Arterial and Venous Sheath Removal
Arterial and Venous Sheath Removal
PREREQUISITE NURSING KNOWLEDGE
• Knowledge of the femoral artery and vein anatomy is important.
• The technique for the percutaneous approach to the insertion of the arterial and venous sheaths should be understood.
• Technical and clinical competence in removal of arterial and venous sheaths is needed.
• Knowledge about anticoagulation and antiplatelet therapy used during interventional procedures is essential.
• Understanding of the technology (i.e., activated clotting time [ACT] machine) used to determine the timing of arterial sheath removal and knowledge of the institution’s standards regarding removal of arterial sheaths are important.
• The importance of peripheral vascular and neurovascular assessment of the affected extremity (e.g., assessment of the quality and strength of the pulse to be accessed and the pulses distal to the access site, assessment for a bruit) should be understood.
• Knowledge about the variety of hemostasis options available should include the following:
 Manual compression alone or in combination with noninvasive hemostasis pads (e.g., Syvek Patch, Marine Polymer Technologies, Inc, Danvers, MA; Clo-Sur P.A.D., Scion Cardio-Vascular, Inc, Miami, FL; D-Stat Dry, Vascular Solutions, Minneapolis, MN).
Manual compression alone or in combination with noninvasive hemostasis pads (e.g., Syvek Patch, Marine Polymer Technologies, Inc, Danvers, MA; Clo-Sur P.A.D., Scion Cardio-Vascular, Inc, Miami, FL; D-Stat Dry, Vascular Solutions, Minneapolis, MN).
 Mechanical compression devices (e.g., CompressAR, Advanced Vascular Dynamics, Portland, OR; FemoStop, Radi Medical Systems, Wilmington, MA; Fig. 77-1).
Mechanical compression devices (e.g., CompressAR, Advanced Vascular Dynamics, Portland, OR; FemoStop, Radi Medical Systems, Wilmington, MA; Fig. 77-1).
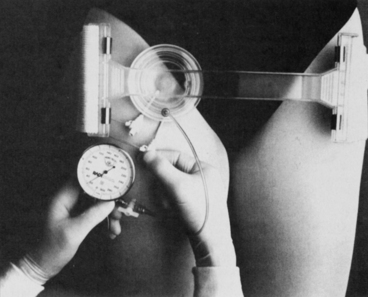
Figure 77-1 FemoStop in the correct position. (From Barbiere C: A new device for control of bleeding after transfemoral catheterization, Crit Care Nurse 15[1]:52, 1995.)
 Collagen plug devices (e.g., VasoSeal, Datascope Corp, Montvale, NJ; Angioseal, St Jude Medical, St Paul, MN).
Collagen plug devices (e.g., VasoSeal, Datascope Corp, Montvale, NJ; Angioseal, St Jude Medical, St Paul, MN).
 Percutaneous suture-mediated closure devices (e.g., Perclose, Abbott Vascular Devices, Redwood City, CA; X-Site, Datascope Corp)
Percutaneous suture-mediated closure devices (e.g., Perclose, Abbott Vascular Devices, Redwood City, CA; X-Site, Datascope Corp)
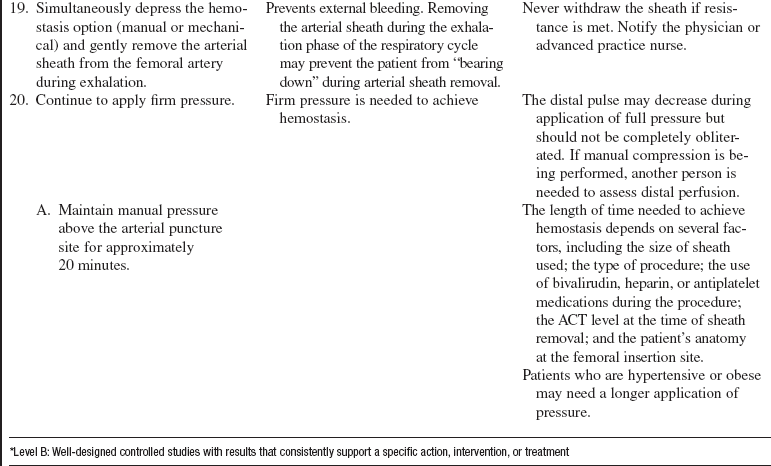
 Percutaneous staple-mediated closure devices (e.g., Angiolink, Medtronic, Santa Rosa, CA; Starclose, Abbott Vascular Devices).
Percutaneous staple-mediated closure devices (e.g., Angiolink, Medtronic, Santa Rosa, CA; Starclose, Abbott Vascular Devices).
• Collagen plug devices, percutaneous suture-mediated closure devices, and percutaneous staple-mediated closure devices can be deployed into the artery by the physician at the end of the catheterization or interventional procedure.
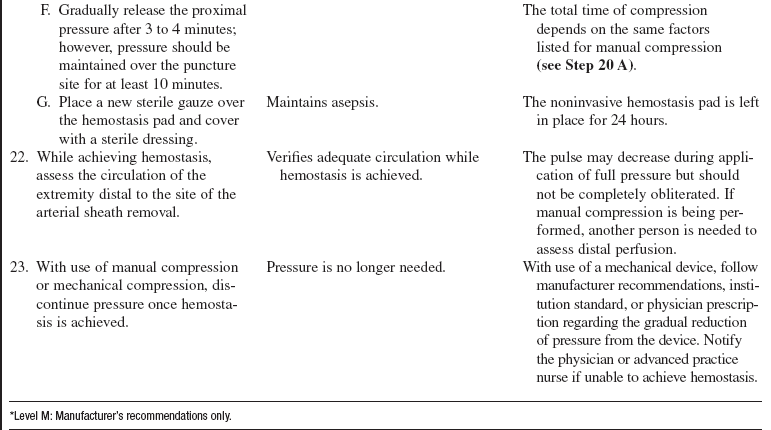
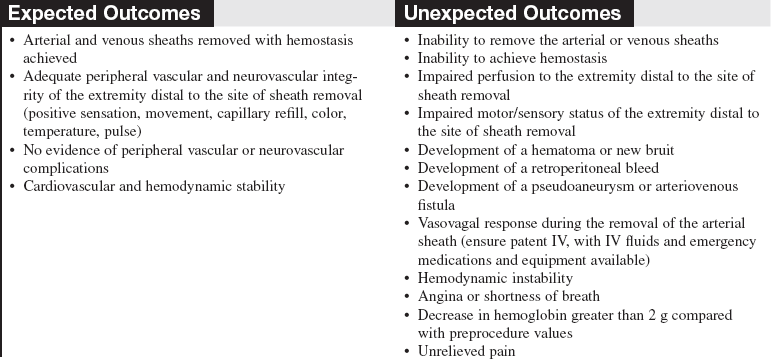
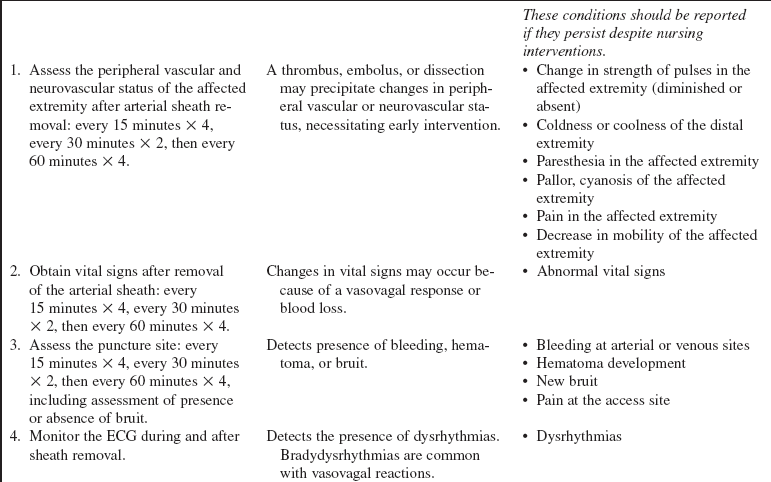
• Sheath removal can be associated with many complications, including the following:
EQUIPMENT
PATIENT AND FAMILY EDUCATION
• Explain the procedure to the patient and the family.  Rationale: This explanation provides information and may help decrease anxiety and fear. This also encourages the patient to ask questions and voice concerns about the procedure.
Rationale: This explanation provides information and may help decrease anxiety and fear. This also encourages the patient to ask questions and voice concerns about the procedure.
• Explain the importance of bed rest, of not lifting the head off the pillow, of maintaining the head of the bed at no higher than 30 degrees, and of keeping the affected extremity straight for a specified time to maintain hemostasis after the procedure.  Rationale: The patient is prepared for what to expect after the procedure, and patient cooperation is elicited to decrease the risk for bleeding, hematoma, and other vascular complications.
Rationale: The patient is prepared for what to expect after the procedure, and patient cooperation is elicited to decrease the risk for bleeding, hematoma, and other vascular complications.
• Explain that the procedure may produce discomfort and that pressure will be felt at the site until hemostasis is achieved. Encourage the patient to report discomfort, and reassure the patient that analgesia and or sedation will be provided.  Rationale: Explanation prepares the patient for what to expect and allays fears.
Rationale: Explanation prepares the patient for what to expect and allays fears.
• After sheath removal, instruct the patient to report any warm, wet feeling or pain at the puncture site. Also, instruct the patient to report any sensory or motor changes in the affected extremity.  Rationale: This aids in the early recognition of complications and identifies the need for additional pain interventions.
Rationale: This aids in the early recognition of complications and identifies the need for additional pain interventions.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess the patient’s medical history for bleeding disorders.  Rationale: Bleeding disorders may increase the risk for bleeding or vascular complications.
Rationale: Bleeding disorders may increase the risk for bleeding or vascular complications.
• Assess the patient’s platelet count, prothrombin time (PT), with international normalized ratio (INR), and partial thromboplastin time (PTT) before sheath removal.  Rationale: Laboratory results should be within acceptable limits to decrease the risk for bleeding after sheath removal.
Rationale: Laboratory results should be within acceptable limits to decrease the risk for bleeding after sheath removal.
• Assess the patient’s complete blood count (CBC).  Rationale: Assessment determines baseline data.
Rationale: Assessment determines baseline data.
• Assess the patient’s ACT before sheath removal.  Rationale: Results should be within acceptable limits to decrease the risk for bleeding after sheath removal.
Rationale: Results should be within acceptable limits to decrease the risk for bleeding after sheath removal.
• Assess the patient’s electrocardiographic (ECG) rhythm and vital signs.  Rationale: Baseline data are established. Collaborate with the advanced practice nurse or physician if the patient’s blood pressure is elevated; elevated blood pressure may need to be treated before sheath removal to achieve and maintain hemostasis.
Rationale: Baseline data are established. Collaborate with the advanced practice nurse or physician if the patient’s blood pressure is elevated; elevated blood pressure may need to be treated before sheath removal to achieve and maintain hemostasis.
• Review the documented baseline assessment of the access site before vascular access, including assessment for presence or absence of bruit.  Rationale: Baseline assessment data are established.
Rationale: Baseline assessment data are established.
• Assess the extremity distal to the sheath for quality and strength of pulses, color, temperature, sensation, and movement.  Rationale: Baseline assessment data are established before sheath removal.
Rationale: Baseline assessment data are established before sheath removal.
• Assess for patency of the intravenous (IV) access and ensure that more than 500 mL of intravenous fluid remains in the IV solution or is readily available.  Rationale: This assessment allows for emergency medication or fluids to be administered if necessary (e.g., vasovagal reaction).
Rationale: This assessment allows for emergency medication or fluids to be administered if necessary (e.g., vasovagal reaction).
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that the patient and the family understand preprocedural teaching. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Administer analgesia or sedation as prescribed before removal of the sheaths.  Rationale: Pain and anxiety are managed.
Rationale: Pain and anxiety are managed.
• Place the patient with the head of the bed flat.  Rationale: This positioning improves the ability to achieve hemostasis.
Rationale: This positioning improves the ability to achieve hemostasis.
• Mark the distal pulses with an indelible marker.  Rationale: Marking facilitates the ability to locate pulses after the procedure.
Rationale: Marking facilitates the ability to locate pulses after the procedure.
• If a mechanical device is used to maintain pressure, position the device under the patient.  Rationale: The device is positioned before sheath removal because patient movement must be minimized after sheath removal.
Rationale: The device is positioned before sheath removal because patient movement must be minimized after sheath removal.
References
1. Baim, DS, Simon, DI, Percutaneous approach including trans-septal and apical puncture Baim DS, ed.. Grossman’s cardiac catheterization, angiography, and intervention. ed 7. Lippincott Williams & Wilkins, Philadelphia, 2006:79–106.
![]() 2. Barbiere, CC, A new device for control of bleeding after transfemoral catheterization. the FemoStop system. Crit Care Nurse 1995; 15:51–53.
2. Barbiere, CC, A new device for control of bleeding after transfemoral catheterization. the FemoStop system. Crit Care Nurse 1995; 15:51–53.
![]() 3. Benson, LM, et al, Determining best practice. comparison of three methods of femoral sheath removal after cardiac interventional procedures. Heart Lung J Acute Crit Care 2004; 34:115–121.
3. Benson, LM, et al, Determining best practice. comparison of three methods of femoral sheath removal after cardiac interventional procedures. Heart Lung J Acute Crit Care 2004; 34:115–121.
![]() 4. Bogart, MA, Time to hemostasis. a comparison of manual versus mechanical compression of the femoral artery. Am J Crit Care 1995; 4:149–156.
4. Bogart, MA, Time to hemostasis. a comparison of manual versus mechanical compression of the femoral artery. Am J Crit Care 1995; 4:149–156.
![]() 5. Bowden, SM, Worrey, JA, Assessing patient comfort . Local infiltration of lidocaine during femoral sheath removal. Am J Crit Care 1995; 4:368–369.
5. Bowden, SM, Worrey, JA, Assessing patient comfort . Local infiltration of lidocaine during femoral sheath removal. Am J Crit Care 1995; 4:368–369.
![]() 6. Coyne, C, et al. Controlled trial of backrest elevation after coronary angiography. Am J Crit Care. 1994; 3:282–288.
6. Coyne, C, et al. Controlled trial of backrest elevation after coronary angiography. Am J Crit Care. 1994; 3:282–288.
![]() 7. Fowlow, B, Price, P, Fung, T, Ambulation after sheath removal. a comparison of 6 and 8 hours of bedrest after sheath removal in patients following a PTCA procedure. Heart Lung J Acute Crit Care 1995; 24:28–37.
7. Fowlow, B, Price, P, Fung, T, Ambulation after sheath removal. a comparison of 6 and 8 hours of bedrest after sheath removal in patients following a PTCA procedure. Heart Lung J Acute Crit Care 1995; 24:28–37.
![]() 8. Jones, T, McCutcheon, H. Effectiveness of mechanical compression devices in attaining hemostasis after femoral sheath removal. Am J Crit Care. 2002; 11:155–162.
8. Jones, T, McCutcheon, H. Effectiveness of mechanical compression devices in attaining hemostasis after femoral sheath removal. Am J Crit Care. 2002; 11:155–162.
![]() 9. Keeling, AW. Reducing time in bed after percutaneous transluminal coronary angioplasty (TIBS III). Am J Crit Care. 2000; 9:185–187.
9. Keeling, AW. Reducing time in bed after percutaneous transluminal coronary angioplasty (TIBS III). Am J Crit Care. 2000; 9:185–187.
![]() 10. Kiat Ang C, et al. Effect of local anesthesia and intravenous sedation on pain perception and vasovagal reactions during femoral arterial sheath removal after percutaneous coronary intervention. Int J Cardiol. 2005; 116:321–326.
10. Kiat Ang C, et al. Effect of local anesthesia and intravenous sedation on pain perception and vasovagal reactions during femoral arterial sheath removal after percutaneous coronary intervention. Int J Cardiol. 2005; 116:321–326.
![]() 11. Koreny, M, et al, Arterial puncture closing devices compared with manual compression after cardiac catheterization: . systematic review and meta-analysis. JAMA 2004; 291:350–357.
11. Koreny, M, et al, Arterial puncture closing devices compared with manual compression after cardiac catheterization: . systematic review and meta-analysis. JAMA 2004; 291:350–357.
![]() 12. Logemann, T, et al. Two versus six hours of bed rest following left-sided cardiac catheterization and a meta-analysis of early ambulation trials. Am J Cardiol. 1999; 84:486–488.
12. Logemann, T, et al. Two versus six hours of bed rest following left-sided cardiac catheterization and a meta-analysis of early ambulation trials. Am J Cardiol. 1999; 84:486–488.
![]() 13. Nikolsky, E, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures. J Am Coll Cardiol. 2004; 44:1200–1209.
13. Nikolsky, E, et al. Vascular complications associated with arteriotomy closure devices in patients undergoing percutaneous coronary procedures. J Am Coll Cardiol. 2004; 44:1200–1209.
![]() 14. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter- related infections, Centers for Disease Control and Prevention. MMWR Recommend Rep. 2002; 51(RR-10):1–29.
14. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter- related infections, Centers for Disease Control and Prevention. MMWR Recommend Rep. 2002; 51(RR-10):1–29.
![]() 15. Rudisill, PT, et al. Study of mechanical versus manual-mechanical compression following various interventional cardiology procedures. J Cardiovasc Nurs. 1997; 11:15–21.
15. Rudisill, PT, et al. Study of mechanical versus manual-mechanical compression following various interventional cardiology procedures. J Cardiovasc Nurs. 1997; 11:15–21.
![]() 16. Simon, A, et al. Manual versus mechanical compression for femoral artery hemostasis after cardiac catheterization. Am J Crit Care. 1998; 7:308–313.
16. Simon, A, et al. Manual versus mechanical compression for femoral artery hemostasis after cardiac catheterization. Am J Crit Care. 1998; 7:308–313.
![]() 17. Sulzbach, LM, Munro, BH, Hirshfeld, JW. A randomized clinical trial of the effect of bed position after PTCA. Am J Crit Care. 1995; 4:221–226.
17. Sulzbach, LM, Munro, BH, Hirshfeld, JW. A randomized clinical trial of the effect of bed position after PTCA. Am J Crit Care. 1995; 4:221–226.
![]() 18. Tagney, J, Lackie, D, Bed-rest post-femoral arterial sheath removal. what is safe practice? A clinical audit. Nurs Crit Care 2005; 10:167–173.
18. Tagney, J, Lackie, D, Bed-rest post-femoral arterial sheath removal. what is safe practice? A clinical audit. Nurs Crit Care 2005; 10:167–173.
![]() 19. Vlasic, W, Almond, D, Massel, D, Reducing bedrest following arterial puncture for coronary interventional procedures-impact on vascular complications. the BAC Trial. J Invasive Cardiol 2001; 13:788–792.
19. Vlasic, W, Almond, D, Massel, D, Reducing bedrest following arterial puncture for coronary interventional procedures-impact on vascular complications. the BAC Trial. J Invasive Cardiol 2001; 13:788–792.
![]() 20. Wadas, TM, Hill, J. Is lidocaine infiltration during femoral sheath removal really necessary. Heart Lung J Acute Crit Care. 1998; 27:31–36.
20. Wadas, TM, Hill, J. Is lidocaine infiltration during femoral sheath removal really necessary. Heart Lung J Acute Crit Care. 1998; 27:31–36.
21. Walker, S, et al. Comparison of complications in percutaneous coronary intervention patients mobilized at 3, 4 and 6 hours after femoral arterial sheath removal. J Cardiovasc Nurs. 2008; 23:407–413.
![]() 22. Walker, SB, Cleary, SR, Higgins, M. Comparison of the FemoStop device and manual pressure in reducing groin puncture site complications following coronary angioplasty and coronary stent placement. Int J Nurs Pract. 2001; 7:366–375.
22. Walker, SB, Cleary, SR, Higgins, M. Comparison of the FemoStop device and manual pressure in reducing groin puncture site complications following coronary angioplasty and coronary stent placement. Int J Nurs Pract. 2001; 7:366–375.
23. Wensley, CJ, et al, Pain relief for the removal of femoral sheath in interventional cardiology patients Art. No. :CD006043. Cochrane Database Syst Rev 2008; 4, doi: 10. 1002/14651858. CD006043. pub2.
![]() Chlan, LL, Sabo, J, Savik, K. Effects of three groin compression methods on patient discomfort, distress, and vascular complications following a precutaneous coronary intervention procedure. Nurs Res. 2005; 54:391–398.
Chlan, LL, Sabo, J, Savik, K. Effects of three groin compression methods on patient discomfort, distress, and vascular complications following a precutaneous coronary intervention procedure. Nurs Res. 2005; 54:391–398.
![]() Christensen, BV, et al. Vascular complications after angiography with and without the use of sandbags. Nurs Res. 1998; 47:51–53.
Christensen, BV, et al. Vascular complications after angiography with and without the use of sandbags. Nurs Res. 1998; 47:51–53.
![]() Cura, FA, et al. Safety of femoral closure devices after percutaneous coronary interventions in the era of glycoprotein IIb/IIIa platelet blockade. Am J Cardiol. 2000; 86:780–782.
Cura, FA, et al. Safety of femoral closure devices after percutaneous coronary interventions in the era of glycoprotein IIb/IIIa platelet blockade. Am J Cardiol. 2000; 86:780–782.
Dressler, DK, Dressler, KK, Caring for patients with femoral sheaths. after percutaneous coronary intervention, sheath removal and site monitoring are the nurse’s responsibility. AJN 2006; 106:64.
Dueling, JHH, et al, Closure of the femoral artery after cardiac catheterization. a comparison of Angio-Seal, StarClose, and manual compression. Cathet Cardiovasc Interv 2008; 71:518–523.
Dumont, CJP, et al. Predictors of vascular complications post diagnostic cardiac catheterization and percutaneous coronary intervention. Dimens Crit Care Nurs. 2006; 25:137–142.
![]() Galli, A, Palatnik, A, Ask the experts. what is the proper activated clotting time (ACT) at which to remove a femoral sheath after PCI? What are the best “protocols” for sheath removal. Crit Care Nurse 2005; 25:88–95.
Galli, A, Palatnik, A, Ask the experts. what is the proper activated clotting time (ACT) at which to remove a femoral sheath after PCI? What are the best “protocols” for sheath removal. Crit Care Nurse 2005; 25:88–95.
Juergens, CP, et al. Vaso-vagal reactions during femoral arterial sheath removal after percutaneous coronary intervention and impact on cardiac events. Int J Cardiol. 2007; 127:252–254.
Kim, M. Vascular closure devices. Cardiol Clin. 2006; 24:277–286.
![]() Nickolaus, MJ, Gilchrist, IC, Ettinger, SM, The way to the heart is all in the wrist. transradial catheterization and interventions. AACN Clin Issues 2001; 12:62–71.
Nickolaus, MJ, Gilchrist, IC, Ettinger, SM, The way to the heart is all in the wrist. transradial catheterization and interventions. AACN Clin Issues 2001; 12:62–71.
Sabo, J, Chlan, LL, Savik, K. Relationships among patient characteristics, comorbidities, and vascular complications post-percutaneous coronary intervention. Heart Lung J Acute Crit Care. 2008; 37:190–195.
![]() Schickel, S, et al, Removal of femoral sheaths by registered nurses. issues and outcomes. Crit Care Nurse 1996; 16:32–36.
Schickel, S, et al, Removal of femoral sheaths by registered nurses. issues and outcomes. Crit Care Nurse 1996; 16:32–36.
![]() Smith, TT, Labriola, R. Developing best practice in arterial sheath removal for registered nurses. J Nurs Care Qual. 2001; 16:61–67.
Smith, TT, Labriola, R. Developing best practice in arterial sheath removal for registered nurses. J Nurs Care Qual. 2001; 16:61–67.