Neurology Emergencies
Edited by Anne-Maree Kelly
8.1 Headache
Anne-Maree Kelly
Introduction
Headache is a common ailment that is often due to a combination of physical and psychological factors. The vast majority are benign and self-limiting and are managed by patients in the community. Only a very small proportion of patients experiencing headache attend emergency departments (ED) for treatment. The challenges are to distinguish potentially life-threatening causes from the more benign and to manage effectively the pain of headache.
Aetiology, pathophysiology and pathology
The structures in the head capable of producing headache are limited. They include:
 the great venous sinuses and their branches and
the great venous sinuses and their branches and
 the basal dura and dural arteries, but to a lesser extent than the other structures.
the basal dura and dural arteries, but to a lesser extent than the other structures.
The bulk of the intracranial contents, including the parenchyma of the brain, the subarachnoid and pia mater and most of the dura mater, are incapable of producing painful stimuli.
The pathological processes that may cause headache are:
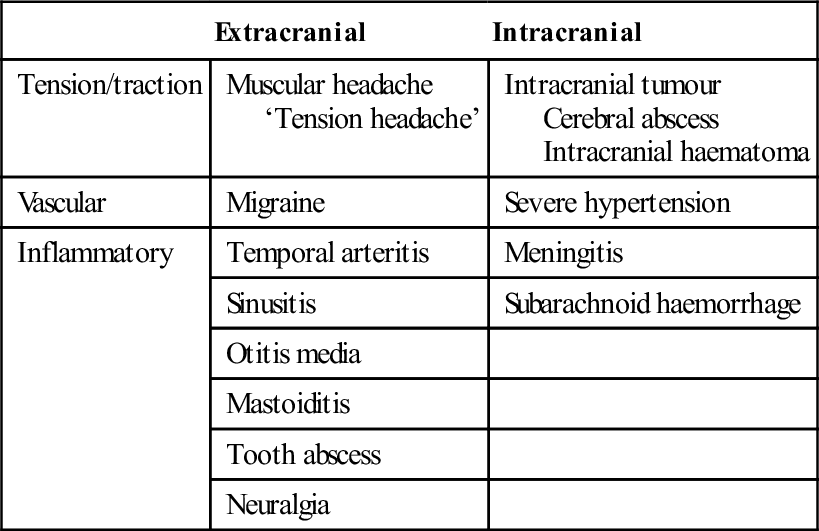
The pathophysiological causes of headache are summarized in Table 8.1.1.
Table 8.1.1
A pathophysiological classification of headache
| Extracranial | Intracranial | |
| Tension/traction | Muscular headache ‘Tension headache’ |
Intracranial tumour Cerebral abscess Intracranial haematoma |
| Vascular | Migraine | Severe hypertension |
| Inflammatory | Temporal arteritis | Meningitis |
| Sinusitis | Subarachnoid haemorrhage | |
| Otitis media | ||
| Mastoiditis | ||
| Tooth abscess | ||
| Neuralgia |
Clinical features
In the assessment of a patient with headache, history is of prime importance. Specific information should be sought about the timing of the headache (in terms of both overall duration and speed of onset), the site and quality of the pain, relieving factors, the presence of associated features, such as nausea and vomiting, photophobia and alteration in mental state, medical and occupational history and drug use.
Intensity of the pain is important from the viewpoint of management but is not a reliable indicator of the nature of underlying pathology. This said, sudden, severe headache and chronic, unremitting or progressive headache are more likely to have a serious cause.
Physical examination should include temperature, pulse rate and blood pressure measurements, assessment of conscious state and neck stiffness and a neurological examination, including funduscopy (where indicated). Abnormal physical signs are uncommon, but the presence of neurological findings makes a serious cause probable. In addition, a search should be made for sinus, ear, mouth and neck pathology and muscular or superficial temporal artery tenderness.
Headache patterns
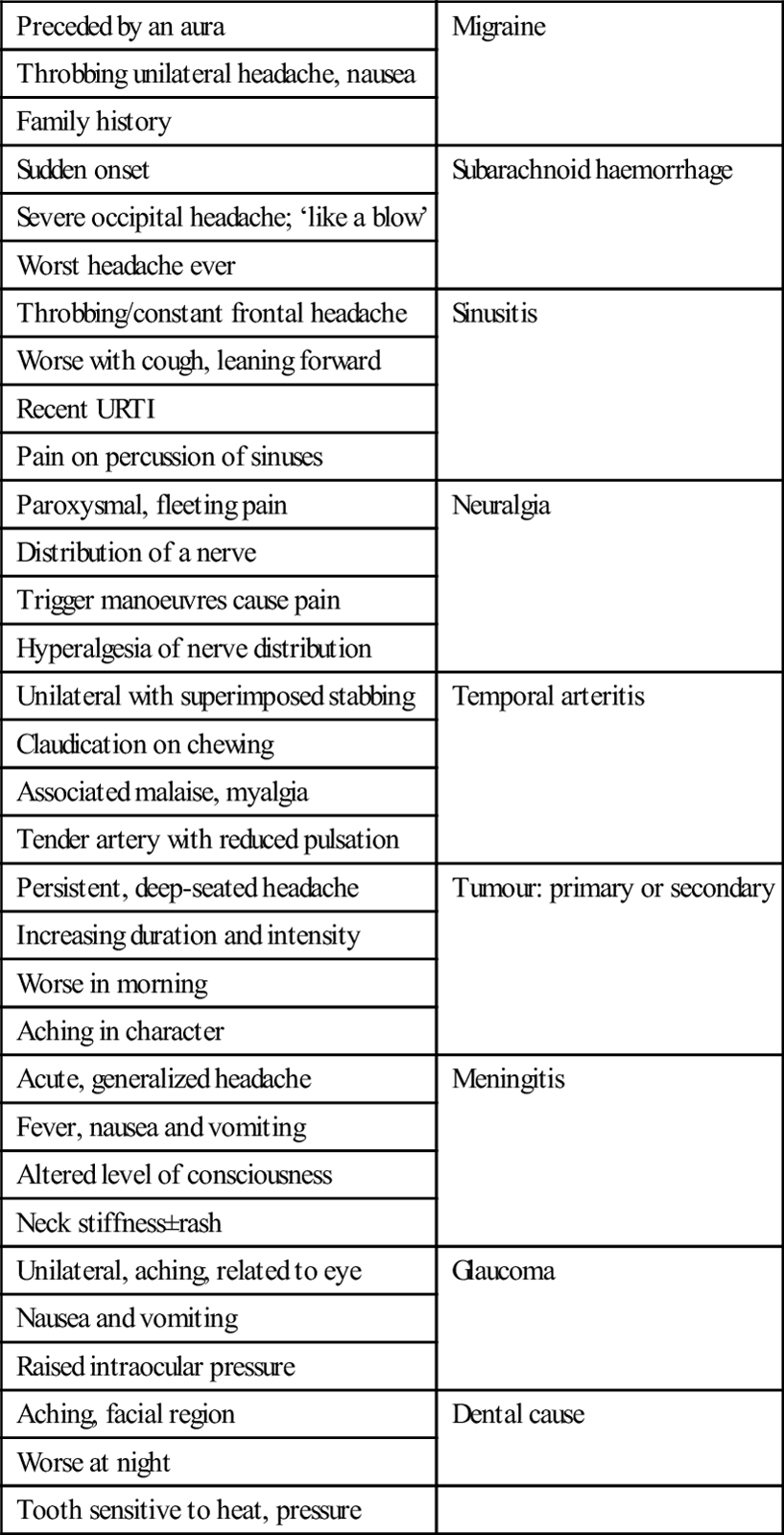
Some headaches have ‘classic’ clinical features: these are listed in Table 8.1.2. It must be remembered that, as with all diseases, there is a spectrum of presenting features and the absence of the classic features does not rule out a particular diagnosis. Every patient must be assessed on their merits and, if symptoms persist without reasonable explanation, further investigation should be undertaken.
Table 8.1.2
Classic clinical complexes and cause of headache
| Preceded by an aura | Migraine |
| Throbbing unilateral headache, nausea | |
| Family history | |
| Sudden onset | Subarachnoid haemorrhage |
| Severe occipital headache; ‘like a blow’ | |
| Worst headache ever | |
| Throbbing/constant frontal headache | Sinusitis |
| Worse with cough, leaning forward | |
| Recent URTI | |
| Pain on percussion of sinuses | |
| Paroxysmal, fleeting pain | Neuralgia |
| Distribution of a nerve | |
| Trigger manoeuvres cause pain | |
| Hyperalgesia of nerve distribution | |
| Unilateral with superimposed stabbing | Temporal arteritis |
| Claudication on chewing | |
| Associated malaise, myalgia | |
| Tender artery with reduced pulsation | |
| Persistent, deep-seated headache | Tumour: primary or secondary |
| Increasing duration and intensity | |
| Worse in morning | |
| Aching in character | |
| Acute, generalized headache | Meningitis |
| Fever, nausea and vomiting | |
| Altered level of consciousness | |
| Neck stiffness±rash | |
| Unilateral, aching, related to eye | Glaucoma |
| Nausea and vomiting | |
| Raised intraocular pressure | |
| Aching, facial region | Dental cause |
| Worse at night | |
| Tooth sensitive to heat, pressure |
Clinical investigations
For the majority of patients with headache no investigation is required. The investigation of suspected subarachnoid haemorrhage and meningitis is discussed elsewhere in this book. If tumour is suspected, the investigations of choice are magnetic resonance imaging (MRI) or a contrast-enhanced computed tomography (CT) scan. An elevated erythrocyte sedimentation rate (ESR) may be supporting evidence for a diagnosis of temporal arteritis. With respect to sinusitis, facial X-rays are of very limited value.
Tension headache
The pathological basis of tension headaches remains unclear, but increased tension of the neck or cranial muscles is a prominent feature. A family history of headaches is common and there is an association with an injury in childhood or adolescence. The most common precipitants are stress and alteration in sleep patterns.
Aspirin, non-steroidal anti-inflammatory agents (NSAIDs) and paracetamol (acetaminophen) have all been shown to be effective in the treatment of tension headaches, with success rates between 50 and 70%. Ibruprofen 400 mg or ketoprofen 25–50 mg appear to be the most effective, followed by aspirin 600–1000 mg and paracetamol 1000 mg.
Migraine
Migraine can be a disabling condition for the sufferer. Most migraine headaches are successfully managed by the patient and their general practitioner, but a small number fail to respond or become ‘fixed’ and sufferers may present for treatment at EDs. As most patients (up to 80% in some studies) have tried oral medications prior to presenting, parenterally administered agents are usually indicated for ED treatment.
Migraine is a clinical diagnosis and, in the ED setting, a diagnosis of exclusion. Other causes of severe headache, such as subarachnoid haemorrhage and meningitis, must be ruled out before this diagnosis is made. Of particular note, the response of a headache to antimigraine therapy should not be used to assume that the cause was migraine. There have been reports that the headaches associated with subarachnoid haemorrhage and meningitis have, on occasion, responded to these agents.
Pathophysiology
The pathophysiology of migraine is complex and not completely understood. It is a chronic neurovascular disorder characterized by dysfunction of the central and peripheral nervous system and intracranial vasculature.
The headache pain of migraine seems to result from the activation of the trigeminovascular system. The triggers for the development of migraine headache are probably chemical and are thought to originate in the brain, the blood vessel walls and the blood itself. These triggers stimulate trigeminovascular axons, causing pain and the release of vasoactive neuropeptides. These neuropeptides act on mast cells, endothelial cells and platelets, resulting in increased extracellular levels of arachidonate metabolites, amines, peptides and ions. These mediators and the resultant tissue injury lead to a prolongation of pain and hyperalgesia.
Serotonin has also been specifically implicated in migraine. By activation of afferents, it causes a retrograde release of substance P. This in turn increases capillary permeability and oedema.
Classification and clinical features
Migraine is defined as an idiopathic recurring headache disorder with attacks that last 4–72 hours. Typical characteristics are unilateral location, pulsating quality, moderate or severe intensity and aggravation by routine physical activity. There is also usually nausea, photophobia and phonophobia.
In some patients, migraine is preceded by an ‘aura’ of neurological symptoms localizable to the cerebral cortex or brainstem, such as visual disturbance, paraesthesia, diplopia or limb weakness. These develop gradually over 5–20 minutes and last less than 60 minutes. Headache, nausea and/or photophobia usually follow after an interval of less than an hour.
Several variant forms of migraine have been defined, including ophthalmoplegic, abdominal, hemiplegic and retinal migraine, but all are uncommon. In ophthalmoplegic migraine, the headache is associated with paralysis of one or more of the nerves supplying the ocular muscles. Horner’s syndrome may also occur. Abdominal migraine manifests as recurrent episodes of abdominal pain for which no other cause is found. Retinal migraine, which is fortunately very rare, involves recurrent attacks of retinal ischaemia which may lead to bilateral optic atrophy. Hemiplegic migraine is a stroke mimic.
Treatment
The complexity of the mechanisms involved in the genesis of migraine suggests that there are a number of ways to interrupt the processes to provide effective relief from symptoms.
A wide variety of pharmacological agents and combinations of agents have been tried for the treatment of migraine, with varying results. Interpreting the evidence is challenging, as the majority of the studies have small sample sizes, compare different agents or combinations of agents, are conducted in settings other than EDs and the outcome measure(s) tested varies widely. For mild to moderate migraine headache in patients who have not taken other medication, aspirin 900 mg combined with metoclopramide 10 mg is effective. Most ED patients, however, have either tried their usual medication or have significant nausea or vomiting making oral therapy inappropriate.
The effectiveness of commonly used agents is summarized in Table 8.1.3. Dosing and administration are summarized in Table 8.1.4. At present, the most effective agents appear to be the phenothiazines (chlorpromazine and prochlorperazine) and the triptans, each of which has achieved>70% efficacy in a number of studies. Note that triptans are contraindicated in patients with a history of ischaemic heart disease, uncontrolled hypertension or with the concomitant use of ergot preparations.
Table 8.1.3
Pooled effectiveness data from ED studies of the treatment of migraine
| Agent | No. of studies | Total patients | Clinical success rate (%) | NNT: clinical success |
| Chlorpromazine (IV) | 6 | 171 | 85 | 1.67 |
| Prochlorperazine (IM or IV) | 6 | 171 | 71 | 2.17 |
| Sumatriptan (6 mg SC) | 21 | 3139 | 71 | 2.17 |
| Ketorolac (IM or IV) | 6 | 155 | 66 | 2.44 |
| Tramadol (IM or IV) | 3 | 191 | 60 | 2.86 |
| Metoclopramide (IV) | 7 | 300 | 58 | 3.03 |
| Magnesium sulphate (IV) | 3 | 117 | 41 | 6.25 |
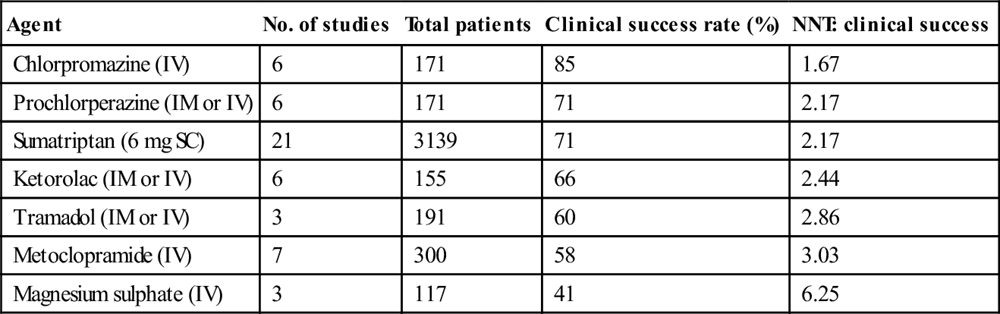
Table 8.1.4
Drug dosing and administration
| Agent | Drug dosing/administration |
| Chlorpromazine (IV) | 12.5 mg intravenously, repeated every 20 minutes as needed to a maximum dose of 37.5 mg, accompanied by 1 L normal saline over 1 hour to avoid hypotension OR 25 mg in 1 L normal saline over 1 hour, repeated if necessary |
| Prochlorperazine (IM or IV) | 10 mg/12.5 mg (depending on packaging) |
| Sumatriptan (SC, IN) | 6 mg SC, 20 mg IN |
| Metoclopramide (IV) | 10–20 mg |
| Ketorolac (IM or IV) | 30 mg IV, 60 mg IM |
| Tramadol (IM) | 100 mg |
Pethidine (meperidine) is not indicated for the treatment of migraine. Its reported effectiveness is only 56%, it has a high rate of rebound headache and it carries a risk of dependence. The data on dihydroergotamine are difficult to interpret because it is often used in combination with other agents (e.g. metoclopramide); however, it has also been shown to be less effective than chlorpromazine and sumatriptan in acute treatment and to have a high rate of unpleasant side effects. Sodium valproate and haloperidol have also shown moderate effectiveness in small studies, but there are insufficient data to draw a valid conclusion or recommend them as treatment options. Lignocaine (lidocaine) has been shown to be no more effective than placebo. The efficacy of intravenous magnesium sulphate (1 or 2 mg) remains unclear. It was shown in a small placebo-controlled trial to be effective but, in another study, the combination of magnesium with metoclopramide was less effective than metoclopramide and placebo.
Rebound or recurrent headache is common in ED patients treated for migraine (approximately 30%). There is evidence that oral or IV dexamethasone, in addition to standard migraine therapy for selected patients, reduces the proportion of patients who experience early recurrence (so-called rebound headache). A meta-analysis of published papers reports a 26% reduction in the relative risk of headache recurrence within 72 hours. Doses used were 10 mg IV or 8 mg orally.
Trigeminal neuralgia
Trigeminal neuralgia is a debilitating condition in which patients describe ‘lightning-’or a ‘hot poker-’like pain that is severe and follows the distribution of the trigeminal nerve. Individual episodes of pain last only seconds, but may recur repeatedly within a short period and can be triggered by minor stimuli, such as light touch, eating or drinking, shaving or passing gusts of wind. It is most common in middle or older age.
Aetiology and pathophysiology
Evidence suggests that the pathological basis of trigeminal neuralgia is demyelination of sensory fibres of the trigeminal nerve in the proximal (CNS) portion of the nerve root or, rarely, in the brainstem, most commonly due to compression of the nerve root by an overlying artery or vein.
Trigeminal neuralgia is classified as classic trigeminal neuralgia (no cause identified) and symptomatic trigeminal neuralgia (secondary to another condition). Characteristics associated with symptomatic trigeminal neuralgia are trigeminal sensory deficits and bilateral involvement.
Clinical investigations
In approximately 15% of cases, there is a structural cause for trigeminal neuralgia. For this reason, there is some support for routine neuroimaging (CT, MRI) in these patients. Electrophysiological assessment of trigeminal reflexes can also be helpful in distinguishing classic from symptomatic trigeminal neuralgia. The choice between the two approaches will depend on availability, expertise, cost and patient and treating clinician preference.
Treatment
The mainstay of therapy for trigeminal neuralgia is carbamazepine. The usual starting dose is 200–400 mg/day in divided doses, increased by 200 mg/day until relief up to a maximum of 1200 mg/day. The average dose required is 800 mg/day. Where available, oxcarbazepine 600–1800 mg/day is an effective alternative. For patients who fail first-line therapy, there is some evidence to support the addition of lamotrigine or a change to baclofen. Referral for consideration of surgery is appropriate in patients who are refractory to medical therapy.
Temporal (giant cell) arteritis
Giant cell arteritis is the most common form of vasculitis in patients aged over 50 years. It affects large and middle-sided blood vessels with a predisposition for the cranial arteries arising from the carotid arteries. Loss of vision is the most common severe complication. Involvement of extracranial arteries including the aorta is more frequent than previously assumed. Inflammation markers in blood are usually elevated, but specific laboratory tests for the diagnosis of giant cell arteritis are not available. Imaging using ultrasonography, magnetic resonance imaging and positron emission tomography can be useful to confirm, localize and assess the extent of vascular involvement. Temporal artery biopsy is the gold standard for diagnosis. Glucocorticoids are still the standard therapy (50–100 mg/day). Patients with acute visual changes secondary to giant cell arteritis should receive parenteral corticosteroid therapy and be admitted until their condition stabilizes.
8.2 Stroke and transient ischaemic attacks
Philip Aplin and Mark Morphett
Introduction
Cerebrovascular disease is the third highest cause of death in developed countries, after heart disease and cancer. A stroke is an acute neurological injury secondary to cerebrovascular disease, either by infarction (80%) or by haemorrhage (20%). The incidence of stroke is steady and, although mortality is decreasing, it is still a leading cause of long-term disability. Transient ischaemic attacks (TIAs) are defined as transient episodes of neurological dysfunction caused by focal brain, spinal cord or retinal ischaemia, without acute infarction. Causes are similar to those of ischaemic stroke, particularly atherosclerotic thromboembolism related to the cerebral circulation and cardioembolism. Diagnosis of the cause of TIAs with appropriate management is important in order to prevent a potentially devastating stroke.
Pathophysiology
Brain tissue is very sensitive to the effects of oxygen deprivation. Following cerebral vascular occlusion, a series of metabolic consequences may ensue, depending on the extent, duration and vessels involved, which can lead to cell death. Reperfusion of occluded vessels may also occur, either spontaneously or via therapeutic intervention, with the potential for reperfusion injury. An area of threatened but possibly salvageable brain may surround an area of infarction. The identification of this so-called ischaemic penumbra and therapeutic efforts to ameliorate the extent of irreversible neuronal damage, have been the subject of ongoing research efforts.
Large anterior circulation ischaemic strokes can be associated with increasing mass effect and intracranial pressure in the hours to days following onset. Secondary haemorrhage into an infarct may also occur, either spontaneously or related to therapy. Clinical deterioration often follows.
Ischaemic strokes
These are the results of several pathological processes (Table 8.2.1):
Table 8.2.1
Ischaemic stroke
Arterial thromboembolism
Carotid and vertebral artery atheroma
Intracranial vessel atheroma
Small vessel disease – lacunar infarction
Haematological disorders – hypercoagulable states
Cardioembolism
Aortic and mitral valve disease
Atrial fibrillation
Mural thrombus
Atrial myxoma
Paradoxical emboli
Hypoperfusion
Severe vascular stenosis or a combination of these factors
Hypotension
Vasoconstriction – drug induced, post-SAH, pre-eclampsia
Other vascular disorders
Arterial dissection
Gas embolism syndromes
Moyamoya disease
Arteritis
Intracerebral haemorrhage
Hypertensive vascular disease
Lipohyalinosis and microaneurysms
Aneurysms
Saccular
Mycotic
Arteriovenous malformations
Amyloid angiopathy
Bleeding diathesis
Anticoagulation
Thrombolytics
Thrombocytopenia/disseminated intravascular coagulation
Haemophilia
Secondary haemorrhage into a lesion – tumour or infarction
Haemorrhagic stroke
Haemorrhagic stroke is the result of vessel rupture into the surrounding intracerebral tissue or subarachnoid space. Subarachnoid haemorrhage is the subject of a separate chapter in this book (see Chapter 8.3).
The neurological defect associated with an intracerebral haemorrhage (ICH) is the consequence of direct brain injury, secondary occlusion of nearby vessels, reduced cerebral perfusion caused by associated raised intracranial pressure and cerebral herniation. The causes of ICH include:
Risk factors for TIA/stroke and prevention
This particularly applies to cerebral ischaemic events, both TIAs and strokes. Non-modifiable risk factors for ischaemic stroke include:
 increasing age: the stroke rate more than doubles for each 10 years above age 55.
increasing age: the stroke rate more than doubles for each 10 years above age 55.
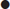 gender: stroke is slightly more common in males than females.
gender: stroke is slightly more common in males than females.
In terms of primary prevention, hypertension is the most important modifiable risk factor. The benefit of antihypertensive treatment in stroke prevention has been well shown. The other major risk factors for atherosclerosis and its complications – diabetes, smoking and hypercholesterolaemia – often contribute to increased stroke risk. These should be managed according to standard guidelines.
The most important cardiac risk factor for TIA and stroke is atrial fibrillation (AF), both chronic and paroxysmal. Warfarin is recommended to prevent cardioembolism where the risk:benefit ratio of anticoagulation (target INR 2.0–3.0) favours this. Prediction tools, such as the CHADS2 and CHA2DS2-VASc scores, have been developed to standardize the approach to primary stroke prevention in patients with non-valvular AF. Recently, an oral direct thrombin inhibitor (dabigatran) has been shown to be non-inferior to warfarin for stroke prevention in a large industry sponsored trial (the RE-LY trial). On the basis of this trial, dabigatran has been approved for use as an alternative to warfarin with rapid uptake of this medication in the community. Those with contraindications to warfarin or very low stroke risk should initially receive aspirin.
A carotid bruit or carotid stenosis found in an otherwise asymptomatic patient is associated with an increased stroke risk. However, the role of carotid endarterectomy in these patients is controversial. While early trials suggested some minor benefit, more recent studies have refuted this and it is increasingly clear that intensive medical therapy in patients with asymptomatic carotid stenosis reduces stroke risk well below that achieved with either endarterectomy or carotid stenting.
Other major cardiac conditions associated with increased TIA/stroke risk include endocarditis, mitral stenosis, prosthetic heart valves, recent myocardial infarction and left ventricular aneurysm. Less common ones include atrial myxoma, a patent foramen ovale and cardiomyopathies.
Secondary prevention involves detection and modification, if possible, of conditions that may have caused a TIA or stroke in order to prevent further events that may result in worse clinical outcomes. As well as the risk factors already mentioned, many other uncommon conditions, such as arterial dissection and prothrombotic states, may cause TIA and stroke. These will be discussed later in the chapter.
Ischaemic stroke syndromes
The symptoms and signs of stroke or TIA correspond to the area of the brain affected by ischaemia or haemorrhage (Table 8.2.2).
Table 8.2.2
| Arterial territory | |||
| Symptom | Carotid | Either | Vertebrobasilar |
| Dysphasia | + | ||
| Monocular visual loss | + | ||
| Unilateral weakness* | + | ||
| Unilateral sensory disturbance* | + | ||
| Dysarthria** | + | ||
| Homonymous hemianopia | + | ||
| Dysphagia** | + | ||
| Diplopia** | + | ||
| Vertigo** | + | ||
| Bilateral simultaneous visual loss | + | ||
| Bilateral simultaneous weakness | + | ||
| Bilateral simultaneous sensory disturbance | + | ||
| Crossed sensory/motor loss | + | ||
In ischaemic brain injury, the history and pattern of physical signs may correspond to a characteristic clinical syndrome according to the underlying cause and the vessel occluded. This has a bearing on the direction of further investigation and treatment decisions. Differentiating between anterior and posterior circulation ischaemia/infarction is important in this respect, but is not always possible on clinical grounds alone.
Determining the cause of the event is the next step. Once again, clues, such as a carotid bruit or atrial fibrillation, may be present on clinical evaluation. For accurate delineation of the site of the brain lesion, exclusion of haemorrhage and assessment of the underlying cause, it is usually necessary to undertake imaging studies.
Patterns of clinical features
Anterior circulation ischaemia
The anterior circulation supplies blood to 80% of the brain and consists of the ICA and its branches, principally the ophthalmic, middle cerebral and anterior cerebral arteries. This system supplies the optic nerve, retina, frontoparietal and most of the temporal lobes. Ischaemic injury involving the anterior cerebral circulation commonly has its origins in atherothrombotic disease of the ICA. Atherosclerosis of this artery usually affects the proximal 2 cm, just distal to the division of the common carotid artery. Advanced lesions may be the source of embolism to other parts of the anterior circulation or cause severe stenosis with resultant hypoperfusion distally if there is inadequate collateral supply via the circle of Willis. This is usually manifest by signs and symptoms in the middle cerebral artery (MCA) territory (Table 8.2.3). Less commonly, lesions of the intracranial ICA and MCA may cause similar clinical features.
Table 8.2.3
Signs of middle cerebral artery (MCA) occlusion
Homonymous hemianopia
Contralateral hemiplegia affecting face and arm more than leg
Contralateral hemisensory loss
Dysphasias with dominant hemispheric involvement (usually left)
Spatial neglect and dressing apraxia with non-dominant hemispheric involvement
Embolism to the ophthalmic artery or its branches causes monocular visual symptoms of blurring, loss of vision and field defects. When transient, this is referred to as amaurosis fugax or transient monocular blindness.
The anterior cerebral artery territory is the least commonly affected by ischaemia because of the collateral supply via the anterior communicating artery. If occlusion occurs distally or the collateral supply is inadequate, then ischaemia may occur. This manifests as sensory/motor changes in the leg – more so than in the arm. More subtle changes of personality may occur with frontal lobe lesions, as may disturbances of micturition and conjugate gaze.
Major alterations of consciousness, with Glasgow coma scores<8, imply bilateral hemispheric or brainstem dysfunction. The brainstem may be primarily involved by a brainstem stroke or secondarily affected by an ischaemic or haemorrhagic lesion elsewhere in the brain, owing to a mass effect and/or increased intracranial pressure.
Posterior circulation ischaemia
Ischaemic injury in the posterior circulation involves the vertebrobasilar arteries and their major branches which supply the cerebellum, brainstem, thalamus, medial temporal and occipital lobes. Posterior cerebral artery occlusion is manifested by visual changes of homonymous hemianopia (typically with macular sparing if the MCA supplies this part of the occipital cortex). Cortical blindness, of which the patient may be unaware, occurs with bilateral posterior cerebral artery infarction.
Depending on the area and extent of involvement, brainstem and cerebellar stroke manifest as a combination of: motor and sensory abnormalities, which may be uni- or bilateral; cerebellar features of vertigo, nystagmus and ataxia; and cranial nerve signs, such as diplopia/ophthalmoplegia, facial weakness and dysarthria. Consciousness may also be affected.
Examples of brainstem stroke patterns include (this list is by no means exhaustive):
Lacunar infarcts
Lacunar infarcts are associated primarily with hypertension and diabetes. They occur in the small penetrating arteries supplying the internal capsule, thalamus and upper brainstem. Isolated motor or sensory deficits are most commonly seen.
Clinical features
History
This includes the circumstances, time of onset, associated symptoms, such as headache, and any resolution/progression of signs and symptoms. It may be necessary to take a history from a relative or friend, particularly in the presence of dysphasia or reduced conscious state. The history of a stroke is usually of acute onset of a neurological deficit over minutes but, occasionally, there may be a more gradual or stuttering nature to a presentation over a period of hours. A past history of similar events suggestive of a TIA should be carefully sought. The presence of a severe headache with the onset of symptoms may indicate ICH or SAH. However, headache may also occur with ischaemic strokes.
A declining level of consciousness may indicate increasing intracranial pressure due to an ICH or a large anterior circulation infarct – so-called malignant MCA infarction. It may also be caused by pressure on the brainstem by an infratentorial lesion, such as a cerebellar haemorrhage.
The possibility of trauma or drug abuse should be remembered along with the past medical and medication history, particularly anticoagulant/antiplatelet therapy. Risk factors for vascular disease, cardiac embolism, venous embolism and increased bleeding should be sought.
In young patients with an acute neurological deficit, dissection of the carotid or vertebral artery should be considered. This is often associated with neck pain and headaches/facial pain with or without a history of neck trauma. Trauma if present may be minor, such as a twisting or hyperextension/flexion injury sustained in a motor vehicle accident, playing sports or neck manipulation.
Cardioembolism tends to produce ischaemic injury in different parts of the brain, resulting in non-stereotypical recurrent TIAs, whereas atherothrombotic disease of the cerebral vessels tends to cause recurrent TIAs of a similar nature, particularly in stenosing lesions of the internal carotid or vertebrobasilar arteries.
Examination
Central nervous system
This includes assessing the level of consciousness, pupil size and reactivity, extent of neurological deficit, presence of neck stiffness and funduscopy for signs of papilloedema and retinal haemorrhage. Quantifying the neurological deficit using a stroke scale, such as the 42-point National Institute of Health Stroke Scale (NIHSS), is useful in the initial assessment and also for monitoring progress in a more objective way than clinical description alone. Strokes with a NIHSS score>22 are classified as severe.
In the case of TIA, all clinical signs may have resolved. The average TIA lasts less than 15 minutes.
Cardiovascular
This includes carotid auscultation and is directed towards findings associated with a cardioembolic source. A carotid bruit in a symptomatic patient is likely to predict a moderate to severe carotid stenosis. Conversely, the absence of a carotid bruit does not exclude significant carotid artery disease as a cause of a TIA or stroke. Major risk factors for cardioembolism that can be identified in the emergency department (ED) include AF, mitral stenosis, prosthetic heart valves, infective endocarditis, recent myocardial infarction, left ventricular aneurysm and cardiomyopathies.
Differential diagnosis (Table 8.2.4)
The acute onset of stroke and TIA is characteristic, however, misdiagnoses (the so-called ‘stroke mimics’) can occur. The most common stroke mimics are seizures (particularly when there is associated Todd’s paresis), hypoglycaemia, systemic infection, brain tumour and toxic metabolic disorders. Others include subdural haematoma, hypertensive encephalopathy, encephalitis, multiple sclerosis, migraine and conversion disorder. This has implications when considering more aggressive stroke interventions, such as thrombolysis.
Table 8.2.4
Differential diagnosis of stroke
Intracranial space-occupying lesion
Subdural haematoma
Brain tumour
Brain abscess
Postictal neurological deficit – Todd’s paresis
Head injury
Encephalitis
Metabolic or drug-induced encephalopathy
Hypoglycaemia, hyponatraemia, etc.
Wernicke–Korsakoff syndrome
Drug toxicity
Hypertensive encephalopathy
Multiple sclerosis
Migraine
Peripheral nerve lesions
Functional
Complications
CNS complications of stroke include:
Clinical investigations
The investigations of TIA and stroke often overlap, but the priorities and implications for management may differ significantly.
General investigations
Standard investigations that may identify contributing factors to stroke/TIA or guide therapy include a complete blood picture, blood glucose, coagulation profile, electrolytes, liver function tests, fasting lipids and, in selected cases, C-reactive protein (CRP). Arterial blood gases should be performed if the adequacy of ventilation is in doubt. An ECG should be performed to identify arrhythmias and signs of pre-existing cardiac disease. Holter monitoring can be considered to identify paroxysmal arrhythmias but has a low yield in unselected patients (i.e. those without any history suggestive of symptomatic arrhythmias or background of structural heart disease). A prothrombotic screen may be indicated, particularly in younger patients. Further investigations depend on the nature of the neurological deficit and other risk factors for stroke that are identified on evaluation, but usually involve a combination of brain, vascular and cardiac imaging.
Imaging in TIAs
Prompt diagnosis and management of patients presenting with TIAs and non-disabling strokes has been shown to reduce the risk of subsequent stroke by up to 80%. Risk stratification for patients presenting with TIAs can guide the urgency of investigations required to determine the underlying cause of the TIA – this is discussed more fully below.
Brain imaging
A head computed tomgraphy (CT) or magnetic resonance imaging (MRI) scan is indicated in all patients with TIA to exclude lesions that occasionally mimic TIA, such as subdural haematomas and brain tumours. CT and, more particularly MRI, may show areas of infarction which match the symptoms of an ischaemic event that, on clinical grounds, has completely resolved. CT is less sensitive than MRI in detecting posterior territory ischaemic lesions, particularly in the brainstem. In TIAs due to AF or another known cardiac source, brain imaging to exclude ICH is necessary prior to commencing anticoagulation. The exception is in cases of emboli from endocarditis in which anticoagulation is contraindicated owing to the increased risk of secondary ICH.
Imaging vessels
Ultrasound: if the aetiology of a TIA is likely to be carotid disease, with or without a carotid bruit, then a carotid ultrasound remains the most commonly utilized initial investigation to investigate the presence and degree of a carotid stenosis.
CT angiography (CTA): CTA is increasingly being used to image vessels in cases of TIA – commonly in conjunction with contrast studies examining cerebral perfusion. Advantages include ease of access and avoidance of further delay waiting for second modality imaging. Disadvantages include exposure to contrast dye and ionizing radiation.
Magnetic resonance imaging and magnetic resonance angiography (MRA): this provides non-invasive imaging of the brain and major cerebral vasculature. MRA can show lesions suggestive of a vascular aetiology for TIAs, such as a stenosis due to atheromatous disease and dissection. MRI/MRA is not routine in TIA work-up but may be indicated in more prolonged TIAs, in patients in whom an uncommon cause is suspected or in younger patients.
Angiography: formal angiography may be indicated in selected cases to confirm high-grade carotid stenosis and to confirm/exclude complete carotid occlusions shown on ultrasound. Angiography and MRI/MRA may be performed to investigate for intracranial cerebrovascular disease. The use of formal angiography has declined in recent years, with greater use of both CT angiography and MRA studies as confirmatory tests where atheroma is found on carotid ultrasound.
Cardiac imaging
If the clinical evaluation indicates that a cardioembolic source is a likely cause of a TIA, echocardiography is a priority. However, if there is no evidence of cardiac disease on clinical evaluation and the ECG is normal, then the yield of echocardiography is relatively low. A transthoracic echocardiogram (TTE) is the first-line investigation in cardiac imaging. A transoesophageal echocardiogram (TOE) is more sensitive than TTE in detecting potential cardiac sources of emboli, such as mitral valve vegetations, atrial/mural thrombi and atrial myxoma. TOE should be considered in patients with inconclusive or normal TTE with ongoing clinical concern of a cardioembolic source or patent foramen ovale. This particularly applies to younger patients with unexplained TIAs/non-disabling stroke.
Imaging in stroke
Brain imaging
Computed tomography: in the setting of completed stroke, the usual first-line investigation is a non-contrast CT scan. The main value of CT is its sensitivity in the detection of ICH and its ready availability. However, CT scans are often normal in the first hours following ischaemic stroke. In only about half of cases will there be changes detected 24 hours after the onset of symptoms.
The early signs of ischaemic stroke include loss of the cortical grey/white matter distinction and hypoattenuation in the affected arterial distribution (e.g. the insular ribbon sign and obscuration of the lenticulostriate territory in MCA infarcts). Occasionally, a hyperdense clot sign will be seen in the region of the MCA. As well as the presence of haemorrhage, the degree of acute ischaemic change, typically change affecting greater than a third of the MCA territory, has been used to exclude patients from thombolytic trials due to possible lack of therapeutic benefit and increased haemorrhage risk. The degree of acute ischaemic change on plain CT can be more reliably quantified by using the ASPECTS score.
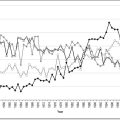
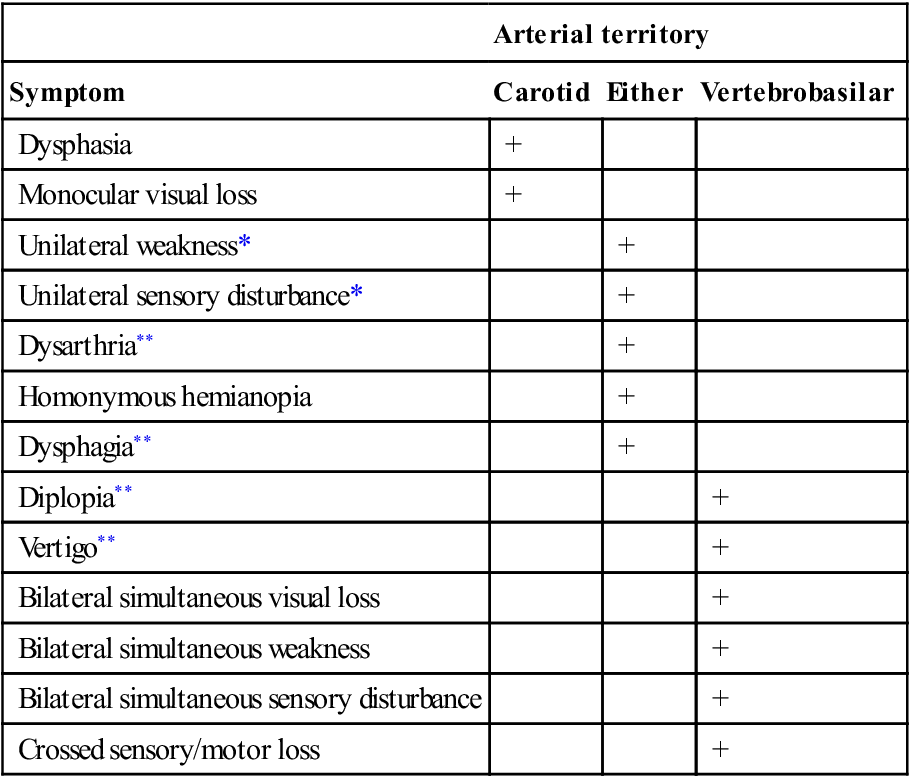
A CT scan should be performed as soon as possible following stroke onset. Urgent CT scanning is indicated in patients with a reduced level of consciousness, deteriorating clinical state, symptoms suggestive of ICH, associated seizures, prior to thrombolytic therapy, in younger patients, in patients who are on warfarin and in cases of diagnostic doubt. A CT scan should also be performed to exclude haemorrhage prior to the commencement of antiplatelet therapies. It should, however, be noted that ICH may be subtle and difficult to diagnose, even for radiologists. CT perfusion/CT angiography: CT perfusion studies are increasingly being used in the setting of acute stroke. Following IV contrast injection, an area of brain is imaged and analysed using computer software with respect to the cerebral blood volume (CBV), cerebral blood flow (CBF) and mean transit time (MTT). Using predetermined cut-offs of these values, the areas of likely irreversibly infarcted brain (infarct core) and at risk ischaemic brain (ischaemic penumbra) can be demonstrated (E-Fig. 8.2.1). A CT angiogram which includes the carotid vessels is also performed to determine if there is a site of large vessel occlusion. This technology is seen as offering an alternative to diffusion/perfusion MRI and MRA as it is more readily available, generally quicker and less subject to artefact. However, the technique is still in evolution and involves iodinated contrast and radiation exposure.
CT angiography (CTA): CTA is the imaging modality of choice in evaluation of primary ICH to identify the underlying cause, such as an aneurysm or AVM. CTA should be performed in cases of stroke due to suspected arterial dissection and basilar artery thrombosis.
Formal angiography is required occasionally in acute stroke. It will be required if intra-arterial therapy, such as embolectomy, is being considered. This only occurs in specialized centres.
MRI: there are many magnetic resonance modalities available for imaging the brain in acute stroke. Even standard MRI is superior to CT in showing early signs of infarction, with 90% showing changes at 24 hours on T2-weighted images. Multimodal MRI typically involves additional modes, such as gradient recalled echo (GRE) and fluid-attenuated inversion recovery (FLAIR) sequences for the detection of acute and chronic haemorrhage and diffusion-weighted imaging (DWI) for the detection of early ischaemia or infarction. MR DWI images show areas of reduced water diffusion in the parts of the brain that are ischaemic and likely to be irreversibly injured. This occurs rapidly after vessel occlusion (less than an hour after stroke onset) and manifests as an area of abnormal high signal in the area of core ischaemia. Hence it is much more sensitive in detecting early ischaemia/infarction than standard T2-weighted MRI modalities or CT. Perfusion-weighted MRI scans (PWI) reveal areas of reduced or delayed cerebral blood flow following MRI contrast injection. This area of the brain is likely to become infarcted if flow is not restored. The DWI and PWI lesions can then be compared. A PWI lesion significantly larger than a DWI lesion is a marker of potentially salvageable brain: the ischaemic penumbra. It is postulated that acute ischaemic stroke patients with this pattern are most likely to benefit from vessel opening strategies, such as thrombolysis. Large areas of diffusion abnormality may also be a marker for increased risk of ICH with thrombolysis. An MRA can be performed at the same time to identify a major vessel occlusion. DWI/PWI imaging is generally considered to be easier to interpret and more reliable than CT perfusion studies. However, MRI may not be as available or feasible. A significant number of patients are unsuitable for MRI and the multimodal imaging takes longer than CT which increases the risk of motion artefact and potential delay to treatment. Radiation and iodinated contrast exposure are absent in MRI.
Recent studies have suggested that MRI is as accurate as CT in diagnosing acute ICH. This is significant as it means that, where facilities are immediately available, CT may be bypassed in acute stroke and MRI can be used both to exclude ICH and to scan for ischaemia/infarction with DWI plus or minus PWI and MRA.
The place of advanced imaging modalities, such as CT perfusion and DWI/PWI MRI, in acute stroke work-up is evolving. For over a decade now it has been hoped that the information provided by these studies will help better select patients who will benefit from aggressive stroke therapies, such as thrombolysis, and extend the current narrow time window for such treatment on the basis of the existence of a significant ischaemic penumbra. They are now a common feature of acute stroke imaging work-up protocols if thrombolysis is being considered. However, at this point, high level evidence of improved clinical outcomes in acute stroke patients based on this approach is lacking. To date, the only published phase III trial (DIAS 2) of thrombolysis 3–9 hours post-onset using penumbral section criteria as shown on advanced imaging CT perfusion or DWI\PWI MRI was negative for the primary outcome of improved neurological outcome. The field of stoke imaging is changing rapidly and ongoing research investigating advanced imaging modalities as a basis for patient selection and mode of treatment is intense.
MRI is indicated in strokes involving the brainstem and posterior fossa where CT has poor accuracy. MRA/MRV is particularly useful in the evaluation of unusual causes of stroke, such as arterial dissection, venous sinus thrombosis and arteritis. Basilar artery thrombosis causes a brainstem stroke with an associated high mortality. If the diagnosis is suspected, urgent specialist consultation should be obtained. If MRA or CTA confirms the diagnosis, aggressive therapies, such as thrombolysis, may improve outcome.
Other investigations
Other investigations may be indicated, particularly in young people, in whom the cause of strokes/TIA may be obscure. These include tests to detect prothrombotic states and uncommon vascular disorders. The list of tests is potentially long and includes a thrombophilia screen, vasculitic and luetic screens, echocardiography and angiography.
Treatment
The treatment of cerebrovascular events must be individualized. It is determined by the nature and site of the neurological lesion and its underlying cause. The benefits and risks of any treatment strategy can then be considered and informed decisions made by the patient or their surrogate. This is particularly the case with the use of more aggressive therapies, such as anticoagulation, thrombolysis and surgery.
Pre-hospital care
The pre-hospital care of the possible stroke patient involves the usual attention to the ABCs of resuscitation and early blood sugar measurement. It is unusual for interventions to be required.
Of potentially greater significance is the development of stroke systems (along the lines of trauma systems) in which the sudden onset of neurological signs and symptoms, identified in the pre-hospital evaluation as being consistent with acute stroke, is used to direct patients to stroke centres with the facilities and expertise to manage them, particularly with regard to the delivery of thrombolytic agents. Closer hospitals without these capabilities may be bypassed.
Pre-hospital evaluation and early hospital triage tools that have been developed for rapid identification of stroke include the Cincinnati Prehospital Stroke Scale or FAST (F – facial movements, A – arm movements, S – speech, and Τ – test) and the Rosier score. Pre-hospital personnel who identify patients with acute onset of neurological deficits consistent with stroke, using these simple scales, can be potentially directed to stroke centres and in-hospital acute stroke responses can be activated so as to expedite assessment and imaging, particularly if thrombolysis is being considered.
General measures
The ED management of a TIA and stroke requires reassessment of the ABCDs and repeated blood glucose testing. Airway intervention may be necessary in the setting of a severely depressed level of consciousness, neurological deterioration or signs of raised intracranial pressure and cerebral herniation.
Hypotension is very uncommon in stroke patients, except in the terminal phase of brainstem failure. Hypertension is much more likely to be associated with stroke because of the associated pain, vomiting and raised intracranial pressure and/or pre-existing hypertension, but rarely requires treatment and usually settles spontaneously. It may be a physiological response to maintain cerebral perfusion pressure in the face of cerebral hypoxia and raised intracranial pressure. The use of antihypertensives in this situation may aggravate the neurological deficit. The recently published SCAST trial of candesartan commenced within 30 hours of symptom onset and continued for 7 days in patients with ischaemic (85%) or haemorrhagic (15%) stroke and systolic blood pressure≥140 mmHg showed no benefit in functional outcomes and suggested possible harm. This study excluded patients receiving thrombolysis. Hypertension in the setting of thrombolytic therapy is managed according to local protocols.
Analgesia is appropriate if pain is thought to be contributory and urinary retention should be excluded prior to commencing antihypertensive therapy.
An elevated temperature can occur in stroke and should be controlled. It should also raise the suspicion of other possible causes for the neurological findings or an associated infective focus. Hyperglycaemia should be treated appropriately, however, intensive euglycaemic therapy is not indicated.
TIA
Risk stratification
As already stated, the main aim in therapy in TIAs and minor strokes is to prevent a major subsequent cerebrovascular event. While traditionally patients presenting with TIAs have been hospitalized for work-up, more recently, there has been a move away from this to a more tailored approach where patients are risk stratified into higher risk (inpatient work-up) and lower risk patients who may be suitable for early outpatient follow up, for example in coordinated rapid access ‘TIA clinics’.
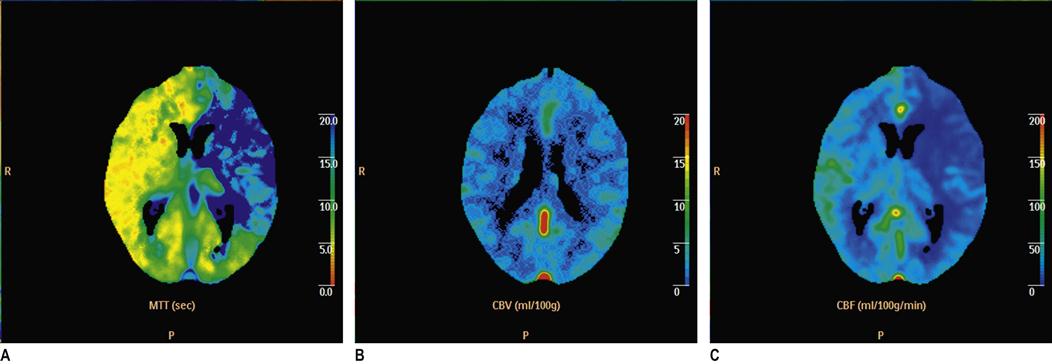
One popular tool for risk stratification in this population is the ABCD2 score. The ABCD2 stroke risk score for TIA has been developed and validated to evaluate the very early risk of a stroke following a TIA. The scoring system is shown in Table 8.2.5. In patients with an ABCD2 score less than 4, there is minimal short-term risk of stroke. With scores of 4–5 and 6–7, the 2-day risk is 4.1%, and 8.1%, respectively. The use of the ABCD2 score is not universally accepted, however, as ongoing validation studies have had mixed results. Other risk stratification strategies, such as the recently published M3T model, use a combination of CT, ECG and carotid ultrasound results to stratify follow-up urgency. Other patient groups are at increased risk of stroke independent of the classical risk stratification systems. These include patients with multiple TIAs within a short period and patients with a probable or proven cardioembolic source.
Table 8.2.5
| ABCD2 | Risk factor | Score |
| Age | Below 60 | 0 |
| Above 60 | 1 | |
| Blood pressure | BP above systolic 140 mmHg, and/or diastolic 90 mmHg on first assessment after TIA | 1 |
| Clinical | Unilateral weakness of face, arm, hand or leg | 2 |
| Speech disturbance without weakness | 1 | |
| Duration | Symptoms lasted>60 mins | 2 |
| Symptoms lasted 10–60 mins | 1 | |
| Symptoms lasted<10 mins | 0 | |
| Diabetes | Presence of diabetes | 1 |
From Johnston SC , Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of a score to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283-92 with permission.
Antiplatelet therapy
Following CT scanning that excludes ICH, aspirin should be commenced at a dose of 300 mg and maintained at 75–150 mg per day in patients with TIAs or minor ischaemic strokes. It has been shown to be effective in preventing further ischaemic events. The ESPRIT trial showed a modest additional benefit from a combination of dipyridamole with aspirin, over aspirin alone. There was no increased risk of bleeding complications but there was a significantly increased rate of withdrawal of patients from the combination arm due to side effects from dipyridamole, principally headache. Clopidogrel may be substituted for aspirin if the patient is intolerant of aspirin or aspirin is contraindicated. There is some evidence that clopidogrel is more effective than aspirin in prevention of vascular events but at greater expense. The combination of aspirin and clopidogrel is not recommended as it does not appear to give any greater therapeutic benefits and there is increased bleeding risk. Anticoagulation with heparin and warfarin has not been shown to be superior to aspirin except in cases of TIA/minor stroke due to cardioembolism (excluding endocarditis).
Anticoagulant therapy
Patients with a cardioembolic source of TIA should be considered for full anticoagulation following neurological consultation and normal brain imaging, with the exception of those with endocarditis in whom the risk of haemorrhagic complications is increased.
Surgery
Trials have demonstrated a beneficial outcome of urgent surgery for symptomatic carotid stenosis in patients with anterior circulation TIAs and minor stroke with a demonstrated carotid stenosis of between 70 and 99%. The benefit of surgery may extend to lesser grades of stenosis down to 50% in selected patients. The patient’s baseline neurological state, co-morbidities and operative mortality and morbidity rate also need to be assessed when considering surgery. The recent CREST trial compared carotid artery stenting (CAS) with endarterectomy (CEA). It revealed slightly superior stroke prevention for CEA in symptomatic patients. In patients with significant co-morbidities, CAS remains an option.
Other medical therapies
Risk factors for stroke and TIAs should be identified and treated. Statins should be considered regardless of cholesterol levels. The benefit of lowering LDL cholesterol levels using atorvastatin in preventing further cerebro- and cardiovascular events following an initial episode of cerebral ischaemia was demonstrated in a recent study.
Ischaemic stroke
A more active approach to the acute management of ischaemic stroke is seen as having the potential to improve neurological outcomes. The ED is the place where these important treatment decisions will largely be made. Most patients with a stroke will require hospital admission for further evaluation and treatment, as well as for observation and rehabilitation. Studies of stroke units show that patients benefit from being under the care of physicians with expertise in stroke and a multidisciplinary team that can manage all aspects of their care.
Aspirin
In two large trials, aspirin, when administered within 48 hours of the onset of stroke, was found to improve the outcomes of early death or recurrent stroke compared to placebo. A CT or MRI scan should be performed to exclude ICH prior to commencing aspirin. Aspirin should be withheld for at least 24 hours in patients treated with thrombolytics.
Thrombolysis
As the most important factor in ischaemic stroke outcome is vessel re-opening, thrombolytic agents are seen as having an important place in the management of acute ischaemic stroke. The evidence on which thrombolysis was originally approved was the NINDs study of 1996. In that study, thrombolysis resulted in improved neurological outcomes in patients receiving tPA compared to placebo, with a 13% absolute increase in the number of patients having good neurological outcomes (numbers needed to treat (NNT)=8). In the thrombolysis group, there was a significant increase in symptomatic intracerebral haemorrhage rate (6.4% versus 0.6% in the placebo group), of which half were fatal, although there was no overall excess mortality. Factors that may be associated with increased haemorrhage risk include increased age (especially>80 years), increased severity of stroke and early CT changes of a large ischaemic stroke. More recently, three other phase III trials of thrombolysis have been published. The DIAS-2 study was described previously in this chapter. ECASS 3 studied IV tPA versus placebo in ischaemic stroke patients 3–4.5 hours from symptom onset. It had a similar design to NINDS but with additional exclusions of patients aged>80, those with severe stroke (NIHSS>25) or acute ischaemic change on plain CT greater than a third of the MCA territory or a history of previous stroke and diabetes. It found a modest but significant improvement in full or very good neurological recovery (Modified Rankin Score [MRS] 0–1), NNT 14. The outcome for full to good neurological recovery (MRS 0–2) was not significantly improved. The risk of a major symptomatic ICH complicating treatment was 5%. The IST-3 trial studied the open label use of IV tPA versus placebo in ischaemic stroke patients from 0 to 6 hours post-onset in whom the treating physician was uncertain if tPA was clearly indicated or not. Over 3000 patients were enrolled over 10 years. The primary outcome of good neurological function at 6 months (Oxford Handicap Score 0–2) was not significantly improved by tPA. There was an excess of early deaths within 7 days in the tPA group but, by 6 months, this was similar in both groups. Symptomatic ICH rate was 7%. The conclusions that can be inferred from these results have been a matter of considerable debate.
Studies of acute stroke patients given tPA outside controlled trials have yielded conflicting results. They suggest that when tPA is used by specialists in well-equipped stroke centres in accordance with strict guidelines, the complication rate for acute stroke patients can be similar to that achieved in trials. The evidence would also strongly suggest that better outcomes are associated with earlier treatment using current treatment guidelines. The percentage of acute ischaemic stroke patients fulfilling the eligibility criteria and receiving thrombolytic treatment is still relatively low. Importantly, protocol violations are associated with an increased risk of poor outcomes, particularly due to haemorrhage. Stroke guidelines in Australia currently recommend the use of tPA if it can be administered in suitable patients up to 4.5 hours post-onset, although every effort should be made to commence treatment as soon as possible.
Patients receiving tPA must be managed in an high dependency type setting. Any deterioration in clinical state, headache or vomiting should instigate cessation of tPA and urgent CT scan. ICH associated with tPA is managed with attention to ABCDs, reversal of thrombolysis according to local protocols and neurosurgical and ICU involvement as required.
Trials of IV thrombolysis are ongoing with the aims of identifying the best agent, identifying patients most likely to benefit from reperfusion therapy, reducing the risk of symptomatic ICH and extending the time window for treatment. As already discussed, this is particularly through the use of advanced imaging modalities, such as CT perfusion and diffusion/perfusion MRI.
Interventional techniques
Occlusions of the internal carotid and proximal middle cerebral arteries have relatively low rates of recanalization with intravenous tPA. Hence a number of interventional therapies are being investigated either as primary therapy or as a rescue technique post-IV tPA and reperfusion failure. These interventional techniques involve the use of clot retrieval devices and/or intra-arterial thrombolysis and are only available in highly specialized centres. The time window of benefit from interventional therapy may be longer than with IV thrombolysis and may be considered in cases of acute ischaemic stroke due to large vessel occlusion where thrombolysis is contraindicated.
Basilar artery thrombosis has a very poor outcome with conservative management. Case series suggest improved outcomes with interventional techniques, which may be indicated up to 12 hours post-symptom onset.
Anticoagulation
Therapeutic anticoagulation with heparin or clexane is associated with increased risk of haemorrhagic transformation in acute ischaemic stroke. Stroke due to endocarditis has a particularly high risk of this complication. Anticoagulation following acute ischaemic stroke should not be commenced in the ED. In cases of stroke due to cardioembolism, the timing and manner of anticoagulation should be determined by stroke physicians.
Neuroprotection
A range of neuroprotective agents has been trialled in the setting of acute stroke in the hope that modulation of the ischaemic cascade of metabolic changes that follows vascular occlusion may result in improved neurological outcomes. At this stage, however, none of these therapies is recommended for the treatment of acute stroke.
Surgery
As for TIAs, patients with non-disabling stroke should be considered for investigation with a vascular imaging modality to detect a significant carotid artery stenosis that may be appropriate for urgent surgery. The use of endovascular stents in carotid surgery is also being developed and studied.
Large anterior circulation infarcts have a significant risk of developing cerebral oedema and raised ICP with associated clinical deterioration, particularly manifest by a declining conscious state with or without progression of other signs. These are termed malignant MCA infarcts. Along with standard measures for managing raised ICP, there may be a place for early decompressive craniotomy in selected cases. Intensive care and neurosurgical consultation may be required.
Intracerebral haemorrhage
Primary ICH is most commonly caused by long-standing hypertension-induced small vessel disease. Hypertensive haemorrhage tends to occur in characteristic locations, such as the basal ganglia, thalamus and cerebellum. Berry aneurysms most commonly arise around the circle of Willis, hence ICH due to aneurysmal rupture is often located around this area. Secondary ICH may occur into an underlying lesion, such as a tumour or infarct, and clinical deterioration may result – so-called symptomatic ICH – but this is not always the case.
The clinical presentation of primary ICH is typical of sudden onset of a neurological deficit with associated headache, collapse/transient loss of consciousness, hypertension and vomiting. However, clinical features alone are unable to differentiate ICH from infarction, hence the requirement for brain imaging to confirm the diagnosis. Both CT and MRI (using gradient echo sequences) are equivalent in the detection of ICH.
Treatment
Primary ICH is a medical emergency with a high mortality (between 35 and 50%), with half of these deaths occurring in the first 2 days. There is also a very high risk of dependency. Haematomas can expand rapidly and there is a significant risk of early neurological deterioration and increasing intracranial pressure. General measures as for TIAs and ischaemic stroke should be initiated, in particular attention to airway and ventilatory support. Treatment of raised ICP in a setting of ICH involves a range of modalities similar to those used in head trauma. These include elevation of the head of the bed, analgesia, sedation, an osmotic diuretic, such as mannitol, and hypertonic saline, hyperventilation, drainage of CSF via ventricular catheter and neuromuscular paralysis.
Current consensus guidelines in ICH recommend cautious and controlled lowering of a persistently raised systolic blood pressure>180–200 mmHg or mean arterial pressure (MAP)>130–150 following specialist consultation. Recommended agents include rapidly titratable intravenous drugs, such as sodium nitroprusside, labetolol or glycerine trinitrate at low initial doses, and with continuous haemodynamic monitoring in a critical care setting. The aim is for a 10–15% reduction in blood pressure. The results of the SCAST trial, as mentioned previously, may alter this recommendation; however, the outcome of more aggressive blood pressure reduction in ICH is still being evaluated. The INTERACT trial of intensive BP reduction within 6 hours of symptom onset showed a reduction in haematoma growth. The effect on clinical outcomes is being studied in the INTERACT 2 trial. Treatment should be individualized and take place in consultation with stroke/neurosurgery/intensive care specialists. Sudden falls in blood pressure and hypotension should be avoided as they may aggravate cerebral ischaemia in the setting of raised ICP, which is often associated with ICH.
Use of recombinant Factor VIIa is not recommended. Steroids are also not indicated in ICH. Anticonvulsant prophylaxis is common practice.
Management of ICH associated with anticoagulation or thrombolysis is a matter of urgency and should be done in consultation with a haematologist and a neurosurgeon. Depending on the clinical situation, agents such as protamine sulphate, vitamin K, prothrombin complex concentrate, fresh frozen plasma (FFP) and tranexamic acid may be indicated. Factor VIIa normalizes the INR rapidly, but with a greater potential for thromboembolism. Platelets should be considered if the patient is on antiplatelet therapy.
Surgical management
Surgical management of ICH depends on the location, cause, neurological deficit and overall clinical state. Early neurosurgical consultation should be obtained. High-level evidence for improved outcomes following drainage of supratentorial haematomas by craniotomy is lacking, but the procedure may be indicated in selected patients, particularly in those with lobar clots within 1 cm of the surface. In patients with deep haemorrhages, craniotomy is generally not recommended.
External ventricular drainage devices (EVDs) may be indicated if hydrocephalus develops.
The presence of a cerebellar haematoma is a particular indication for surgery, with a potential for a good neurological recovery. A variety of other techniques, such as minimally invasive haematoma evacuation, are under investigation.
8.3 Subarachnoid haemorrhage
Pamela Rosengarten
Introduction
Patients with headache account for approximately 1% of all emergency department (ED) visits and, of these, 1–4% have a final diagnosis of subarachnoid haemorrhage (SAH). Early accurate diagnosis of aneurysmal SAH is imperative, as early occlusion of the aneurysm has been shown to reduce early complications of re-bleeding and vasospasm and improve outcome.
Epidemiology and pathology
SAH is the presence of extravasated blood within the subarachnoid space. The incidence in Australia is approximately 10 cases per 100 000 patient-years, but is significantly higher (around 20 per 100 000) in Japan and Finland, for reasons that are unclear. Although incidence increases with age, about half of those affected are aged under 55, the condition being most common in the 45–64 age group.
The most common cause of SAH is head trauma which is dealt with elsewhere in this book. Non-traumatic or spontaneous SAH results from rupture of a cerebral aneurysm in approximately 85% of cases, non-aneurysmal perimesencephalic haemorrhage in 10% and the remaining 5% from other rare causes including rupture of mycotic aneurysms, intracranial arterial dissection, arteriovenous malformations, vasculitis, central venous thrombosis, bleeding diatheses, tumours and drugs, such as cocaine, amphetamines and anticoagulants.
Aneurysms
Intracranial aneurysms are not congenital. Rather, they develop during the course of life. An estimate of the frequency for an adult without risk factors is 2.3%, with the proportion increasing with age. Most aneurysms will never rupture, but the risk increases with size. Paradoxically, as the vast majority of aneurysms are small, most aneurysms that rupture are small. An aneurysm of the posterior circulation is more likely to rupture than one of comparable size in the anterior circulation.
Risk factors can be divided into those that are modifiable and those that are not. Modifiable risk factors include cigarette smoking, hypertension, sympathomimetic drug use (e.g. cocaine) and excessive alcohol intake. Non-modifiable factors include history of previous aneurysmal SAH, a family history of first-degree relatives with SAH, inherited connective tissue disorders (particularly polycystic kidney disease and neurofibromatosis), sickle cell disease and α1-antitrypsin deficiency.
Non-aneurysmal perimesencephalic haemorrhage
This type of SAH is defined by the characteristic distribution of blood in the cisterns around the midbrain in combination with normal angiographic studies. It usually carries a relatively benign prognosis. A small proportion of patients with this distribution of blood may have a ruptured aneurysm of a vertebral or basilar artery.
Clinical features
History
Examination
There is a wide spectrum of clinical presentations, the level of consciousness and clinical signs being dependent on the site and extent of the haemorrhage:
Patients are categorized into clinical grades from I to V, according to their conscious state and neurological deficit. Two grading schemes, that of Hunt and Hess and that of the World Federation of Neurosurgeons, which is preferred, are depicted in Table 8.3.1. The higher the score, the worse the prognosis.
Table 8.3.1
Clinical grading schemes for patients with SAH
| Grade | Grading scheme of Hunt and Hess | Grading scheme of WFNS | |
| GCS | Motor deficit | ||
| 1 | No symptoms or minimal headache, slight nuchal rigidity | 15 | No |
| 2 | Moderate to severe headache, no neurological deficit other than cranial nerve palsy | 13–14 | No |
| 3 | Drowsy, confused, mild focal deficit | 13–14 | Yes |
| 4 | Stupor, moderate to severe hemiparesis, vegetative posturing | 7–12 | Yes or no |
| 5 | Deep coma, decerebration, moribund | 3–6 | Yes or no |
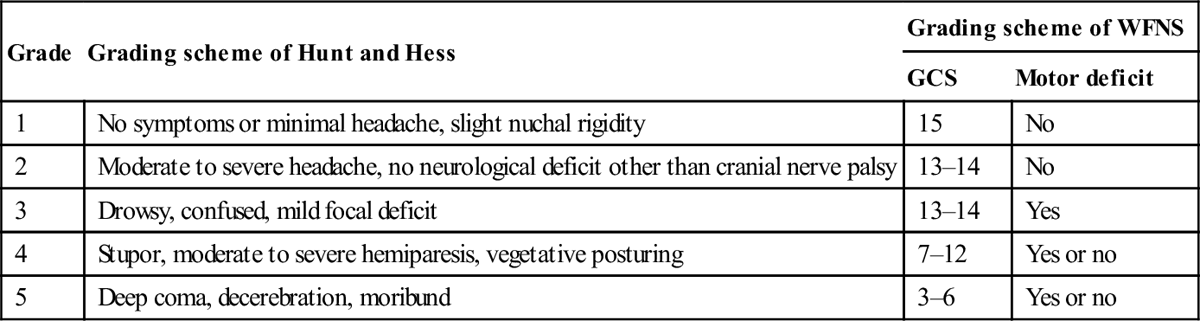
WFNS: World Federation of Neurosurgeons; GCS: Glasgow coma score. Reproduced with permission from Sawin PD, Loftus CM. Diagnosis of spontaneous subarachnoid hemorrhage. Am Fam Phys 1997;55:145–56.
Differential diagnosis
Important differential diagnoses include benign thunderclap headache (40%), migraine, cluster headache, headache associated with sexual exertion, vascular headaches of stroke, intracranial haemorrhage, venous thrombosis and arterial dissection, meningitis, encephalitis, acute hydrocephalus, intracranial tumour and intracranial hypotension.
Clinical investigations
Imaging
Computed tomography (CT)
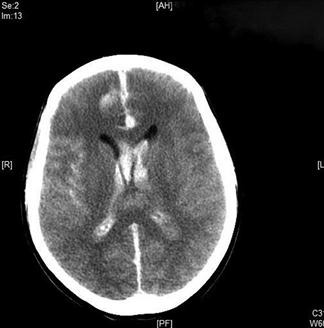
Non-contrast brain CT scan is the initial investigation of choice. In the first 24 hours after haemorrhage it can demonstrate the presence of subarachnoid blood in more than 95% of cases (Fig. 8.3.1). The sensitivity, however, decreases with time owing to the rapid clearance of blood, with only 80% of scans positive at 3 days and 50% positive at 1 week. CT will also demonstrate the site and extent of the haemorrhage, indicate the possible location of the aneurysm and demonstrate the presence of hydrocephalus and other pathological changes.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) with FLAIR (fluid attenuated inversion recovery) proton density, diffusion weighted imaging and gradient echo sequences is reliable in demonstrating early SAH and is superior to CT in detecting extravasated blood in the days (up to 40 days) following haemorrhage. Availability and logistical considerations, including longer procedure time, make MRI impractical for use in the initial diagnostic work-up of SAH, but it may be considered in patients who present late.
CT angiography
CT angiography (CTA) is the preferred angiographic technique once SAH has been identified. Compared to catheter angiography, it has a sensitivity of 98% for cerebral aneurysms, is readily available and has a lower complication rate. It should be performed as soon as the diagnosis is made. Where diagnosis has been made by CT, CTA should preferably be performed while the patient is still in the scanner. CTA is usually of sufficient quality to allow planning of endovascular or neurosurgical interventions. It is important to note that small aneurysms<3 mm may not reliably be detected on CTA and so further investigations may be warranted in CTA-negative SAH.
A CT/CTA approach has been suggested as an alternate diagnostic strategy to CT/LP (lumbar puncture) in the diagnosis of SAH. However, this approach focuses on identifying an aneurysm, rather than the presence of intracranial haemorrhage. The consequence of this strategy may be that the aneurysm detected is an incidental finding, as aneurysms are known to occur in about 2.5% of the normal population. This would then result in unnecessary investigation and treatment of an asymptomatic aneurysm and so is currently not supported.
Cerebral angiography
Cerebral angiography is the gold standard for confirming the presence of an aneurysm, its location and the presence of vasospasm and was previously the preferred angiographic test. It is not, however, without risk. Neurological complications occur in ≈1.8% of cases, with re-rupture of an aneurysm reported in 2–3%. It is also less available than CTA. These factors have seen it become less favoured and used in selected cases only.
Magnetic resonance angiography
MR angiography is currently useful as a screening tool for the diagnosis of intracranial aneurysms in patients at increased risk.
Further imaging when no cause for SAH is found
In patients where SAH is present and no cause is found, then the distribution of extravasated blood on the CT scan should be reviewed. If this conforms to the perimesencephalic distribution of non-aneurysmal haemorrhage, then no further investigations may be warranted. If, however, an aneurysmal pattern of haemorrhage is present, then a second CTA is recommended as occasionally an aneurysm may have gone undetected on the original test.
Lumbar puncture
Lumbar puncture is necessary when there is clinical suspicion of SAH, the CT scan is negative, equivocal or technically inadequate and no mass lesion or signs of raised intracranial pressure are found. In about 3–5% of patients with SAH, the CT scan will be normal. Although it has been suggested that a negative CT scan performed in the first 6 hours following headache onset is sufficient to exclude a diagnosis of SAH, evidence is inadequate to support this practice and so cannot be recommended to replace a CT/LP strategy.
The diagnosis of SAH, then, is dependent on the finding of red blood cells not due to traumatic tap or red blood cell breakdown products within the CSF. Lumbar puncture should be delayed for at least 6 and preferably 12 hours after symptom onset to allow bilirubin to be formed from cell breakdown in SAH. Detection of bilirubin and xanthochromia is the only reliable method of distinguishing SAH from a traumatic tap. Proceeding to angiographic studies in every patient with bloodstained CSF would be expected to identify an incidental finding of a small unruptured aneurysm in about 2%.
It is important to measure the opening pressure when performing a lumbar puncture, as CSF pressure may be elevated in SAH or in other conditions, such as intracranial venous thrombosis or pseudotumour cerebri, or low in spontaneous intracranial hypotension.
Xanthochromia, the yellow discoloration of CSF caused by the haemoglobin breakdown products oxyhaemoglobin and bilirubin due to lysis of red blood cells, is generally agreed to be the primary criterion for diagnosis of SAH and differentiates between SAH and traumatic tap. It is usually present within 6 hours of SAH and has been demonstrated in all patients with SAH between 12 hours and 2 weeks following the haemorrhage. Xanthochromia is not reliably detected by visual examination of centrifuged CSF. Spectrophotometric analysis of CSF for bilirubin is considered the most sensitive means of detecting xanthochromia.
Controversy exists as to the optimal timing of lumbar puncture. Early lumbar puncture within 12 hours may have negative or equivocal CSF findings, whereas delayed lumbar puncture may result in an increased risk of early re-bleeding as well as having practical implications for the ED. In general, at least 6–12 hours should have elapsed between the onset of headache and lumbar puncture. Although detection of xanthochromia is indicative of SAH, it does not entirely rule out traumatic lumbar puncture and can occur in extremely bloody taps (>12 000 RBC/mL) or where the lumbar puncture has been repeated after an initial traumatic tap.
Other studies of the CSF, such as three tube cell counts, D-dimer assay and detection of erythrophages, have been found to be inconsistent in differentiating SAH from traumatic tap.
General investigations
General investigations to be performed include full blood examination, erythrocyte sedimentation rate, urea, electrolytes including magnesium, blood glucose, coagulation screen, chest X-ray and 12-lead ECG. ECG changes are frequently present and include ST and T-wave changes which may mimic ischaemia, QRS and QT prolongation and arrhythmias. Cardiac biomarkers, including troponin, may also be elevated.
Complications
Early complications
Late complications
Treatment
The management of SAH requires general supportive measures, particularly airway protection and blood pressure control, as well as specific management of the ruptured aneurysm and the complications of aneurysmal haemorrhage.
General measures
 Close observation of Glasgow coma scale (GCS) and vital signs.
Close observation of Glasgow coma scale (GCS) and vital signs.
 In all patients, maintain oxygenation and circulation ensuring adequate (euvolaemic) blood volume.
In all patients, maintain oxygenation and circulation ensuring adequate (euvolaemic) blood volume.
 Effective glucose control, importantly avoiding hyper- and hypoglycaemia.
Effective glucose control, importantly avoiding hyper- and hypoglycaemia.
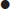 Treatment of hydrocephalus by ventricular drainage may be required.
Treatment of hydrocephalus by ventricular drainage may be required.
Specific treatment
Prevention of re-bleeding
Obliteration of the ruptured aneurysm by endovascular coiling or surgical clipping should be performed as early as possible to prevent re-bleeding, remove clot, reduce the incidence of early complications and improve outcomes.
Endovascular occlusion, achieved by placing detachable coils in aneurysms under radiological guidance (coiling), has largely replaced surgical occlusion as the method of choice for prevention of re-bleeding in suitable cases. The method of treatment, however, depends on anatomical considerations, as aneurysms are not equally amenable to this option. In aneurysms that are suitable to treatment by either modality, 4-year outcome has been demonstrated to be better with coiling, although there are higher aneurysm recurrence and re-bleeding rates.
Surgical clipping is now a second-line option for most patients. It is usually done early – within 3 days, and preferably within 24 hours.
Antifibrinolytic agents, including ε-aminocaproic acid, which inhibit clot lysis, reduce the incidence of re-bleeding after initial aneurysmal rupture. Short-term therapy (<72 hours) may be indicated in patients without medical contraindications who have an unavoidable delay in obliteration of the aneurysm and a significant risk of re-bleeding. Although not affecting clinical outcome, their use has been associated with an increase in deep venous thrombosis but not pulmonary embolism.
Prevention of delayed cerebral ischaemia
Cerebral ischaemia is often gradual in onset and involves the territory of more than one cerebral artery. Peak frequency is at 5–14 days after SAH. Nimodipine, a calcium channel antagonist, improves clinical outcome in SAH, with a relative risk reduction of 18% and an absolute risk reduction of 5.1%. The current standard regimen is nimodipine 60 mg orally every 4 hours for 3 weeks. It should be commenced within 48 hours of haemorrhage.
Other treatments including magnesium sulphate, the statins and antiplatelet agents have not been demonstrated to improve clinical outcomes.
There are no definitive treatments for delayed cerebral ischaemia, although improving cerebral perfusion by maintenance of euvolaemia and induced hypertension is recommended where blood pressure and cardiac status permit. In vasospasm unresponsive to medical management, emergency cerebral angiography with intra-arterial vasodilator infusion or transluminal balloon angioplasty may be considered where focal vessel narrowing is demonstrated.
Prognosis
SAH has a 40–60% mortality rate from the initial haemorrhage, with up to one-third of survivors having a significant neurological deficit. The most important prognostic factor is the clinical condition at the time of presentation, with coma and major neurological deficits generally being associated with a poor prognosis. Survival rates have been reported at 70% for grade I, 60% for grade II, 50% for grade III, 40% for grade IV and 10% for grade V SAH.
It is worth noting, however, that survival without brain damage is possible even after respiratory arrest. Even patients who make a good recovery may suffer cognitive and psychosocial dysfunction.
Aneurysm screening in patients who have survived aneurysmal SAH is advocated, as these patients are at increased risk of new or recurrent aneurysmal bleeds.
Incidental unruptured aneurysms
If an unruptured aneurysm is found incidentally, it raises the dilemma of the risk–benefit rationale between intervention and conservative management. Factors taken into account include age, aneurysm size and location, gender, country, co-morbidity and family history. Such patients should be referred to a neurosurgical service for advice and counselling.
Conclusion
Clinical suspicion of the diagnosis of SAH gained from a history of sudden, severe or atypical headache demands a full investigation, including brain CT scan and, if necessary, lumbar puncture. Once SAH has been diagnosed, urgent neurosurgical referral and management are required.
8.4 Altered conscious state
Ruth Hew
Introduction
Consciousness is variously termed lucidness, orientation, awakeness and mentation in the context of patients in the emergency department (ED). None of these terms is a comprehensive definition of consciousness. Neither does the Glasgow coma scale (GCS) (Table 8.4.1), commonly used but developed for head-injured patients in a time when computed tomography (CT) scanning was not yet available, truly measure what we as clinicians mean by conscious state. Ultimately, consciousness is an amalgam of alertness, orientation and clarity of cognition or mentation. Of note, isolated absence or derangement of cognition and mentation (e.g. caused by dementia or psychiatric illness or both) does not result in the clinical entity of altered conscious state. Both these clinical presentations are considered elsewhere. In the same way, the lack of orientation in and of itself does not constitute a clinical alteration in conscious state.
Table 8.4.1
The GCS is scored between 3 and 15, 3 being the worst and 15 the best. It is composed of three parameters: best eye response, best verbal response, best motor response, as given below
Best eye response (score out of 4)
1 – No eye opening
2 – Eye opening to pain
3 – Eye opening to verbal command
4 – Eyes open spontaneously
Best verbal response (score out of 5)
1 – No verbal response
2 – Incomprehensible sounds
3 – Inappropriate words
4 – Confused
5 – Orientated
Best motor response (score out of 6)
1 – No motor response
2 – Extension to pain
3 – Flexion to pain
4 – Withdrawal from pain
5 – Localizing pain
6 – Obeys commands
In the clinical context, an alteration in conscious state requires a drop in alertness and ‘awakeness’. This drop in alertness may result in a corresponding loss of orientation and/or a clouding of cognition, thereby altering a patient’s response to environmental stimuli or provocation. The reverse, an increase in alertness, could also be considered an alteration in conscious state but, in practice, is most often due to pharmacological agents or a mood elevating psychiatric illness and is beyond the scope of this discussion.
Pathophysiology
The level of consciousness describes the rousability of the individual, whereas the content of consciousness may be assessed in terms of the appropriateness of the individual’s response. Broadly speaking, the first is a brainstem function and the second is an attribute of the forebrain.
The physical portions of the brain involved in consciousness consist of the ascending arousal system that begins with monoaminergic cell groups in the brainstem and culminates in extensive diffuse cortical projections throughout the cerebrum. En route there is input and modulation from both thalamic and hypothalamic nuclei, as well as basal forebrain cell groups.
The integration of the brainstem and the forebrain is illustrated by individuals who have an isolated pontine injury. They remain awake, but the intact forebrain is unable to interact with the external world, hence the aptly named ‘locked-in syndrome’. At the other end of the spectrum are individuals in a persistent vegetative state who, in spite of extensive forebrain impairment, appear awake but totally lack the content of consciousness. These clinical extremes emphasize the important role of the brainstem in modulating motor and sensory systems through its descending pathways and regulating the wakefulness of the forebrain through its ascending pathways.
Differential diagnosis
Given the pathophysiology, the causes of an altered conscious state are myriad and include any cause of insult or injury either directly or indirectly to the brain (Table 8.4.2). Direct injury resulting in structural insults could be traumatic or non-traumatic, e.g. subdural haemorrhage, stroke. Indirect injury could encompass any change in the metabolic and chemical milieu of the brain resulting in a depression of neuronal function, e.g. sepsis, hyper- or hypoglycaemia, drug ingestion.
Table 8.4.2
Causes of Alteration in Conscious State
STRUCTURAL INSULTS
Supratentorial
Haematoma
epidural
subdural
Cerebral tumour
Cerebral aneurysm
Haemorrhagic CVA
Infratentorial
Cerebellar AVM
Pontine haemorrhage
Brainstem tumour
METABOLIC INSULTS
Loss of substrate
Hypoxia
Hypoglycaemia
Global ischaemia
Shock
hypovolaemia
cardiogenic
Focal ischaemia
TIA/CVA
vasculitis
DERANGEMENT OF NORMAL PHYSIOLOGY
Hypo- or hypernatraemia
Hyperglycaemia/hyperosmolarity
Hypercalcaemia
Hypermagnesaemia
Addisonian crisis
Seizures
status epilepticus
post-ictal
post-concussive
Hypo- or hyperthyroidism
Co-factor deficiency
Metastatic malignancy
Psychiatric illness
Dementia
TOXINS
Drugs
alcohol
illicit
prescription
Endotoxins
subarachnoid blood
liver failure
renal failure
Sepsis
systemic
Focal
meningitis
encephalitis
Environmental
hypothermia/heat exhaustion
altitude illness/decompression
envenomations
Structural insults, commonly focal intracranial lesions that exert direct or indirect pressure on the brainstem and the more caudal portions of the ascending arousal system, tend to produce lateralizing neurological signs that can assist in pinpointing the level of the lesion. As there is little space in and around the brainstem, any extrinsic or intrinsic compression will rapidly progress through coma to death, unless the pressure on the brainstem is relieved surgically or pharmacologically.
Metabolic insults, commonly due to systemic pathology that affects primarily the forebrain (although direct depression of the brainstem may also occur), seldom result in lateralizing signs. The appropriate treatment is the correction of the underlying metabolic impairment. Naturally, as in all clinical practice, there are no absolute distinctions. Uncorrected, any of the metabolic causes can eventually cause cerebral oedema and herniation, leading thence to brainstem compression with lateralizing signs, coma and death.
Clinical assessment
In approaching a patient with an altered conscious state, the initial imperatives are to ensure the safety of the airway, the breathing and the circulation and to determine the cause for the alteration with a view to correcting the rapidly reversible causes and offering supportive care while working through the remainder. To this end, assessment and management must proceed concurrently. As in other time critical situations, the primary and secondary survey approach often proves useful, seeking to identify and correct the primary insult while preventing or minimizing secondary injury, such as hypoxia, acidosis and raised intracranial pressure.
Primary survey and resuscitation
An assessment of the airway, breathing and circulation of the patient is urgent to ensure that life-saving measures, such as airway and ventilation support, can be instituted. Initially, supplemental oxygen and non-invasive monitoring should be applied and attention paid to the absolute value and trends in GCS and vital signs. Normotension should be the goal of blood pressure monitoring and this may require inotropic or antihypertensive support.
Early endotracheal intubation may be required if the GCS is less than 8 or ventilatory effort is inadequate. Mechanical ventilation to maintain a pCO2 of 30–35 mmHg may assist in correcting underlying acidosis and reducing intracranial pressure; however, over-correction may itself be detrimental. Accurate end-tidal CO2 monitoring correlated to arterial pCO2 is required. In the setting of trauma, spinal precautions should be maintained until any possibility of trauma is excluded or until clearance of the spine can be obtained.
As hypoglycaemia is an easily identified and corrected cause of altered conscious state, an early bedside glucose determination is vital.
In certain patient populations, pinpoint pupils and a depressed respiratory response may suggest a diagnosis of opiate toxicity and the administration of naloxone as a diagnostic and therapeutic tool. Often this has already been attempted by the pre-hospital services where the practice of intranasal naloxone administration has significantly reduced the risk of needlestick injuries. Although the adverse reactions to naloxone in initial doses is small, the greater risk is in the unmasking of the proconvulsant or proarrhythmic effects of other drugs ingested or injected in combination with opioids. Thus, naloxone should not be given unless clinically indicated. There is also the potential of introducing diagnostic bias as a percentage of non-opioid users also appear to respond clinically to naloxone. The routine use of the ‘coma cocktail’ of 50% dextrose, naloxone and thiamine intravenously is no longer advocated.
Secondary survey
Following initial assessment and resuscitation, it is important to complete the assessment by obtaining a full history, conducting a full examination and performing any adjunctive investigations. This will assist in identifying the cause of the condition and planning further management.
History
This is often difficult with an obtunded or confused patient and may need to rely heavily on ancillary sources, such as first responders, carers, primary physicians, medical records and patient alert identification.
It is crucial to establish the events leading up to the presentation with specific questioning about prodromal events (e.g. ingestions, IV drug usage, trauma, underlying illness, medications, allergies) and associated seizures and abnormal movements. For example, the presence or absence of a headache and its onset and duration might aid in the clinical diagnosis of a subarachnoid haemorrhage and a history of head injury with loss of consciousness would increase the likelihood of an extra-axial intracranial collection. Patients who are taking anticoagulants also have an increased risk of intracranial haemorrhage with minimal trauma. Patients with a history of hepatic failure may require specific treatment for hepatic encephalopathy.
In the elderly, dementia, itself a progressive illness, may be exacerbated by delirium caused by an acute illness and often, only a careful, corroborated history from care providers and the passage of time will allow the two to be distinguished. Often, the most helpful portion of the history lies in ascertaining the patient’s usual pattern of behaviour and the degree and time course of any changes that have occurred. In these patients, it is important to remember that dementia as a cause of altered conscious state is a diagnosis of exclusion.
Examination
A general physical examination, bearing in mind the various differential diagnoses, is very important. In the absence of adequate history, a thorough physical examination may offer the only pointers to initial treatment. Vital signs may suggest sepsis or other causes of shock. A keen sense of smell might detect fetor hepaticus or the sweet breath of ketosis. A bitter almond scent is pathognomonic of cyanide poisoning. Of note, alteration of consciousness can be attributed to alcoholic intoxication only by the process of exclusion. Thus the characteristic odour of alcoholic liquor is indicative but cannot be presumed to be diagnostic. A bedside blood glucose determination is mandatory as deficits are easily correctable.
Neurological examination must be as comprehensive as possible. There are several obstacles to this. Initial resuscitation measures, such as endotracheal intubation, will reduce the ability of the patient to cooperate with the examination and language difficulties will be accentuated as the neurological examination is strongly language orientated. Thus patients who do not share a common language with clinicians and those with dysphasia may be disadvantaged. Also, sensory modalities are difficult to assess in patients with impaired mentation, although these deficits are often paralleled by deficits in the motor system.
The aim of the neurological examination is, primarily, to differentiate structural and non-structural causes; secondly, to identify groups of signs that may indicate specific diagnoses, such as meningitis; and, finally, to pinpoint the location of any structural lesion. Therefore, emphasis needs to be placed on signs of trauma, tone, reflexes, pupillary responses and eye signs, as well as serial calculation of GCS. Circumstances permitting, some or all of the neurological examination should be documented before the patient receives neuromuscular paralysing agents.
Signs of trauma need to be documented and spinal precautions taken as indicated. Palpation of the soft tissues and bones of the skull may detect deformity or bruising and a haemotympanum may herald a fracture of the base of the skull.
Hypotonia is common in acute neurological deficits. Specific examination of anal sphincter tone will uncover spinal cord compromise and is crucial in trauma patients who have a depressed level of consciousness. An upgoing Babinski response is indicative of pyramidal pathology and asymmetry of the peripheral limb reflexes may help to ‘side’ a lesion. Conversely, heightened tone in the neck muscles (neck stiffness) may indicate meningitis or subarachnoid haemorrhage.
Pupillary responses and eye signs may also be useful to differentiate metabolic and structural insults and, more importantly, to detect incipient uncal herniation. Intact oculocephalic reflexes and preservation of the ‘doll’s eyes’ response indicates an intact medial longitudinal fasciculus and, by default, an intact brainstem, suggesting a metabolic cause for coma. There are four pairs of nuclei governing ocular movements and they are spread between the superior and inferior midbrain and the pons. The pattern of ocular movement dysfunction can be used to pinpoint the site of a brainstem lesion (Table 8.4.3). Likewise, specific testing of the oculovestibular reflex and the cranial nerve examination can be used to locate precisely a brainstem lesion but is of limited use in the emergency setting except as a predictor of herniation (Tables 8.4.3 and 8.4.4).
Table 8.4.3
Ocular responses to cold caloric testing of the oculovestibular reflex
| Response | Cerebrum | Medial longitudinal fasciculus | Brainstem |
| Bilateral nystagmus | Intact | Intact | Intact |
| Bilateral conjugate deviation towards the stimulus | Metabolic dysfunction | Intact | Intact |
| No response | Structural or metabolic dysfunction | ||
| Ipsilateral dysconjugate deviation | Structural dysfunction |
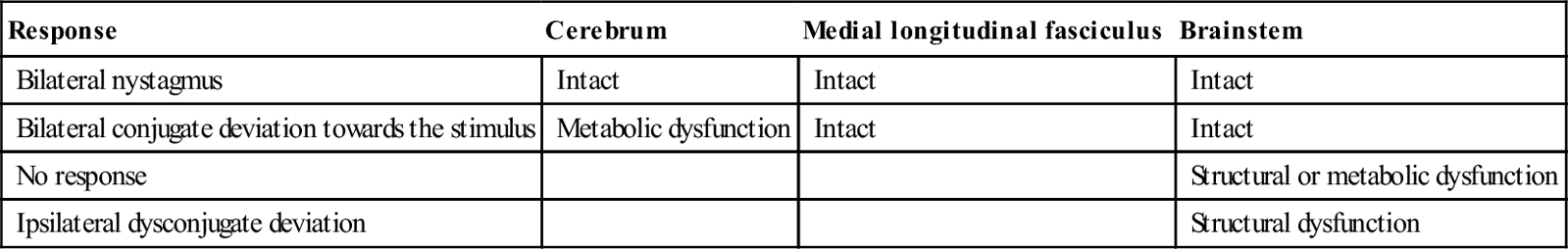
Table 8.4.4
More generally, skin examination may reveal needle tracks suggestive of drug use, envenomation bite marks or a meningococcal rash. Mucosal changes, such as cyanosis or the cherry-red glow of carbon monoxide poisoning, can be diagnostic. Cardiac monitoring and cardiovascular examination should identify rhythm disturbances, the murmurs of endocarditis and valvular disease or evidence of shock from myocardial ischaemia or infarction. Respiratory patterns may aid in identifying the site of the lesion (see Table 8.4.4). Abdominal examination may detect organomegaly, ascites, bruits or pulsatile masses.
Collections of physical signs, particularly into toxidromes, such as those due to anticholinergic or serotoninergic drugs, should be sought as these are not uncommon causes of alterations in conscious state with or without psychiatric symptoms. Further information on toxidromes can be found in the relevant chapters in this text.
Clinical investigations
Given the breadth of differential diagnoses, these must be guided by the preceding history and examination and their timing dictated by the resuscitation imperatives. The following is an extensive list of possible investigations but there is no suggestion that they should all be performed in every patient with an altered conscious state without due consideration of the patient’s context and condition. A comprehensive history and thorough examination are key to the appropriate choice of investigations.
Haematology
A full blood examination may reveal anaemia, immunocompromise, thrombocytopaenia, inflammation or infection, but is rarely diagnostically specific. In the setting of trauma, a bedside haemoglobin determination can direct immediate blood-product replacement. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are non-specific acute-phase reactants and single determinants are not initially useful, although they may later be followed to monitor resolution of the illness or response to therapy. Coagulation profiles are particularly useful in haematological and liver disease or if patients are taking anticoagulants, such as warfarin, and should lower the threshold for radiological imaging of the brain.
Biochemistry
Serum electrolyte levels aid in the differentiation of the various hypo- and hyper-elemental causes of coma. Electrolyte imbalances may also be secondary to the causative insult and may not need specific correction. In hypotensive patients, a high to normal sodium and low potassium suggests primary or secondary Addisonian crisis.
Cardiac markers, liver, renal and thyroid function tests may confirm focal organ dysfunction. The last may not always be readily available, but hypothyroidism should be considered in the hypothermic patient and hyperthyroidism in the presence of tremor and tachyarrhythmias.
Serum glucose provides confirmation of bedside testing. Serum lactate determinations may reveal a metabolic acidosis and reflect the degree of tissue hypoxia which, again, may be primary or secondary. Creatinine kinase and myoglobinuria are useful to determine the presence and extent of rhabdomyolysis and to predict the likelihood of requiring dialysis. Serum and urine osmolarity may be useful in toxic ingestions, such as ethylene glycol, and in other hyperosmolar states.
Blood gas analysis may give important information regarding acid–base balance, a useful marker of severity of disease and, along with the anion gap and the serum electrolytes, can help distinguish between the various types and causes of acidosis and alkalosis. Knowledge of the partial pressures of oxygen and carbon dioxide is vital to resuscitative efforts.
Microbiology
Sepsis is a major metabolic cause of conscious state alteration and may present with no localizing symptoms or signs, especially in the elderly. In this case, blood cultures – preferably multiple sets obtained before antibiotic therapy – may be the only means of isolating the causative organism. Naturally, system-specific specimens, such as sputum, urine and cerebrospinal fluid, should be collected when clinically indicated. A bedside dipstick of the urine sample can provide valuable information to begin initial treatment. Although as a rule specimens should be obtained prior to therapy, in suspected meningitis or encephalitis, the administration of antibiotics or antiviral agents should not be delayed while a lumbar puncture/CT scan is performed.
Specific laboratory testing
Based on information from the history and examination, specific drug assays and urine screens may be indicated. These may include over-the-counter or prescribed medications, such as paracetamol, lithium or theophylline, or drugs of addiction, such as amphetamine or opiates. Routine urine drug screens are of very limited overall value and no help to emergency management.
Venom detection kits can be used in specific clinical situations and evidence of systemic envenomation can be screened for with other tests, such as coagulation profiles and creatinine kinase.
Imaging
Intracranial imaging is best achieved with a plain CT of the head which, if normal and concern regarding intracranial pathology persists, may be followed by a contrast-enhanced scan or magnetic resonance imaging (MRI). The latter has a higher sensitivity for encephalitis and cerebral vasculitis, although it may not always be easily accessible from the ED. Also, the technical constraints of MRI require a stable patient. Emergency CT angiography has a role in the delineation of cerebral aneurysms and interventional angiography can provide therapeutic options, particularly in a patient who is progressing towards herniation. A normal CT scan does not completely exclude treatable intracranial infection or subarachnoid haemorrhage. Therefore, depending upon the patient’s conscious state and the level of clinical suspicion, a lumbar puncture may further assist with diagnosis. However, it must be emphasized that, in suspected intracranial infection, an obtunded patient should be treated empirically with appropriate antiviral agents and antibiotics and the lumbar puncture deferred till the risk of herniation is minimized.
A chest X-ray may reveal primary infection or malignancy. In a patient with an altered conscious state and any suspicion of head trauma, a full cervical spine series is mandatory. Inadequate plain films should be supplemented by CT imaging of the cervical spine, as allowed by resuscitation imperatives while spinal immobilization is maintained. Imaging of the rest of the spine and the pelvis should be guided by clinical assessment.
Other tests
The 12-lead ECG can highlight rate and rhythm disturbances. Specific changes, such as the U wave of hypokalaemia, the J wave of hypothermia and focal infarction and ischaemic patterns, serve to confirm and offer pointers to the cause of the conscious state alteration. It is worth noting that intracranial bleeding, such as subarachnoid haemorrhage, can be associated with an ischaemic-looking ECG. Care is required in cases of depressed level of consciousness with ECG changes, as the use of thrombolysis or anticoagulation based on the ECG in the presence of intracranial bleeding may well be fatal.