathy, verbal fluency, decision-making, perseveration, and inflexibility frequently follow damage to the frontal lobes.1,19
Personality and Behavioral Changes
In many respects the changes in behavior and personality that follow TBI, particularly when there is frontal lobe involvement, are more disabling than are the cognitive changes (Table 81-7).1,20 They limit an individual’s ability to participate in therapy, they are a source of significant stress on families and caretakers,20 and they can prevent the individual from returning home and returning to work.21 The individual may appear to move about aimlessly, become preoccupied with seemingly trivial matters, or perseverate on topics or concerns in an obsessional manner. The individual may fail to be aware of, or take in, information from the environment or alter his or her behavior in response to feedback. As a result the individual may have a difficult time learning from his or her mistakes. Because of deficits in executive function, several functions (e.g., planning, sequencing, and initiating behavior in a goal-directed manner) become difficult. Looking ahead and anticipating the implications or consequences of one’s actions becomes difficult following frontal lobe damage; this can interfere with participation in therapy because the individual fails to grasp the long-term benefits of treatment in the absence of more immediate gratification. Deficits in the modulation of affect, self-awareness, and self-monitoring result in behavior that is socially inappropriate. Temper outbursts and mood swings, often dysphoric in nature, are common after temporal lobe damage.1 Flatness of affect and indifference, belligerence and aggression, childishness, euphoria and abnormal jocularity, irritability and reduced tolerance for frustration, disinhibition, and lack of empathy may arise, interfering with normal social relationships.22 Individuals have a difficult time identifying and initiating activities that foster social interaction. Previously active people will be content to sit and watch television for hours on end. Individuals may begin to avoid social gatherings because the demands of selectively attending, shifting attention, self-monitoring, and comprehending language and emotive expression are overwhelming. All of these deficits are subject to the effects of fatigue and environmental variables (such as supportive structure, level of sensory stimulation, and degree of familiarity). An individual’s behavior is seen as erratic and difficult to predict. Social isolation becomes common as friends and even family withdraw from behavior that can be disruptive, embarrassing, and even dangerous. The burden these behaviors place on families who are already stressed by changes in financial resources and the demands of physical care and increased dependence can be enormous.1
Table 81-7 Personality and Behavioral Changes Associated with Traumatic Brain Injury
| Aggression | Need for immediate gratification |
|---|---|
| Apathy | Mood lability |
| Withdrawal | Erratic and difficult to predict |
| Lack of goal-directed activity | Temper outbursts and mood swings |
| Lack of empathy | Abnormal jocularity |
| Distractible | Irritability and reduced tolerance for frustration |
| Difficulty learning from mistakes | Disinhibition |
| Impulsivity |
Aggressive behavior is perhaps the most disruptive of the behavioral changes observed after TBI. Agitated, combative, disinhibited behavior is common during the initial stages of posttraumatic delirium23 and is one of the defining features of the Ranchos Los Amigos Level IV. Agitated behavior at this stage tends to be reactive in nature, and often arises in response to seemingly minor or trivial stimuli. It is neither planned nor serves a purpose other than to eliminate the source of irritation. The behavior is often explosive and occurs with little buildup or warning. Brief outbursts alternate with long periods of calm. Where the individual is aware of the behavior, he or she is often upset or embarrassed by it.24 Early agitation tends to resolve as cognition improves.
Agitated and aggressive behavior can persist beyond the acute phase of recovery; it has been observed following severe TBI in 31% to 71% of cases studied and in 5% to 71% of cases involving mild TBI.23 Agitated and aggressive behaviors persist in 31% to 71% of patients from 1 to 15 years after injury.24 Aggressive behavior has been associated with damage to the inferior orbital surface of the frontal lobes, the anterior temporal lobes, the hypothalamus, and limbic structures.23,24 Changes in neurotransmitter levels (particularly serotonin, norepinephrine, dopamine, acetylcholine, and gamma-aminobutyric acid [GABA]) have also been associated with impulsive and aggressive behavior.23–25 A preinjury history of psychiatric illness, attention-deficit disorder (ADD), aggressive behavior, poor social function, or alcohol or drug abuse has been identified as a risk factor for aggressive behavior following TBI.23–25
Aggressive behavior may also occur as a result of a mood disorder, psychosis, or seizure disorder (Table 81-8),24,26 although aggressive behavior occurring in the context of seizure disorders can take different forms.24 Ictal or postictal behavior tends to be less focused or directed and is accompanied by an altered level of consciousness. Following the outburst the individual is likely to express regret and remorse when informed about the behavior. Interictal aggression tends to be more directed and is less ego-dystonic. Delirium resulting from hypoxia, electrolyte imbalance, metabolic disorders, dehydration, and infection can trigger aggressive behavior.24 Drugs and medications can produce aggressive behavior. Alcohol, barbiturates, benzodiazepines, analgesics, steroids, antidepressants, amphetamines, antipsychotics, and anticholinergic drugs can contribute to sedation and to disinhibition that leads to increased irritability and aggression (Table 81-9).24 Aggressive behavior may be learned as a result of an environmental response to the behavior. Increased attention or the withdrawal of unpleasant demands or stimuli may reinforce and increase, or maintain, aggressive behavior.
Table 81-8 Potential Sources of Aggression in Traumatic Brain Injury
Table 81-9 Medications Associated with Aggression in Traumatic Brain Injury
Psychiatric Disorders
Psychiatric disorders, particularly mood disorders, are found more frequently in individuals with TBI than they are in the general population; they are associated with longer recovery time, worse outcomes, and higher mortality rates as compared to those who have suffered a TBI, but without psychiatric disturbances.1,27–30 Koponen and colleagues31 found that 48% of patients developed an Axis I disorder (most commonly major depression, alcohol abuse or dependence, panic disorder, specific phobias, and psychotic disorder) after TBI (Table 81-10). Roughly one-fourth of the subjects developed an Axis II personality disorder (avoidant, paranoid, or schizoid) after the injury.31
Table 81-10 Psychiatric Disorders Associated with Traumatic Brain Injury
Depression is the most common psychiatric disorder observed after TBI, with a frequency rate ranging from 26% to 77%.32 Presence of depression is associated with poor psychosocial outcome, increased psychological distress, and a greater number and intensity of perceived postinjury symptoms.33 Depression has been found to develop acutely within the first month of the injury.34,35 In other cases, the onset is delayed.34 Symptoms may resolve within the first 6 months34 or may persist for many years.36 Depression has not been associated with the severity of injury or with cognitive impairment. A preinjury history of substance abuse, worse premorbid social function, less than a high school education, and an unstable work history predict depression after TBI.34,35
Individuals with TBI should be considered at greater risk for depression as a reaction to the loss of capabilities and competencies, changes in social supports, capacity for work, increased financial and medical concerns, loss of role and income, and decreased quality of life.37 In addition to these psychosocial factors, depression is associated with the neurophysiological changes and changes in neurotransmitter levels that follow TBI. While no single structure is responsible for the development of depression, depression during the acute stage of recovery has been closely associated with damage to left frontal and basal ganglia regions.34
Individuals with TBI are at higher risk for thoughts of suicide, suicide attempts, and suicide.28,37,38 TBI and suicide share risk factors: age (midteens to midtwenties); gender (males more than females); lower socioeconomic level; presence of drug or alcohol use; and psychological disturbance (Table 81-11).37 Key features of TBI (such as cognitive and motor disturbances, emotional lability and impulsivity, rigidity and hyperactivity, poor problem-solving ability, inability to identify alternatives, and heightened aggression and hostility) increase the risk for suicide. Individuals with TBI who have attempted suicide have not differed significantly from suicide attempters without TBI in terms of age of first suicide attempt, suicidal ideation, suicidal intent, number of attempts, or maximum lethality.28 When comparing attempters with nonattempters, Oquendo and co-workers28 found that those who attempted suicide had higher levels of aggression and hostility, were more likely to have a substance abuse history, and were more likely to have a Cluster B personality disorder than nonattempters. Presence of profound feelings of hopelessness, despair, worthlessness, and loss of sense of integrity; relationship breakdown; and problems with isolation contribute to the risk for suicide.37,38
Table 81-11 Risk Factors for Suicide Following Traumatic Brain Injury
| Depression | Cognitive and motor disturbances |
|---|---|
| Profound feelings of hopelessness, despair, worthlessness | Emotional lability |
| Loss of sense of integrity | Impulsivity |
| Prior history of attempt | Inflexibility |
| Male | Hyperactivity |
| Midteens to midtwenties | Poor problem-solving ability |
| Lower socioeconomic level | Inability to identify alternatives |
| Drug or alcohol use | Heightened aggression and hostility |
| Cluster B personality disorder | Relationship breakdown |
| Social isolation |
Evaluating patients with a history of TBI for depression is complicated by the fact that the neurovegetative signs of depression (e.g., sleep disturbance, changes in appetite, anhedonia, and loss of libido) frequently occur as a result of TBI. At the same time, deficits in self-awareness can interfere with an individual’s ability to identify symptoms of depression.34,39 Depression must be distinguished from an adjustment disorder, posttraumatic stress disorder (PTSD), organic apathy, and emotional lability. Pathological laughing or crying, which can occur with focal prefrontal lesions, occurs suddenly, uncontrollably, and may or may not be mood congruent, but is recognized by the individual as disproportionate to the mood or precipitating stimuli. Such an individual tends to have more anxiety, more aggression, and worse social function than an individual without this syndrome. Apathy (marked by lack of motivation, absence of emotional reaction, and difficulty with initiation of actions) frequently follows frontal lobe damage. Apathy can co-occur with depression.34
The diagnosis of depression is more convincing when the psychological symptoms of depression (e.g., presence of depressed affect, irritability, ruminations, feelings of hopelessness and worthlessness, and having difficulty enjoying activities) are manifest. These factors may distinguish depressed from nondepressed patients following TBI.35,36,40,41 Depression should be diagnosed via a semistructured or structured psychiatric interview. Because cognitive deficits may involve awareness, memory, self-monitoring, expression, and language comprehension, information from family and caretakers is invaluable.
Anxiety disorders are found at all stages of recovery: immediately following the injury, during the postacute phase, and in those who have persistent problems. Generalized anxiety disorder (GAD), panic attacks, obsessive-compulsive disorder (OCD), simple phobias, acute stress disorder, and PTSD have all been observed following TBI.42 For most of the disorders the presence or severity of the anxiety disorder has not been associated with the severity of injury. The exception to this is PTSD, in which an inverse relationship has been observed (i.e., PTSD is more likely in individuals with a mild TBI than it is in individuals with severe TBI).42 Anxiety is frequently accompanied by depression and by alcohol dependence. Anxious patients experience higher levels of functional disability and report higher levels of injury and cognitive impairment.42,43
Anxiety may develop in the early stages of recovery when the individual has trouble with simple, previously automatic tasks (such as dressing and bathing) or sees significant decline in areas that were particular strengths (due to the cognitive and functional changes that have taken place). The immediate environment becomes unfamiliar and unpredictable as a consequence of problems with memory and information processing. The individual loses a sense of competence and confidence in his or her ability to control the immediate environment. Fears arise regarding the permanence of the individual’s deficits and his or her ability to return to previous roles and activities. Avoidance behaviors may arise. Somatic complaints (e.g., vertigo, headaches, complex partial seizures) that frequently accompany TBI may be interpreted by the individual as symptoms of anxiety. Alcohol withdrawal may be mistaken for primary anxiety. At the same time, anxiety may develop as a result of damage to the temporal lobes, the frontal lobe pathways connecting to the caudate nucleus, the hippocampus, and the amygdala. Increases or dysregulation of circulating cortisol or catecholamines may produce primary anxiety.43 Damage to the orbitofrontal cortex, anterior cingulate, and caudate nucleus has been associated with the development of OCD. Orbitofrontal, cingulate, and medial temporal cortical areas, which are frequently damaged in TBI, have been associated with panic attacks.43
OCD occurs after TBI at rates (0.5% to 7.8%) similar to those for the general population.44 Unfortunately, research has focused on single cases or small group studies, which limits what is known about the impact of demographic variables, the severity of TBI, or co-morbidity. Fully developed OCD following TBI in the absence of preinjury OCD is rare.44 In diagnosing OCD following TBI, it is important to appreciate the role that cognitive dysfunction may play in the development of obsessions and compulsive or ritualistic behavior. The rituals may be an effort to compensate for poor memory and problem-solving ability. The slowness, indecisiveness, and avoidance may reflect a realistic appraisal, based on the individual’s postinjury experiences, of the individual’s ability to carry out tasks accurately and independently and make good decisions.
PTSD following TBI often occurs in the context of a traumatic, life-threatening event (such as a motor vehicle accident or an assault). While there is debate as to whether or not patients with amnesia for the traumatic event can go on to develop PTSD (with its hallmark of reexperiencing the original trauma), PTSD following severe TBI is well documented even in cases where the individual has no explicit or episodic memory of the event.42,43,45 In cases where there is amnesia for the trauma, the individual may be less likely to have intrusive reexperiencing of the event in the form of flashbacks or nightmares. The individual can, however, experience heightened emotional reactivity in response to trauma-related stimuli. Memories may have been formed at a more emotional level and stored through mechanisms mediated by the amygdala, independent from explicit memories mediated by more vulnerable hippocampal pathways.43 Individuals may also react to stimuli from events before the LOC or after regaining consciousness. PTSD is often accompanied by depression and anxiety. Diagnosing PTSD following TBI can be complicated by the overlap in symptoms between the two disorders. The PTSD symptoms of dissociative amnesia, disassociation, depersonalization, derealization, reduced drive, altered consciousness, and confusion resemble posttraumatic amnesia and delirium that follow TBI.43
Psychosis is not a typical feature of TBI, though it does occur at a higher rate in this population than in the general population (0.7% to 9.8% versus 0.8% lifetime incidence).46 The presence of a first-degree relative with schizophrenia, premorbid pathology, temporal lobe epilepsy, depression, or mania is associated with post-TBI psychosis.46 That psychosis can occur as a result of TBI is not surprising given that the areas of the brain associated with schizophrenia (the prefrontal cortex, temporal lobes, and hippocampus) are all vulnerable to injury. Posttraumatic psychosis can develop at any time following the injury. Visual hallucinations and delusions are a common feature of posttraumatic delirium.47 Following the episode of delirium, psychosis is more likely to be seen months to years after the injury.47 It is associated with more moderate to severe injury and is seen more frequently following damage to the left hemisphere and to the temporal lobes.46,48–50 Content-specific delusions and misidentification syndromes (such as Capgras’ syndrome [a loved one has been replaced by an identical-appearing imposter] or reduplicative paramnesia [a familiar place exists in another location simultaneously]) are associated with right hemisphere damage.50 There may be a prodromal period of bizarre or antisocial behavior, social withdrawal, affective instability, and deterioration in overall function.49 Delusions are more common than hallucinations.48 When hallucinations occur, auditory hallucinations are more common than visual hallucinations. Positive symptoms of schizophrenia are more common than negative symptoms.50 Delusions tend to involve themes of persecution, ideas of reference, grandiosity, and religiosity.49,50 Concerns that the individual’s thoughts are not his or her own, and that thoughts are being inserted or withdrawn or being broadcast, are also common.49 Agitated or assaultive behaviors can occur with psychosis.23
Alcohol may be a contributing factor in a majority of cases of TBI. An elevated BAL at the time of injury is associated with a longer hospitalization, a longer period of agitation, greater cognitive impairment at the time of discharge, and worse overall outcome.51 Alcohol and drug use contribute to the development of psychiatric symptoms and worsen existing symptoms, especially those associated with TBI.52 Within the TBI population, alcohol abuse or dependence has been found to occur more frequently among patients who developed a mood disorder. At the same time, patients with a history of alcohol abuse who relapsed were at higher risk for developing a subsequent mood disorder. Alcohol and TBI appear to interact to produce a greater degree of disruption in neural circuitry involved in reward systems, and mood and executive function. Individuals with alcohol abuse or dependence and mood disorders tend to have a more difficult time resuming a productive life following a TBI.53 Postinjury patterns of alcohol use vary.54 Not all who had problems before the injury resumed drinking. Some who did not have problems before the injury develop problems after the injury. Prior alcohol use and drinking problems are predictive of postinjury patterns of use.54 Those with significant preinjury histories of alcohol-related problems are 10 times more likely to have significant problems after injury compared to normal drinkers.54 Most important, drinking patterns tend to remain stable over time and are reasonably well established by the end of the first year; therefore, evaluation and treatment for alcohol problems during the first year following the TBI is critical.54
DIAGNOSIS
Diagnosing psychiatric disorders following TBI can be complicated, because many disorders may have preceded the injury. Moreover, there is a good deal of overlap between the symptoms of mental disorders and the cognitive and behavioral changes that follow TBI. Many of the risk factors for TBI in terms of gender, education, socioeconomic status (SES), and substance abuse are the risk factors for many primary mental disorders. Further complicating the diagnostic task is the fact that a history of TBI is not unusual in individuals with primary mental disorders. For example, there is a higher rate of TBI in those with schizophrenia than in nonschizophrenic family members.46
The DSM-IV-TR classifications involving mental disorders caused by general medical conditions provide helpful guidelines in distinguishing between primary mental disorders and the mental disorders that are secondary to the injury (Table 81-12). The diagnosis requires that the change in affect and behavior is a direct physiological consequence of a medical condition.55 This can be difficult to determine in the case of mild TBI because there may not be evidence of physiological damage on CT, MRI, or EEG. The connection can be based on timing; that is, the symptoms were not present before the injury, were exacerbated by the injury, or improved as the injury resolved. The later the onset, the more difficult it is to make the case that the initial injury is responsible for the disorder. Further, the diagnosis may be used if the features of the symptoms are atypical of the primary disorder. This may take the form of unusual age of onset, unusual features, or disproportionately severe symptoms. A final consideration in assigning this diagnosis is that the symptoms are neither related to substance use nor observed exclusively in the context of delirium, which is a common feature of the acute stages of recovery. Where these diagnoses are used, the medical condition, TBI, is coded under Axis III.
Table 81-12 Mental Disorders Caused By a General Medical Condition Associated with Traumatic Brain Injury
| 293.0 | Delirium caused by a general medical condition |
| 294.0 | Amnestic disorder caused by a general medical condition |
| 293.83 | Mood disorder caused by a general medical condition |
| 293.89 | Anxiety disorder caused by a general medical condition |
| 293.8x | Psychotic disorder caused by a general medical condition |
| 293.81 | Psychotic disorder caused by a general medical condition with delusions |
| 293.82 | Psychotic disorder caused by a general medical condition with hallucinations |
EVALUATION
A comprehensive evaluation is needed when assessing an individual for psychiatric disorders following a TBI. Arlinghaus and co-workers51 recommended using a bio-psycho-social approach. Clinicians are encouraged to base treatment more on an individual’s symptoms.56 In most cases, medication plays a crucial role, but it will not be the only intervention needed. Disorders, and the need for medication, will change in response to neuroplastic healing and changes that accompany the passage of time as TBI has an impact on interpersonal relationships, family interactions, and work and leisure capacities.51 It is further recommended that family members and other sources of corroborating information (such as medical records and police reports) be included in the evaluation process since many individuals with TBI will be poor historians because of difficulties with memory and awareness.12,46
The evaluation begins with a detailed medical history, including history of birth and development. The history of the injury and the ensuing recovery process are reviewed. The source of the injury (e.g., motor vehicle accident, fall, or assault), the type of injury (open versus closed, focal versus diffuse), the level of injury (as measured by the GCS), the presence and duration of LOC and PTA, and the presence and location of complications (e.g., skull fracture, cerebral contusions, extradural hematomas) are identified.12 The assessment considers the presence of symptoms associated with TBI.51 CT and MRI scans, EEG, and neuropsychological reports are reviewed for further evidence of TBI and its functional impact. As in any psychiatric evaluation, the medical history is reviewed for prior psychiatric history, neurological injury, alcohol or substance abuse, dementia, seizure disorders, ADD, and learning disabilities. The social history identifies level of educational and occupational achievement; sources of family and social support; legal, educational, occupational, or social problems; emotional adjustment, temperament, and coping mechanisms; and family history.51 Current medications are reviewed (such as antipsychotics, antidepressants, anticonvulsants, stimulants, and benzodiazepines) because some can affect cognition and behavior.
In conducting the neurological evaluation, the traditional Mini Mental State Examination (MMSE) will likely reveal abnormalities and there will be focal neurological findings in cases of moderate and severe TBI. In mild cases of TBI, however, the findings are likely to be essentially normal. Arlinghaus and colleagues51 recommended supplementing the MMSE with tasks sensitive to frontal dysfunction (such as clock drawing [“Draw a clock, enter the numbers, and set the hands for 10 past 11 or 10 to 2”]) that involves attention, visuospatial function, registration of information, recall and planning, and sequencing. Additionally, a measure of verbal fluency that assesses the ability to generate novel responses, to self-monitor, and to use working memory, should be included (“Name all of the words you can think of in 60 seconds that begin with a specified letter, with the exception of proper nouns, numbers, and words with the same beginning, but different endings”). The ability to shift set and sequence information can be measured by asking the individual to continue an alternating sequence (e.g., 1a, 2b, 3c) verbally or as part of Luria maneuvers (left fist into right palm, then left palm on right palm, then left side of hand into right palm) or Trails B or “go-no-go” task (say “one” when two fingers are held up, “two” when one finger is held up).22,51 In evaluating the mental status of patients with moderate to severe injury, issues of competence and the possible need for guardianship or substituted judgment need to be considered.
A structured or semistructured psychiatric interview has been recommended when assessing for specific psychiatric symptoms.50 Kennedy and colleagues32 raise concerns over the use of structured psychiatric interviews based on the degree of training needed and the length of time needed to administer them. The specific focus on psychiatric symptomatology can make the interviews unpalatable for patients with TBI who may already be sensitive to the idea that their symptoms are “all in their head.”32 The Neurobehavioral Functioning Inventory (NFI) developed specifically for use with individuals with TBI is suggested as an alternative. It is able to assess emotional function in the broader context of neurobehavioral function in a variety of settings.32 Because of the problems associated with self-report in the TBI population, the NFI should be accompanied by a more structured interview (Table 81-13).36
Table 81-13 Structured Assessment Tools for Patients with Traumatic Brain Injury
Another approach to the assessment of the behavioral and emotional changes following TBI is through the use of a functional assessment of behavior.22,57 The assessment is predicated on the assumption that behavior is a function of the interaction between the cognitive changes that result from the brain injury, environmental factors, and psychological causes, and is likely to vary across settings. Behavior is observed in a variety of settings and conditions. The assessment identifies the antecedent conditions in which the behavior occurs; describes the behavior in objective, measurable terms; and describes the environmental response to the behavior or consequences of the behavior. This approach considers the individual’s learning history (before and after injury), the individual’s cognitive and behavioral repertoire, and the social and environmental context of the behavior and sources of intended and unintended reinforcement for the behavior.
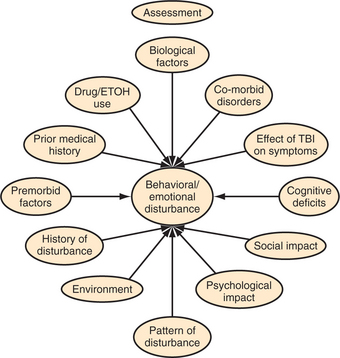
In the end, the psychiatric assessment aims to generate an understanding of the timeline of the development of psychiatric symptoms relative to the injury; the nature of the TBI; changes in affect, behavior, and cognition; the contribution of preinjury personality on postinjury behavior; and the interface between the injury and the psychiatric symptoms (Figure 81-1). The goal is not only to identify which symptoms are present, but also to understand how these symptoms interact with each other and interfere with the individual’s efforts to reintegrate into the world around him or her.
TREATMENT
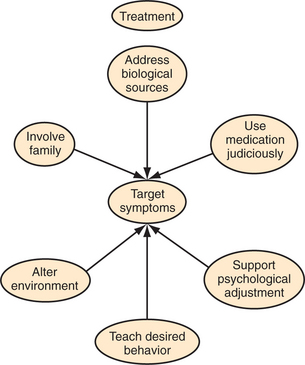
A limited body of well-designed randomized controlled clinical trials (RCTs) exists to guide the use of medications for the treatment of emotional and behavioral disturbances following TBI. The majority of such studies involve small groups, retrospective record reviews, or single case studies.58,59 The general consensus of the research is that medications that are effective in treating primary psychiatric disorders are, for the most part, similarly effective in treating the symptoms of these disorders in the context of TBI.56 Before introducing new medications, possible medical causes should be eliminated, the environment should be altered, and psychosocial interventions should be supplemented (Figure 81-2).30,33 For example, treatment of infections, dehydration, constipation, urinary retention, and pain should be initiated; abnormali-ties of sodium, ammonia, or blood sugar levels should be addressed; sleep/wake cycles should be restored; and medications should be reviewed to make sure therapeutic levels have been reached or that redundant or unnecessary drugs are stopped or reduced.40 Attention should be paid to antihistaminergic and anticholinergic effects (e.g., sedation, impaired memory and learning, impaired attention, dry mouth, constipation, tachycardia, urinary retention, diplopia, confusion, and hypotension) caused by many psychotropic medications, because these will exacerbate existing problems that result from TBI. Medications that are least likely to lower seizure threshold or cause sedation should be chosen. Because patients with TBI are more sensitive to the side effects of medications, a “start low and go slow” approach is recommended (Table 81-14). That is, start with doses one-half to one-third of the dose traditionally used and increase it slowly until therapeutic effects are achieved.30,46 In general, therapeutic doses will be comparable to those used in traditional psychiatric patients.56
Table 81-14 Medication Side Effects of Concern for Patients with Traumatic Brain Injury
An effort should be made to determine the lowest effective dose to minimize the risk for side effects and to avoid the complications of interactions between psychoactive medications (Table 81-15). It is not unusual to see patients prescribed an anticonvulsant to control seizures, a stimulant to improve attention and concentration, an antidepressant to address depression and anxiety symptoms, and an antipsychotic. While the overlap in medications can help augment or facilitate drug efficacy, it can also lead to potentiation and to toxic blood levels. Some medications will reduce or increase serum blood levels of other medications. Antiseizure medications can lower antidepressant levels.33,56 Fluoxetine can raise plasma levels for phenytoin, valproic acid, and carbamazepine.56 Anticholinergic effects can be enhanced in the presence of neuroleptics or antiparkinsonian agents.33,56 Where possible, changes should be made one at a time to best evaluate the effects of the changes. Because of the changing nature of the recovering brain, there is an ongoing need to routinely reassess psychotropic medications. Clinical judgment and a cost-versus-benefit analysis will be needed to determine how long medications should be continued once desired effects have been achieved.34,56 Trial reductions or drug holidays are recommended as ways of verifying the continued need for medication.56
Table 81-15 Medication Guidelines for Patients with Traumatic Brain Injury
Medications
Antidepressants
No research indicates which of the available antidepressants is most effective in treating depression in the context of TBI.33,58,59 However, selective serotonin reuptake inhibitors (SSRIs) appear to be effective, because they affect sleep regulation, headaches, sexual activity, depression, anxiety, appetite, arousal, vigilance, distractibility, neural reward systems, and working memory.58 In addition to treating depression, the SSRIs are also effective in treating psychological distress, anger, aggression, psychomotor speed, overall cognitive efficiency, and various forms of memory.33,56,58 They are also effective for the treatment of anxiety disorders (including OCD, panic disorders, and PTSD).42,43,56,58,60 By treating underlying depression, the SSRIs can reduce agitation and anxiety.60 SSRIs are preferred over the older tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs) because of their demonstrated effectiveness, improved safety profile, and lower incidence of anticholinergic effects and orthostatic hypotension (as well as the risk of a hypertensive crisis associated with MAOIs).33 There is no consensus on which of the SSRIs is most effective thus selection of a specific agent is typically guided by its side-effect profile and the clinician’s familiarity.58 Caution does need to be used when prescribing SSRIs due to syndromes of excess serotonin and the risk of discontinuation syndromes. When high doses or multiple serotonergic agents are used, there is the risk for a serotonin syndrome (including an altered mental status, neuromuscular abnormalities, disturbances in autonomic function, agitation, myoclonus, hyperreflexia, diaphoresis, tremor, diarrhea, and lack of coordination). The SSRI discontinuation syndrome (including profound restlessness, bizarre ideation, headache, nausea, lowered mood, anxiety, insomnia, lethargy, or dizziness) may occur when an SSRI is stopped abruptly.58
TCAs, which tend to be highly anticholinergic and antihistaminergic, can lower the seizure threshold and have a greater potential for lethal overdose.33,34,58 Among the TCAs, nortriptyline and desipramine are used more commonly following TBI because of their more favorable side-effect profile.33 Use of MAOIs is often problematic because of the need to comply with complex dietary restrictions.58 Electroconvulsive therapy (ECT) is also seen as an effective treatment, particularly in cases of severe or treatment-refractory depression, though caution is urged in a population that already has problems with confusion and memory deficits.30,34,58,59
Anxiolytics
Benzodiazepines.
While benzodiazepines are typically used to treat anxiety in non-TBI patients, their long-term use in patients with TBI is generally not recommended.46 The short half-life of lorazepam can make it effective for agitation or anxiety; however, it can significantly increase sedation, and further impair memory and motor function56 and lead to behavioral disinhibition.42,43 Use of benzodiazepines in individuals with histories of drug or alcohol abuse or dependence should be avoided given the potential for abuse and dependence.43,56
Buspirone is another alternative23,42,43 that has been found to improve depression, anxiety, somatic preoccupation, and distractability.42,43,56 It has less impact on cognition than other anxiolytics and has less potential for abuse.42,56 Its effects may not be seen for several weeks, which limits its usefulness for acute anxiety.23 It can cause headaches, dizziness, and, paradoxically, increased anxiety.42,56
Propranolol, a beta-blocker, has the best body of evidence to support its use in managing agitation and aggression following TBI.46 It tends to reduce the intensity, but not necessarily the frequency, of agitated behavior.46 It can lead to hypotension, bradycardia, and depression.23
Anticonvulsants
Anticonvulsants have been used to prevent seizures following TBI. Current recommendations for prophylactic use suggest that they be discontinued if no seizures have been observed after the first week following injury.56,59 Several anticonvulsants (such as valproic acid, lamotrigine, and gabapentin) have been used to good effect to reduce agitation and aggression.23,60 While valproic acid has Food and Drug Administration (FDA) approval for treatment of acute mania and is generally well tolerated (with fewer cognitive side effects), selection of specific anticonvulsants is typically based on side-effect profiles.23 The more traditional anticonvulsants (phenobarbital, phenytoin, and carbamazepine) tend to sedate, and interfere with cognition.61 Levetiracetam can increase agitation and other problematic behaviors (including assaultive behavior).62–64
Antipsychotics
Psychosis outside of delirium is unusual after TBI. Antipsychotics are used more frequently to control aggression in patients with brain injuries.24 Indications for such use include agitation with and without psychosis, extreme agitation or rage, and mania associated with TBI.59,65 They, along with the short-acting benzodiazepines (such as lorazepam), are effective agents in rapidly controlling acute agitation. The use of neuroleptics in this population is controversial. Human and animal research indicates that they interfere with neural plasticity and the recovery process, are associated with longer periods of PTA, and worsen outcomes.50,56,65 Use of the typical neuroleptics risks extrapyramidal side effects (EPS) and neuroleptic malignant syndrome (NMS),65 and some are anticholinergic and interfere with orthostasis, alertness, cognition, and initiation.56,65 Atypical antipsychotics (such as quetiapine, olanzapine, and risperidone) are preferred because of their greater effectiveness in treating positive and negative symptoms and associated lower risk of EPS, NMS, and anticholinergic effects.46,50,65 They can interfere with glucose metabolism and contribute to weight gain and hyperlipidemia as part of a metabolic syndrome. Starting doses should be approximately half the standard starting dose and increased slowly. Starting doses are similar to those used for geriatric patients.50
Stimulants
Disorders of attention and arousal can contribute to behavioral disturbances. Psychostimulants such as methylphenidate and dextroamphetamine, and the activating antidepressants (e.g., modafinil), are routinely used to improve arousal, speed of processing, attention, apathy, irritability, impulsivity, and fatigue.56,66 They reduce agitation, depression, and anxiety by improving the underlying cognitive dysfunction that contributes to behavioral problems. Reductions in anger and psychopathology can occur even without improvements in cognition66 and seem to have the additional benefit of increasing overall neuronal recovery.56 Side effects are generally dose dependent, occur at higher levels, and resolve with a reduction in dosage. None of these medications has been found to alter the risk for seizures.56
Environmental Interventions
Alterations of the environmental context of the patient are crucial in managing disturbing behaviors. Anxiety, agitation, and aggression can be responses to environmental stressors or overwhelming task demands. Providing the patient with structure and routine, simplifying tasks, reducing or increasing levels of stimulation, and removing environmental distractions, irritations, or triggers can all help reduce confusion and agitation. Providing the patient with a prosthetic environment helps compensate for the cognitive losses that result from TBI.22 Daily schedules, memory aids, computers, and personal digital assistants (PDAs); prompting and cuing; and proactive, collaborative problem solving are examples of supportive interventions that reduce demand on a compromised cognitive system and increase the patient’s opportunities for success and self-control. Formal training can be used to replace missing skills or increase alternative behaviors.22 Changing antecedent conditions and the environmental response to behavior can help decrease maladaptive or dangerous behavior and increase safer, more adaptive behavior.57 These measures can be more time-consuming and labor intensive and require more training of families or staff than the use of medications. However, there are few negative side effects and many potential psychological benefits in terms of increasing the patient’s sense of control, success, and self-confidence, as well as those of the family and caregiving team.
Psychotherapy
The essential tasks of psychotherapy for patients with TBI involve maintaining a sense of hope during the recovery process, mourning the losses that have occurred, identifying remaining strengths and assets, and restoring a sense of self that incorporates the changes imposed by the brain injury.67 Patients whose injuries are permanent will need help in setting realistic goals and redefining the ways they work, love, and play.67,68 Deficits in attention, memory, awareness, self-monitoring, expressive and receptive language, impulse control, abstraction, and frustration tolerance affect the therapeutic process.67 Therapists need to take an active and directive role in therapy. Education about the impact of brain injury, the process of recovery, and the patient’s role in this recovery process are important for both the patient and the patent’s family and caretakers.69 Cognitive limitations are addressed through the use of memory aids (such as recording sessions, taking notes, generating summaries of main themes in the session, and reviewing the last session at the start of a new session).70 Sessions may be shorter and more frequent to maximize attention and avoid overloading the patient. Homework to be carried out between sessions allows opportunities to practice skills in a variety of settings to promote awareness and generalization. Coping, relaxation, and stress management skills are taught to reduce anxiety and frustration. The therapist engages in a practical problem-solving approach to identify and rehearse options. Language is kept concrete, and the patient’s comprehension is routinely assessed.70 Family members are often included for at least a portion of sessions to verify patient experience and to provide additional feedback on performance. This is an opportunity to assess and support the patient’s center of support, because families and caretakers will also need education and psychological support. Unrealistic goals identified by the patient are addressed by identifying smaller short-term goals in the service of achieving more distant goals. This increases the patient’s chances of success and assists in developing a more accurate sense of capabilities. A distinction is made between psychological denial and neurologically based anosognosias. Denial protects against overwhelming affect. Lack of awareness, however, serves no psychological purpose.67 Denial will often improve with the passage of time as the patient regains areas of competence and control, making the experience of TBI less overwhelming. The lack of awareness is likely to improve with improved neurological function and interaction with the real world. Resolution of denial will typically lead to improvements in depression and anxiety, whereas improved awareness can actually trigger these emotions. Both need to be approached carefully to avoid overwhelming the patient. Families, who often have been traumatized by the injury and become overly protective, will need support and guidance in helping the patient engage in the reasonable risk-taking activities needed to restore a sense of competence and independence.
1 Lezak MD. Neuropsychological assessment, ed 3. New York: Oxford University Press, 1995.
2 Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control, 2006.
3 Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: a public health perspective. J Head Trauma Rehabil. 1991;14:602-615.
4 Kraus JF, Chu LD. Epidemiology. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
5 Consensus Project: Rehabilitation of persons with traumatic brain injury. JAMA. 1999;282:974-983.
6 Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76-94.
7 Gennarelli TA, Graham DI. Neuropathology. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
8 Rosenthal M, Ricker J. Traumatic brain injury. In: Frank RG, Elliott TR, editors. Handbook of rehabilitation psychology. Washington, DC: American Psychological Association, 2002.
9 Lehr RP. Therapy, neuroplasticity, and rehabilitation. In: Ashley MJ, editor. Traumatic brain injury: rehabilitative treatment and case management. New York: CRC Press, 2004.
10 Selzer E. Regeneration and plasticity in neurologic dysfunction. In: Lazar RB, editor. Principles of neurologic rehabilitation. New York: McGraw-Hill, 1998.
11 Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine: Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8:86-87.
12 McAllister TW, Arciniegas D. Evaluation and treatment of postconcussive symptoms. NeuroRehabilitation. 2002;27:265-283.
13 Rees PM. Contemporary issues in mild traumatic brain injury. Arch Phys Med Rehabil. 2003;84:1885-1894.
14 McCullagh S, Feinstein A. Cognitive changes. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
15 Bleiberg J, Garmoe WS, Halpern EL, et al. Consistency of within-day and across-day performance after mild brain injury. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:247-253.
16 Levin HS. Memory dysfunction after head injury. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill, 1997.
17 Alexander MP. Aphasia: clinical and anatomic aspects. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill, 1997.
18 Benson DF, Miller BL. Frontal lobes: clinical and anatomic aspects. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill, 1997.
19 Kimberg DY, D’Esposito M, Farah MJ. Frontal lobes: cognitive neuropsychological aspects. In: Feinberg TE, Farah MJ, editors. Behavioral neurology and neuropsychology. New York: McGraw-Hill, 1997.
20 Hoofien D, Gilboa A, Vakil E, Donovick PJ. Traumatic brain injury (TBI) 10-20 years later: a comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Injury. 2001;15:189-209.
21 Hanks RA, Temkin N, Machamer J, Dikmen SS. Emotional and behavioral adjustment after traumatic brain injury. Arch Phys Med Rehabil. 1999;80:991-997.
22 Hart T, Jacobs HE. Rehabilitation and management of behavioral disturbances following frontal lobe injury. J Head Trauma Rehabil. 1993;8:1-12.
23 Kim E. Agitation, aggression, and disinhibition syndromes after traumatic brain injury. NeuroRehabilitation. 2002;17:297-310.
24 Silver JM, Yudofsky SC, Anderson KE. Aggressive disorders. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
25 Tanteno A, Jorge R, Robinson RG. Clinical correlates of aggressive behavior after traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2003;15:155-160.
26 Baguley IJ, Cooper J, Felmingham K. Aggressive behavior following traumatic brain injury: how common is common? J Head Trauma Rehabil. 2006;21:45-56.
27 Borgaro SR, Prigatano GP, Kwasnica C, Rexer JL. Cognitive and affective sequelae in complicated and uncomplicated mild traumatic brain injury. Brain Injury. 2003;17:189-198.
28 Oquendo MA, Friedman JH, Grunebaum MF, et al. Suicidal behavior and mild traumatic brain injury in major depression. J Nerv Ment Dis. 2004;192:430-434.
29 Mooney G, Speed J. The association between mild traumatic brain injury and psychiatric conditions. Brain Injury. 2001;15:865-877.
30 Jorge R, Robinson RG. Mood disorders following traumatic brain injury. Int Rev Psychiatry. 2003;15:317-327.
31 Koponen S, Taiminen T, Portin R, et al. Axis I and II psychiatric disorders after traumatic brain injury: a 30-year follow-up study. Am J Psychiatry. 2002;159(8):1315-1321.
32 Kennedy RE, Livingston L, Riddick, et al. Evaluation of the Neurobehavioral Functioning Inventory as a depression screening tool after traumatic brain injury. Head Trauma Rehabil. 2005;20:512-526.
33 Alderfer BS, Arciniegas DB, Silver JM. Treatment of depression following traumatic brain injury. J Head Trauma Rehabil. 2005;20:544-562.
34 Robinson RG, Jorge RE. Mood disorders. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
35 Dikmen SS, Bombardier CH, Machamer JE, et al. Natural history of depression in traumatic brain injury. Arch Phys Med Rehabil. 2004;85:1457-1464.
36 Seel RT, Kreutzer JS. Depression assessment after traumatic brain injury: An empirically based classification method. Arch Phys Med Rehabil. 2003;84:1621-1628.
37 Kuipers P, Lancaster A. Developing a suicide prevention strategy based on the perspectives of people with brain injuries. J Head Trauma Rehabil. 2000;15:1275-1284.
38 Simpson G, Tate R. Clinical features of suicide attempts after traumatic brain injury. J Nerv Ment Dis. 2005;193:680-685.
39 Starkstein SE, Lischinsky A. The phenomenology of depression after brain injury. NeuroRehabilitation. 2002;17:105-113.
40 Glenn M. A differential diagnostic approach to the pharmacological treatment of cognitive, behavioral, and affective disorders after traumatic brain injury. J Head Trauma Rehabil. 2002;17:273-283.
41 Babin PR. Diagnosing depression in persons with brain injuries: a look at theories, the DSM-IV and depression measures. Brain Inj. 2003;17:889-900.
42 Hiott DW, Labatte L. Anxiety disorders associated with traumatic brain injuries. NeuroRehabilitation. 2002;17:345-355.
43 Warden DL, Labbate LA. Posttraumatic stress disorder and other anxiety disorders. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
44 Berthier ML, Kulisevsky J, Gironell A, Lopez OL. Obsessive-compulsive disorder and traumatic brain injury: behavioral, cognitive, and neuroimaging findings. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:23-31.
45 Parker R. Recommendations for the revision of DSM-IV diagnostic categories for co-morbid posttraumatic stress disorder and traumatic brain injury. NeuroRehabilitation. 2002;17:131-143.
46 Corcoran C, McAllister TW, Malaspina D. Psychotic disorders. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
47 Trzepacz PT, Kennedy RE. Delirium and posttraumatic amnesia. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
48 Fujii D, Ahmed I. Characteristics of psychotic disorder due to traumatic brain: an analysis of case studies in the literature. J Neuropsychiatry Clin Neurosci. 2002;14:130-140.
49 Zhang Q, Sachdev PS. Psychotic disorder and traumatic brain injury. Curr Psychiatry Rep. 2003;5:197-201.
50 McAllister TW, Ferrell RB. Evaluation and treatment of psychosis after traumatic brain injury. NeuroRehabilitation. 2002;17:357-368.
51 Arlinghaus KA, Shoaib AM, Price TR. Neuropsychiatric assessment. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
52 Miller NS, Adams J. Alcohol and drug disorders. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
53 Jorge RE, Starkstein SE, Arndt S, et al. Alcohol misuse and mood disorders following traumatic brain injury. Arch Gen Psychiatry. 2005;62:742-749.
54 Bombardier C, Temkin NR, Machamer J, Dikman SS. The natural history of drinking and alcohol-related problems after traumatic brain injury. Arch Phys Med Rehabil. 2003;84:185-191.
55 Diagnostic and statistical manual of mental disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association, 2000.
56 Silver JM, Arciniegas DB, Yudofsky SC. Psychopharmacology. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
57 Yody BB, Schaub C, Conway J, et al. Applied behavior management and acquired brain injury: approaches and assessment. J Head Trauma Rehabil. 2000;15:1041-1060.
58 Zafonte RD, Cullen N, Lexell J. Serotonin agents in the treatment of acquired brain injury. J Head Trauma Rehabil. 2002;17:322-334.
59 Glenn MB, Wroblewski B. Twenty years of pharmacology. J Head Trauma Rehabil. 2005;20:51-61.
60 Deb S, Crownshaw T. The role of pharmacotherapy in the management of behavior disorders in traumatic brain injury patients. Brain Inj. 2004;18:1-31.
61 Fleminger S, Greenwood RJ, Oliver DL. Pharmacological management for agitation and aggression in people with acquired brain injury. Cochrane Database of Systemic Reviews. 2003;3:1-74.
62 Hurtado B, Koepp MJ, Saner JW, Thompson PJ. The impact of levetiracetam on challenging behavior. Epilepsy Behav. 2006;8:588-592.
63 Bodtkorb E, Klees TM, Nakken KO, et al. Levetiracetam in adult patients with and without learning disability: focus on behavioral adverse effects. Epilepsy Behav. 2004;5:231-235.
64 Dinklacker V, Dietl T, Widman G, et al. Aggressive behavior of epilepsy patients in the course of levetiracetam add-on therapy: report of 33 mild to severe cases. Epilepsy Behav. 2003;4:537-547.
65 Elovic EP, Lansang R, Li Y, Ricker JH. The use of atypical antipsychotics in traumatic brain injury. J Head Trauma Rehabil. 2003;18(2):177-195.
66 Whyte J, Vaccaro M, Grieb-Neff P, Hart T. Psychostimulant use in the rehabilitation of individuals with traumatic brain injury. J Head Trauma Rehabil. 2002;17:284-299.
67 Pollack IW. Psychotherapy. In: Silver JM, McAllister TW, Yudofsky SC, editors. Textbook of traumatic brain injury. Washington, DC: American Psychiatric Publishing, 2005.
68 Prigatano GP. Work, love, and play after brain injury. Bull Menninger Clin. 1989;53:414-431.
69 Mateer CA, Sira CS, O’Connell ME. Putting Humpty Dumpty together again: the importance of integrating cognitive and emotional interventions. J Head Trauma Rehabil. 2005;20:62-75.
70 Laatsch L. Application of cognitive rehabilitation techniques in psychotherapy. In: Langer KG, Laatsch L, Lewis L, editors. Psychotherapeutic interventions for adults with brain injury or stroke: a clinician’s treatment resource. Madison: Psychosocial Press, 1999.