CHAPTER 73 Neuropsychiatric Dysfunction
PRINCIPLES OF DIFFERENTIAL DIAGNOSIS
Emotional and cognitive processes are based on brain structure and physiology. Abnormal behavior can be attributable to the complex interplay of social influences, physical environment, and neural physiology. Psychosis, mania, depression, disinhibition, obsessive-compulsive disorder (OCD), and anxiety can all occur as a result of neurological disease and be indistinguishable from the idiopathic forms.1,2 Neurological conditions must be considered in the differential diagnosis of any person with psychiatric symptoms.
Neuropsychiatric dysfunction can be correlated with altered function in anatomical regions. Any disease, toxin, drug, or process that affects a particular region can be expected to show changes in behavior that are mediated by the circuits within that region. The limbic system and the frontal-subcortical circuits are the ones most commonly involved in neuropsychiatric dysfunction. Understanding this neuroanatomical conceptual framework can guide lesion localization and creation of a differential diagnosis. For example, the Kluver-Bucy syndrome3 (manifest by placidity, apathy, visual and auditory agnosia, hyperorality, and hypersexuality) involves injury to bilateral medial temporal-amygdalar regions. Common causes of this syndrome include herpes encephalitis,4 traumatic brain injury, frontotemporal dementias, and late-onset or severe Alzheimer’s disease. Brain trauma, ischemic disease, demyelination, abscesses, and tumors, as well as degenerative dementias, can also result in behavioral disinhibition. Damage to any portion of the circuit (between the orbital frontal cortex, ventral caudate, anterior globus pallidus, or medial dorsal thalamus) can result in disinhibition.5–7
Mood disorders, paranoia, disinhibition, and apathy derive from dysfunction in the limbic system and basal ganglia, structures that are phylogenetically more primitive.8 Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) studies suggest that similar regions of abnormality are involved in acquired forms of depression, mania, OCD, and psychosis, as compared with primary psychiatric presentations.9–14 Table 73-1 summarizes neuropsychiatric symptoms and their anatomical correlates. Additionally, the developmental phase during which a neurological illness occurs has an impact on the frequency with which some neuropsychiatric syndromes occur. In addition, adults with posttraumatic brain injury tend to have a higher rate of depression and anxiety. In contrast, difficulties secondary to posttraumatic brain injury in children often involve deficits in attention, hyperactivity, irritability, aggressivity, and oppositional behavior.15 When temporal lobe epilepsy or Huntington’s disease begins in adolescence, a higher incidence of psychosis is noted than when these conditions develop later in life.2,16 Early onset of multiple sclerosis and stroke is also associated with a higher incidence of depression.17
Table 73-1 Neuropsychiatric Symptoms and Corresponding Neuroanatomy
| Depression | Frontal lobes, left anterior frontal cortex, anterior cingulate gyrus, subgenu of the corpus callosum, basal ganglia, and left caudate |
| Mania | Inferomedial and ventromedial frontal cortex, right inferomedial frontal cortex, anterior cingulate, caudate nucleus, thalamus, and temporal-thalamic projections |
| Apathy | Anterior cingulate gyrus, nucleus accumbens, globus pallidus, and thalamus |
| Obsessive-compulsive disorder | Orbital or medial frontal cortex, caudate nucleus, and globus pallidus |
| Disinhibition | Orbitofrontal cortex, hypothalamus, and septum |
| Paraphilia | Medial temporal cortex, hypothalamus, septum, and rostral brainstem |
| Hallucinations | Unimodal association cortex, orbitofrontal cortex, paralimbic cortex, limbic cortex, striatum, thalamus, and midbrain |
| Delusions | Orbitofrontal cortex, amygdala, striatum, and thalamus |
| Psychosis | Frontal lobes and left temporal cortex |
Patients with Alzheimer’s disease, Huntington’s disease, and frontotemporal dementias can develop multiple co-existing symptoms (e.g., irritability, agitation, apathy, depression, delusions, or psychosis) (Table 73-2). Once present, these symptoms tend to persist or recur, but little research data are available on response rates to specific treatments. The complex relationship between behavioral changes and a caregiver’s ability to cope also plays a role in illness management and in nursing home placement.18,19 Behavioral disturbances in patients with neurological illness have been related to the severity of caregiver distress.20
Table 73-2 Neurological Disorders and Associated Prominent Behavioral Features
| Neurological Disorder | Associated Behavioral Disturbances |
|---|---|
| Alzheimer’s disease | Depression, irritability, anxiety, apathy, delusions, paranoia, and psychosis |
| Lewy body dementia | Fluctuating confusion, hallucinations, delusions, depression, and rapid eye movement (REM) sleep behavior disorder (RBD) |
| Vascular dementia | Depression, apathy, and psychosis |
| Parkinson’s disease | Depression, anxiety, drug-associated hallucinations and psychosis, and RBD |
| Frontotemporal dementia | Early impaired judgment, disinhibition, apathy, depression, delusions, and psychosis |
| Progressive supranuclear palsy | Disinhibition and apathy |
| Traumatic brain injury | Depression, disinhibition, apathy, and irritability; psychosis uncommon |
| Huntington’s disease | Depression, irritability, delusions, mania, apathy, obsessive-compulsive disorder, and psychosis |
| Corticobasalganglionic degeneration | Depression, irritability, RBD, and alien-hand syndrome |
| Epilepsy | Depression and psychosis |
| Human immunodeficiency virus infection | Apathy, depression, mania, and psychosis |
| Multiple sclerosis | Depression, irritability, anxiety, euphoria, and psychosis |
| Amyotrophic lateral sclerosis | Depression, disinhibition, apathy, and impaired judgment |
PRINCIPLES OF NEUROPSYCHIATRIC EVALUATION
A number of important principles must be taken into account when evaluating and treating a patient with behavioral disturbances (Table 73-3).
The medical evaluation of affective illness and psychotic disorders must be individualized based on the patient’s family history, social environment, habits, risk factors, age, gender, clinical history, and examination findings. A careful review of the patient’s medical history and a general physical examination, as well as a neurological examination, should be performed to assess for possible neurological and medical causes. The most basic evaluation should include vital signs (blood pressure, pulse, respiratory rate, and temperature) and a laboratory evaluation that minimally includes a complete blood cell count, electrolyte panel, serum glucose, blood urea nitrogen, creatinine, calcium, serum total protein, albumin, liver function assessment, thyroid function assessment, and additional laboratory testing as clinically indicated. Consideration should be given to checking the patient’s oxygen saturation on room air (especially in the elderly) . Neurological abnormalities suggested by the clinical history or identified on examination, especially those attributable to the central nervous system (CNS), should prompt further evaluation for neurological and medical causes of psychiatric illness. A clear consensus is not available as to when neuroimaging is indicated as a part of the evaluation of new-onset depression in patients without focal neurological complaints and a normal neurological examination. This must be individualized based on clinical judgment. Treatment-resistant depression should prompt reassessment of the diagnosis and evaluation to rule out secondary causes of depressive illness. A careful history to rule out a primary sleep disorder, such as sleep apnea, should be considered in the evaluation of refractory depressive symptoms.23 When new-onset psychosis occurs in the absence of identifiable infectious/inflammatory, metabolic, toxic, or other causes, magnetic resonance imaging (MRI) of the brain should be incorporated into the evaluation. Approximately 5% to 10% of such patients have findings on brain MRI that identify potential neurological contributions (particularly in those age 65 and older). The MRI will help exclude lesions (such as demyelination, ischemic disease, neoplasm, congenital structural abnormalities, or evidence of metabolic storage diseases) in limbic, paralimbic, and frontal regions that may not be associated with neurological abnormalities on elemental examination.24 An electroencephalogram (EEG) should be considered to evaluate for complex partial seizures, if there is a history of intermittent, discreet, or abrupt episodes of psychiatric dysfunction (usually confusion, spells of lost time, or psychotic symptoms), stereotypy of hallucinations, automatisms (e.g., lip smacking or repetitive movements) associated with episodes of psychiatric dysfunction (or confusion), or a suspicion of encephalopathy (or delirium). Sensitivity of the EEG for detection of seizure activity is highest when the patient has experienced the specific symptoms while undergoing the study. Selected cases may require 24-hour or longer EEG monitoring to capture a clinical event to clarify whether a seizure disorder is present.
COGNITIVE-BEHAVIORAL NEUROANATOMY
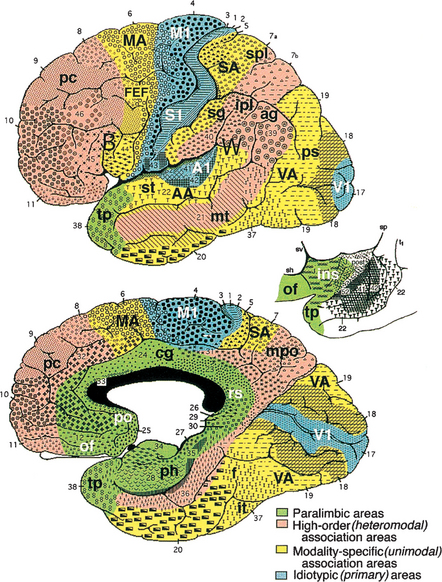
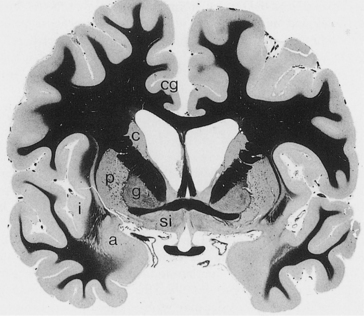
The cerebral cortex can be subdivided into five major functional subtypes: primary sensory-motor, unimodal association, heteromodal association, paralimbic, and limbic. The primary sensory areas are the point of entry for sensory information into the cortical circuitry. The primary motor cortex conveys complex motor programs to motor neurons in the brainstem and the spinal cord. Processing of sensory information occurs as information moves from primary sensory areas to adjacent unimodal association areas (Figure 73-1). The unimodal and heteromodal cortices are involved in perceptual processing and motor planning. The complexity of processing increases as information is then transmitted to heteromodal association areas that receive input from more than one sensory modality. Examples of heteromodal association cortices include the prefrontal cortex, the posterior parietal cortex, parts of the lateral temporal cortex, and portions of the parahippocampal gyrus. These cortical regions have a six-layered architecture. Further cortical processing occurs in areas designated as paralimbic. These regions demonstrate a gradual transition of cortical architecture from the six layered to the more primitive and simplified allocortex of limbic structures. The paralimbic regions consist of orbitofrontal cortex, insula, temporal pole, parahippocampal cortex, and cingulate cortex. Cognitive, emotional, and visceral inputs merge in this region. The limbic subdivision is composed of the hippocampus, amygdala, substantia innominata, prepiriform olfactory cortex, and septal area (Figure 73-2). These structures are to a great extent reciprocally interconnected with the hypothalamus. The limbic region is intimately involved with regulation of emotion, memory, and motivation, as well as autonomic and endocrine function. The highest level of cognitive processing occurs in regions referred to as transmodal areas. These areas are composed of heteromodal, paralimbic, and limbic regions that are collectively linked, in parallel, to other transmodal regions. Interconnections amongst transmodal areas (such as Wernicke’s area, the posterior parietal cortex, and the hippocampal-enterorhinal complex) allow integration of distributed perceptual processing systems that results in perceptual recognition (such as scenes and events becoming experiences and words taking on meaning).8
Cortical Networks
Five distinct cortical network regions govern various aspects of cognitive function: the language network, which includes transmodal regions or “epicenters” in Broca’s and Wernicke’s areas; the spatial awareness network, which is based on transmodal regions in the frontal eye fields and posterior parietal area; the memory and emotion network, which is located in the hippocampal-enterorhinal region and amygdala; the executive function–working memory network, which is based on transmodal regions in the lateral prefrontal cortex and possibly the inferior parietal cortices; and the face-object recognition network, which is based on temporopolar and midtemporal cortices.25
Frontal-Subcortical Networks
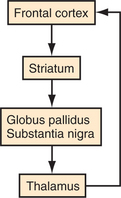
Five frontal-subcortical circuits subserve cognition, behavior, and movement. Disruption of these networks at the cortical or subcortical level can be associated with similar neuropsychiatric symptoms. Each of these circuits shares the same components (Figure 73-3): frontal cortex; striatum (caudate, putamen, ventral striatum); globus pallidus and substantia nigra; and thalamus (which then projects back to frontal cortex).6,7 There are also integrative connections to and from other subcortical and distant cortical regions related to each circuit. Neurotransmitters (e.g., dopamine [DA], glutamate, gamma-aminobutyric acid [GABA], acetylcholine, norepinephrine, and serotonin) are involved in various aspects of neural transmission and modulation through the circuits. The frontal-subcortical networks are named according to their site of origin or function. Somatic motor function is subserved by the motor circuit that originates in the supplementary motor area. Oculomotor function is governed by the oculomotor circuit that originates in the frontal eye fields. Three of the five circuits are most intimately involved in cognitive and behavioral changes: the dorsolateral prefrontal, the orbitofrontal, and the anterior cingulate circuits. The dorsolateral prefrontal circuit governs executive function, including the ability to plan and maintain attention, problem solve, learn, retrieve remote memories, sequence the temporal order of events, shift cognitive and behavioral sets, and generate motor program.26 Executive dysfunction is a principle component of subcortical dementias. Deficits identified in subcortical dementias include slowed information processing, memory retrieval deficits, mood and behavioral changes, gait disturbance, dysarthria, and other motor impairments. Vascular dementias, Parkinson’s disease, and Huntington’s disease are a few of the conditions that affect this circuit.
The anterior cingulate circuit is involved in motivated behavior. Lesions in this circuit result in apathy, abulia, and akinetic mutism. There also is a reported decrease in the ability to understand new thoughts and to participate in the creative thought process.8,27 Some conditions that may affect this circuit include Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, head trauma, brain tumors, cerebral infarcts, and obstructive hydrocephalus.
BRAIN DISORDERS, ANATOMY, AND THEIR ASSOCIATION WITH PSYCHOSIS
A wide variety of neurological conditions, medications, and toxic exposures can be associated with psychosis. No consensus is available in the literature regarding precise localization of various psychotic syndromes. Schizophrenia has been associated with frontal lobe dysfunction and with abnormal regulation of subcortical DA28 and glutamate systems.29 Functional imaging studies in patients with schizophrenia show decreased cerebral blood flow (CBF) in the dorsolateral prefrontal cortex during specific cognitive tasks.30,31 Patients with schizophrenia (who have prominent negative symptoms) display reduced glucose utilization in the frontal lobes.32 Functional imaging studies suggest that disruption in distributed functional circuits (e.g., related to the dorsolateral prefrontal cortex, the orbitofrontal cortex, the medial frontal cortex, the anterior cingulate gyrus, the thalamus, the temporal lobe subregions, and the cerebellum)33 is important in the development of schizophrenia. Several conditions (such as Huntington’s disease, Parkinson’s disease, frontotemporal degenerations, and stroke) are commonly associated with frontal and subcortical dysfunction. Psychosis can also be associated with these conditions. Dorsolateral and medial frontal hypoperfusion on functional imaging has also been demonstrated in a subset of Alzheimer’s disease patients with delusions.11
BRAIN DISORDERS, ANATOMY, AND THEIR ASSOCIATION WITH DEPRESSION
Studies suggest that anterior temporal paralimbic and orbitofrontal regions are involved in the mediation of primary and acquired depression. Functional imaging studies of unmedicated patients with familial depression reveal increased CBF and glucose metabolism in the amygdala, orbital cortex, and medial thalamus, and decreased CBF and glucose metabolism in the dorsomedial/dorsal anterolateral prefrontal cortex and the anterior cingulate cortex.34 Damage to the prefrontal cortex from stroke, tumor, or striatum from degenerative diseases (such as Parkinson’s and Huntington’s), is associated with depression.34,35 Functional imaging studies of subcortical disorders, such as these, reveal hypometabolism in paralimbic regions (e.g., the anterior temporal cortex and anterior cingulate) that are correlated with the depression seen in these patients.36,37 Depression in patients with Parkinson’s disease, Huntington’s disease, and epilepsy has been correlated with reduced metabolic activity in the orbitofrontal cortex and the caudate nucleus.38,39
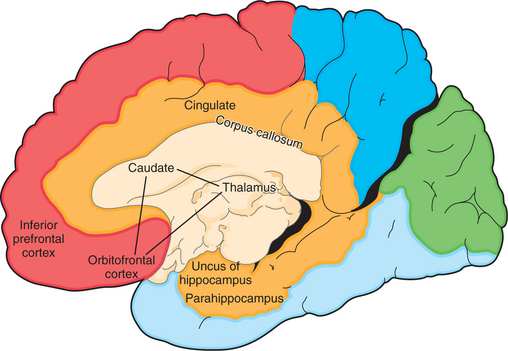
Mayberg36 proposed that primary depression was due to dysfunction in a network that included two known pathways: the orbitofrontal–basal ganglia–thalamic circuit and the basotemporal limbic circuit that links the orbitofrontal cortex and the anterior temporal cortex by the uncinate fasciculus. Portions of this are illustrated in Figure 73-4.
CLINICAL SYMPTOMS AND SIGNS SUGGESTING NEUROLOGICAL DISEASE
Many neurological conditions have associated psychiatric symptoms. Psychiatrists and neurologists need to be intimately acquainted with features of the clinical history and the examination that indicate the need for further investigation. Table 73-4 outlines some key historical features that raise the suspicion of an underlying neurological condition. Table 73-5 reviews some key areas of the review of systems that can be helpful when assessing for neurological and medical causes of psychiatric symptoms. Table 72-1 reviews abnormalities in the elemental neurological examination associated with diseases that can exhibit significant neuropsychiatric features.
Table 73-4 Historical Features Suggesting Neurological Disease in Patients with Psychiatric Symptoms
Table 73-5 Review of Systems with Possible Neuropsychiatric Relevance and Related Neurological Conditions
PSYCHIATRIC MANIFESTATIONS OF NEUROLOGICAL DISEASE
Virtually any process that affects the neuroanatomical circuits described previously can result in behavioral changes and psychiatric symptoms at some point in the natural history of a condition. Psychiatric symptoms may be striking and precede (by years) any neurological manifestation. Table 73-6 lists conditions that can be associated with psychosis or depression. More detailed information regarding the evaluation, natural history, pathology, and specific treatment recommendations for these conditions is beyond the scope of this chapter.
Table 73-6 Selected Neurological and Systemic Causes of Depression and Psychosis
| Category | Disorders |
|---|---|
| Head trauma | Traumatic brain injury |
| Subdural hematoma | |
| Infectious | Lyme disease |
| Prion diseases | |
| Viral infections/encephalitides (HIV infection/encephalopathy, herpes encephalitis, CMV, EBV, etc.) | |
| Whipple’s disease | |
| Cerebral malaria | |
| Encephalitis | |
| Systemic infection | |
| Inflammatory | Lupus |
| Sjögren’s syndrome | |
| Temporal arteritis | |
| Hashimoto’s encephalopathy | |
| Sydenham’s chorea | |
| Sarcoidosis | |
| Neoplastic | Primary or secondary cerebral neoplasm |
| Systemic neoplasm | |
| Pancreatic cancer | |
| Paraneoplastic encephalitis | |
| Endocrine/acquired metabolic | Hepatic encephalopathy |
| Uremia | |
| Dialysis dementia | |
| Hypoparathyroidism/hyperparathyroidism | |
| Hypothyroidism/hyperthyroidism | |
| Addison’s disease/Cushing’s disease | |
| Postpartum | |
| Vitamin deficiency: vitamin B12, folate, niacin, vitamin C | |
| Gastric bypass–associated nutritional deficiencies | |
| Hypoglycemia | |
| Vascular | Stroke |
| Multiinfarct dementia | |
| Central nervous system vasculitis | |
| Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) | |
| Degenerative | Alzheimer’s disease |
| Lewy body disease | |
| Frontotemporal dementia | |
| Parkinson’s disease | |
| Progressive supranuclear palsy | |
| Huntington’s disease | |
| Corticobasalganglionic degeneration | |
| Multisystem atrophy/striatonigral degeneration/olivopontocerebellar atrophy | |
| Idiopathic basal ganglia calcifications/ | |
| Fahr’s disease | |
| Demyelinating/dysmyelinating | Multiple sclerosis |
| Acute disseminated encephalomyelitis | |
| Adrenoleukodystrophy | |
| Metachromatic leukodystrophy | |
| Inherited metabolic | Wilson’s disease |
| Tay-Sachs disease | |
| Adult neuronal ceroid lipofuscinosis | |
| Niemann-Pick type C | |
| Acute intermittent porphyria | |
| Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes | |
| Epilepsy | Ictal |
| Interictal | |
| Postictal | |
| Forced normalization | |
| Postepilepsy surgery | |
| Medications | Analgesics |
| Antiarrhythmics | |
| Anticonvulsants | |
| Anticholinergics | |
| Antibiotics | |
| Antineoplastic agents | |
| Dopamine agonists | |
| Antihypertensives | |
| Sedatives/hypnotics | |
| Steroids | |
| Corticosteroids | |
| Androgens | |
| Oral contraceptives | |
| Drugs of abuse | Amphetamines |
| Cocaine | |
| Marijuana | |
| Alcohol | |
| Hallucinogens | |
| MDMA (Ecstasy) | |
| Phencyclidine (PCP) | |
| Dextromethorphan | |
| Drug withdrawal syndromes | Alcohol |
| Barbiturates | |
| Benzodiazepines | |
| Amphetamines | |
| Toxins | Heavy metals |
| Inhalants | |
| Other | Normal pressure hydrocephalus |
| Ionizing radiation exposure | |
| Decompression sickness |
CMV, Cytomegalovirus; EBV, Epstein-Barr virus; HIV, human immunodeficiency virus; MDMA, 3,4-methylenedioxymethamphetamine.
TREATMENT PRINCIPLES
Each condition has subtleties associated with its prognosis, treatment, and management that should be carefully re-viewed before proceeding with a treatment plan. In general, patients with an underlying neurological condition tend to be more susceptible to the adverse reactions of psychotropic medications, particularly extrapyramidal and cognitive side effects. These adverse reactions tend to be minimized with initiation of medications at low doses and gentle titration of doses. When clinically indicated, atypical neuroleptics are often preferred (due to fewer adverse side effects) over conventional antipsychotics. Electroconvulsive therapy (ECT) can also be useful as an option for treatment of refractory mood disorders or in circumstances wherein medications cannot be tolerated. Individual and family supportive psychotherapy is often helpful. Other options to consider for treatment of refractory depression (or other behavioral disorders such as OCD) include vagal nerve stimulation,40–43 transcranial magnetic stimulation,44 deep brain stimulation,45 and stereotactic ablative surgery.46–49
CONCLUSIONS
With the advancements in neuroscience, and our new understanding of the substrates of cognition and emotional behavior, the traditional boundaries between neurology and psychiatry have become obsolete. The future of psychiatric and neurological care, training, and research will inevitably require effective collaboration between both disciplines.50,51
1 Robinson RG, Travella JI. Neuropsychiatry of mood disorders. In: Fogel BS, Schiffer RB, Rao SM, editors. Neuropsychiatry. Philadelphia: Williams & Wilkins, 1996.
2 Trimble MR. The psychosis of epilepsy. New York: Raven Press, 1991.
3 Kluver H, Bucy PC. Preliminary analysis of function of the temporal lobes in monkeys. Arch Neurol Psychiatr. 1939;42:979-1000.
4 Lilly R, Cummings JL, Benson DF, Frankel M. The human Kluver-Bucy syndrome. Neurology. 1983;33(9):1141-1145.
5 Mendez MF, Adams NL. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349-354.
6 Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873-880.
7 Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53(2):647-654.
8 Mesulam MM. Behavioral neuroanatomy. Large scale networks, association cortex, frontal syndromes, the limbic system and hemisphere specializations. In: Mesulam MM, editor. Principles of behavioral and cognitive neurology. New York: Oxford University Press, 2000.
9 Saxena S, Brody AL, Schwartz JM, et al. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry. 1998;173(suppl 35):26-37.
10 Baxter LRJr. Positron emission tomography studies of cerebral glucose metabolism in obsessive compulsive disorder. J Clin Psychiatry. 1994;55(suppl):54-59.
11 Hirono N, Mori E, Ishii K, et al. Alteration of regional cerebral glucose utilization with delusions in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1998;10(4):433-439.
12 Baxter LRJr, Schwartz JM, Phelps ME, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46(3):243-250.
13 Schwartz JM, Baxter LRJr, Mazziotta JC, et al. The differential diagnosis of depression. Relevance of positron emission tomography studies of cerebral glucose metabolism to the bipolar-unipolar dichotomy. JAMA. 1987;258(10):1368-1374.
14 Rubinsztein JS, Fletcher PC, Rogers RD, et al. Decision-making in mania: a PET study. Brain. 2001;124(pt 12):2550-2563.
15 Geraldina P, Mariarosaria L, Annarita A, et al. Neuropsychiatric sequelae in TBI: a comparison across different age groups. Brain Inj. 2003;17(10):835-884.
16 Morris M. Psychiatric aspects of Huntington’s disease. In: Harper PS, editor. Huntington’s disease. Philadelphia: WB Saunders, 1991.
17 Rickards H. Depression in neurological disorders: Parkinson’s disease, multiple sclerosis, and stroke. J Neurol Neurosurg Psychiatry. 2005;76(suppl 1):i48-i52.
18 Smith GE, O’Brien PC, Ivnik RJ, et al. Prospective analysis of risk factors for nursing home placement of dementia patients. Neurology. 2001;57(8):1467-1473.
19 de Vugt ME, Stevens F, Aalten P, et al. A prospective study of the effects of behavioral symptoms on the institutionalization of patients with dementia. Int Psychogeriatr. 2005;17(4):577-589.
20 Kaufer DI, Cummings JL, Christine D, et al. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46(2):210-215.
21 Aarsland D, Perry R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson’s disease and parkinsonian dementias. J Clin Psychiatry. 2005;66(5):633-637.
22 Papapetropoulos S, Mash DC. Psychotic symptoms in Parkinson’s disease. From description to etiology. J Neurol. 2005;252(7):753-764.
23 Haba-Rubio J. Psychiatric aspects of organic sleep disorders. Dialogues Clin Neurosci. 2005;7(4):335-346.
24 Walterfang M, Wood SJ, Velakoulis D, et al. Diseases of white matter and schizophrenia-like psychosis. Aust N Z J Psychiatry. 2005;39(9):746-756.
25 Mesulam MM. From sensation to cognition. Brain. 1998;121(pt 6):1013-1052.
26 Duffy JD, Campbell JJ. The regional prefrontal syndromes: theoretical and clinical overview. J Neuropsychiatry Clin Neurosci. 1994;6:379-387.
27 Chow TW, Cummings JL. Frontal-subcortical circuits. In: Miller B, Cummings JL, editors. The human frontal lobes. New York: Guilford Publications, 1999.
28 Goldman-Rakic PS, Castner SA, Svensson TH, et al. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl). 2004;174(1):3-16.
29 Weinberger DR. Genetic mechanisms of psychosis: in vivo and postmortem genomics. Clin Ther. 2005;27(suppl A):S8-S15.
30 Andreasen NC. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science. 1996;275:1586-1593.
31 Lehrer DS, Christian BT, Mantil J, et al. Thalamic and prefrontal FDG uptake in never medicated patients with schizophrenia. Am J Psychiatry. 2005;162(5):931-938.
32 Tamminga CA, Thaker GK, Buchanan R, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49(7):522-530.
33 Schultz SK, Andreasen NC. Schizophrenia. Lancet. 1999;353(9162):1425-1430.
34 Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004(225):re5.
35 Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11(2):240-249.
36 Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193-207.
37 Ketter TA, George MS, Kimbrell TA, et al. Functional brain imaging, limbic function, and affective disorders. Neuroscientist. 1996;2:55-65.
38 Mayberg HS. Frontal lobe dysfunction in secondary depression. J Neuropsychiatry Clin Neurosci. 1994;6(4):428-442.
39 Mayberg HS, Starkstein SE, Sadzot B, et al. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Ann Neurol. 1990;28(1):57-64.
40 Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev. 2005;29(3):493-500.
41 Elger G, Hoppe C, Falkai P, et al. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Res. 2000;42:203-210.
42 Rush AJ, George MS, Sackeim HA, et al. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatr. 2000;47:276-286.
43 Marangell LB, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for major depressive episodes: one year outcomes. Biol Psychiatr. 2002;51:280-287.
44 Rosa MA, Gattaz WF, Pascual-Leone A, et al. Comparison of repetitive transcranial magnetic stimulation and electroconvulsive therapy in unipolar non-psychotic refractory depression: a randomized, single-blind study. Int J Neuropsychopharmacol. 2006;9(6):667-676.
45 Skidmore FM, Rodriguez RL, Fernandez HH, et al. Lessons learned in deep brain stimulation for movement and neuropsychiatric disorders. CNS Spectr. 2006;11(7):521-536.
46 Dougherty DD, Weiss AP, Cosgrove GR, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99(6):1010-1017.
47 Montoya A, Weiss AP, Price BH, et al. Magnetic resonance imaging–guided stereotactic limbic leukotomy for treatment of intractable psychiatric disease. Neurosurgery. 2002;50(5):1043-1049. 1049-1052 (discussion)
48 Dougherty DD, Baer L, Cosgrove GR, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159(2):269-275.
49 Price BH, Baral I, Cosgrove GR, et al. Improvement in severe self-mutilation following limbic leucotomy: a series of 5 consecutive cases. J Clin Psychiatry. 2001;62(12):925-932.
50 Price BH, Adams RD, Coyle JT. Neurology and psychiatry: closing the great divide. Neurology. 2000;54(1):8-14.
51 Cunningham MG, Goldstein M, Katz D, et al. Coalescence of psychiatry, neurology, and neuropsychology: from theory to practice. Harvard Rev Psychiatry. 2006;14(3):127-140.