Digestive Emergencies
Edited by Anne-Maree Kelly
7.1 Dysphagia
Graeme Thomson
Introduction
Dysphagia is a broad term encompassing the many forms of difficulty with deglutition (swallowing). The main issues are to determine the likely cause, to identify those patients at risk of significant complications, to treat those causes that are amenable to acute intervention and to refer appropriately for further investigations and treatment.
Dysphagia may be associated with odynophagia (pain on swallowing). Globus is a related term that means the sensation of a lump in the throat. This is rarely of psychological origin. Since the advent of sophisticated investigative techniques, it has been recognized that, in the great majority of cases, there is an identifiable physical cause.
Aetiology
Problems may occur with any of the three stages of swallowing: oral, pharyngeal or oesophageal. Oral and pharyngeal causes may be grouped as transfer dysphagia and oesophageal problems may be referred to as transport dysphagia. Passage of food may be obstructed by a physical barrier, such as a tumour, or a disorder of muscle coordination, such as a neurological deficit.
In addition to diseases, several drugs are recognized as inducing dysphagia. They include tetracyclines, non-steroidal anti-inflammatory drugs, ascorbic acid, quinidine, ferrous sulphate and potassium chloride.
Clinical features
Symptoms may appear suddenly or develop insidiously. If insidious, there may be an acute precipitating event leading to presentation, often complete or partial obstruction owing to the impaction of a food bolus in the oesophagus. This may present as pain, a feeling of a lump in the neck or central chest, severe retching or drooling and an inability to swallow saliva. Patients may report increasing difficulty swallowing solids and then fluids but, in some cases, there may be no previous history of dysphagia.
Where a neurological disorder is causing difficulty initiating swallowing, there may be other neurological deficits. The voice may have changed. Regurgitation of food from the mouth or nose, coughing or frank aspiration may be evident when the patient eats. It should be assumed that patients with recent cerebrovascular events or bulbar dysfunction have dysphagia until formal assessment of swallowing and airway protection can be undertaken.
Examination should focus on testing cranial nerve function plus careful examination of the mouth, neck, chest and abdomen. Hydration status and nutritional status should be evaluated.
Perforation may be suspected if there is a history of ingestion of a corrosive substance or sharp object or if pain is a prominent feature. There may be evidence of surgical emphysema in the neck. If presentation is delayed there may be signs of sepsis.
Clinical investigations
Investigations are directed by the history and likely aetiology. For oropharyngeal and upper oesophageal lesions, a lateral X-ray of the soft tissues of the neck may reveal a lesion impinging on the oesophagus. An impacted dense bone or other solid foreign body may also be seen. For suspected mid- and lower-oesophageal lesions, frontal and lateral chest radiographs may reveal a fluid level, a mediastinal tumour, tuberculous lesions or an aneurysm of the thoracic aorta. Oesophageal perforation may also be detected. If food bolus obstruction is suspected a Gastrografin swallow may reveal the site and degree of obstruction.
Computed tomography (CT) scanning and endoscopy may also be indicated but, in most cases, they can be deferred and performed on a semi-elective basis. A video-fluorographic swallowing study is the best semi-elective investigation. It may reveal structural abnormalities as well as disorders of muscular coordination. Manometry is less reliable.
Laboratory investigations are guided by likely aetiology and complications, but should include basic biochemistry and full blood examination looking for electrolyte disturbances and anaemia.
Treatment
Definitive treatment depends on the underlying cause and will rarely be completed in the emergency department (ED). The degree of oesophageal obstruction, the acuity of onset and the presence of complications dictate the need for emergency treatment. Patients with high-grade obstruction should have oral fluids and food withheld and should be given intravenous fluids if the obstruction persists for more than a few hours.
For food bolus obstruction, intravenous glucagon may relax the oesophageal muscles enough to allow a bolus to pass through. This is less likely to be successful if the bolus is a piece of meat. An initial dose of 1 mg may be followed by a 2 mg dose if necessary. Complications are rare, but include allergy, nausea and hypotension. Phaeochromocytoma is a contraindication to the use of glucagon. Sublingual glyceryl trinitrate may be used as an alternative to glucagon, but hypotension is more likely. After glucagon, a gas-producing substance may be given in an attempt to dilate the oesophagus. Aerated drinks are adequate for this purpose. This technique should be used with great caution because a patient with upper oesophageal obstruction will be at greater risk of aspiration if given a foaming substance. This approach should be avoided if there is any suspicion of perforation. Endoscopic removal will be required in many cases, but this is usually attempted after a period of expectant treatment.
Bones or similar foreign bodies impacted in the pharynx can often be removed in the ED. Topical anaesthetic sprays may suppress the pharyngeal reflexes adequately to allow direct or indirect laryngoscopy and removal with forceps. Removal may immediately relieve the dysphagia, but symptoms due to local oedema or abrasions may persist.
Oesophageal or pharyngeal perforation is a serious complication requiring cover with broad-spectrum antibiotics and urgent surgical referral.
Odynophagia may be relieved by parenteral or topical analgesia. Oral administration of a viscous preparation of lignocaine will ease the pain caused by luminal inflammatory disorders. The maximum recommended dose is 300 mg and should be reduced in the elderly, who may be more affected by systemic absorption.
If a patient with known chronic dysphagia presents to the emergency department for an unrelated reason, care should be taken to avoid giving food or fluids that may be aspirated. Water is associated with a high aspiration risk.
Disposition
Appropriate disposition depends on the likely aetiology and the presence of complications. Admission is indicated for patients at risk of airway compromise, severe haemorrhage, sepsis or those with high-grade oesophageal obstruction. It will also be indicated when dysphagia is part of a broader disease process.
If a food bolus has passed spontaneously, the patient should be referred for semi-elective endoscopy.
7.2 Approach to abdominal pain
Kim Chai Chan and Eillyne Seow
Introduction
The assessment of patients with abdominal pain is challenging because:
 the degree of pain may not be commensurate with the severity of the disease
the degree of pain may not be commensurate with the severity of the disease
 the absence of abnormal vital signs cannot rule out a serious underlying condition
the absence of abnormal vital signs cannot rule out a serious underlying condition
 a large number of potential differential diagnoses may need to be considered.
a large number of potential differential diagnoses may need to be considered.
The emergency department (ED) approach to acute abdominal pain emphasizes disposition over diagnosis. It is more important to recognize an acute abdomen than to identify the exact cause of the pain.
Epidemiology, pathophysiology and differential diagnosis
It has been estimated that abdominal pain accounts for approximately 5–10% of all ED visits. A significant proportion (18–42%) of these patients will require admission. The elderly (aged 60 and over) are over-represented in the admitted patient group. In one study of elderly patients presenting with abdominal pain, at least 50% were hospitalized and about 30–40% eventually required surgery. Up to 40% of patients were initially misdiagnosed and the overall mortality was about 10%.
Abdominal pain may result from:
 Visceral pain: this pain is poorly localized and may be colicky, intermittent and recurrent in nature. Stimulation of nociceptors investing the visceral peritoneum causes visceral pain. For example, when hollow organs are distended or when capsules covering solid organs are stretched. Visceral pain localizes to the abdominal region that correlates with the embryonic segments of the viscera:
Visceral pain: this pain is poorly localized and may be colicky, intermittent and recurrent in nature. Stimulation of nociceptors investing the visceral peritoneum causes visceral pain. For example, when hollow organs are distended or when capsules covering solid organs are stretched. Visceral pain localizes to the abdominal region that correlates with the embryonic segments of the viscera:
 foregut structures (stomach, duodenum, liver, biliary tract, pancreas) localize to the upper abdomen
foregut structures (stomach, duodenum, liver, biliary tract, pancreas) localize to the upper abdomen
 midgut structures (small bowel, proximal colon, appendix) localize to the periumbilical region and
midgut structures (small bowel, proximal colon, appendix) localize to the periumbilical region and
 hindgut structures (distal colon, genitourinary tract) localize to the lower abdomen.
hindgut structures (distal colon, genitourinary tract) localize to the lower abdomen.
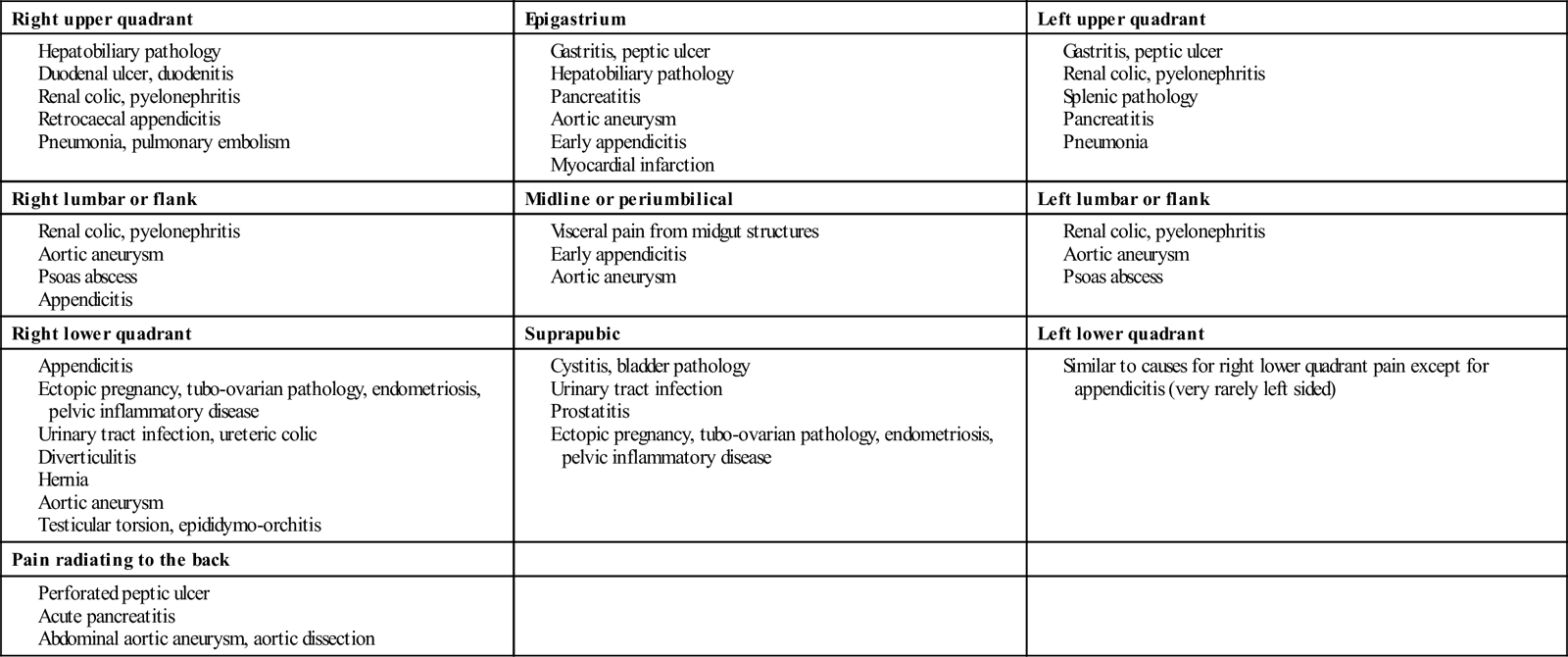
 Somatic pain: this pain is well localized and is often constant and intense. Somatic pain results from local irritation of the parietal peritoneum. It is localized more specifically to the area of pathology. Differential diagnosis of pain by location is shown in Table 7.2.1. It is, however, important to recognize that the area of pain does not always correspond to the supposed anatomical location of the underlying pathology, e.g. acute appendicitis may present as suprapubic or flank pain.
Somatic pain: this pain is well localized and is often constant and intense. Somatic pain results from local irritation of the parietal peritoneum. It is localized more specifically to the area of pathology. Differential diagnosis of pain by location is shown in Table 7.2.1. It is, however, important to recognize that the area of pain does not always correspond to the supposed anatomical location of the underlying pathology, e.g. acute appendicitis may present as suprapubic or flank pain.
Both visceral and somatic pain may manifest as referred pain. Some examples are:
 shoulder pain due to diaphragmatic irritation
shoulder pain due to diaphragmatic irritation
Table 7.2.1
Differential diagnosis of pain by location (list is not exhaustive)
| Right upper quadrant | Epigastrium | Left upper quadrant |
| Right lumbar or flank | Midline or periumbilical | Left lumbar or flank |
| Right lower quadrant | Suprapubic | Left lower quadrant |
| Pain radiating to the back | ||
Causes of diffuse abdominal pain
Generalized diffuse pain that is poorly localized may be due to benign causes (e.g. gastroenteritis, constipation and menstrual cramps) or from life-threatening conditions (Table 7.2.2).
Table 7.2.2
Some potentially life-threatening causes of generalized, diffuse abdominal pain
Haemoperitoneum from any cause, e.g. ruptured abdominal aortic aneurysm, ruptured ectopic pregnancy, trauma
Mesenteric ischaemia
Perforated viscus
Peritonitis (any cause)
Pancreatitis
Bowel obstruction
Diverticulitis
Inflammatory bowel disease
Metabolic disorders (e.g. diabetic ketoacidosis), sickle cell crisis, typhoid fever
Adapted from Gray-Eurom K, Deitte L. Imaging in the adult patient with non-traumatic abdominal pain. Emerg Med Pract 2007;9:2 with permission.
Extra-abdominal causes of abdominal pain
There are a number of extra-abdominal causes for abdominal pain that must be considered along with abdominal causes (Table 7.2.3).
Table 7.2.3
Extra-abdominal causes of abdominal pain
Thoracic
Myocardial infarction/unstable angina
Pneumonia
Pulmonary embolism
Herniated thoracic disc (neuralgia)
Genitourinary
Testicular torsion
Systemic
Diabetic ketoacidosis
Alcoholic ketoacidosis
Uraemia
Sickle cell disease
Systemic lupus erythematosus
Vasculitis
Hyperthyroidism
Porphyria
Glaucoma
Toxic
Methanol poisoning
Heavy metal poisoning
Scorpion bite
Black widow spider bite
Abdominal wall
Muscle spasm
Muscle haematoma
Herpes zoster
Infections
Strep pharyngitis (more often in children)
Mononucleosis
Adapted from Purcell TB. Nonsurgical and extraperitoneal causes of abdominal pain. Emerg Med Clin N Am 1989;7:721 with permission.
Clinical features
Vital signs and general condition
During triage, a rapid assessment is made by looking at the patient’s general condition as well as vital signs. Obviously ill patients, those in severe pain or with abnormal vital signs should be given priority. However, it is not possible to rule out life-threatening causes of abdominal pain by the absence of abnormal vital signs. It has been estimated that up to 7% of patients with normal vital signs may have an underlying life-threatening process and this percentage increases in the elderly. Tachycardia may be absent in patients with autonomic dysfunction, in the elderly and in patients on medications that may blunt the cardiac response to illness or volume loss. Similarly, the elderly, the immunocompromised or those in severe septic shock may sometimes not mount a febrile response. Even in the immunocompetent, fever may not always accompany acute inflammatory conditions.
History
An accurate, focused history often provides the best clue to the possible aetiology of abdominal pain. Clinical impression derived from the history will direct decisions regarding further diagnostic work-up.
Patient demographics and background history
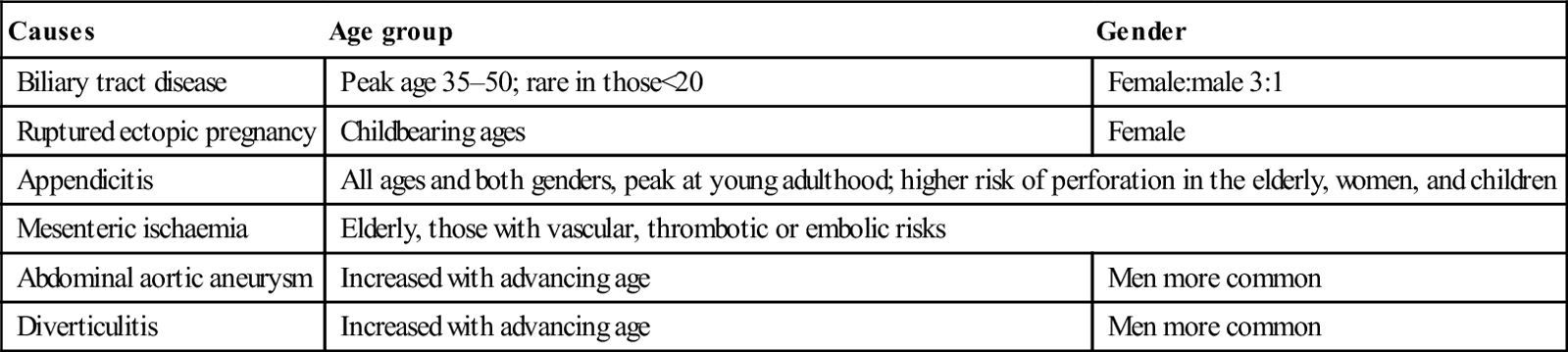
 Age and gender: the likelihood of certain conditions is higher in patients of a specific age and gender (Table 7.2.4). For women of childbearing age, it is important to ascertain the presence or absence of pregnancy.
Age and gender: the likelihood of certain conditions is higher in patients of a specific age and gender (Table 7.2.4). For women of childbearing age, it is important to ascertain the presence or absence of pregnancy.
 Background history: key questions in the background history are:
Background history: key questions in the background history are:
Table 7.2.4
Common causes of abdominal pain according to age group and gender
| Causes | Age group | Gender |
| Biliary tract disease | Peak age 35–50; rare in those<20 | Female:male 3:1 |
| Ruptured ectopic pregnancy | Childbearing ages | Female |
| Appendicitis | All ages and both genders, peak at young adulthood; higher risk of perforation in the elderly, women, and children | |
| Mesenteric ischaemia | Elderly, those with vascular, thrombotic or embolic risks | |
| Abdominal aortic aneurysm | Increased with advancing age | Men more common |
| Diverticulitis | Increased with advancing age | Men more common |
Pain attributes
The nature and time course of pain are key clues to diagnosis. The following attributes should be noted:
 Onset and progress of abdominal pain over time (Table 7.2.5): acute vascular events and rupture of hollow viscus typically presents with maximal pain at the onset. Ureteric and biliary colic also often presents with severe pain in the early stages. This is in contrast to pain from inflammatory processes, such as acute appendicitis, which tends to progress and ‘mature’ over hours.
Onset and progress of abdominal pain over time (Table 7.2.5): acute vascular events and rupture of hollow viscus typically presents with maximal pain at the onset. Ureteric and biliary colic also often presents with severe pain in the early stages. This is in contrast to pain from inflammatory processes, such as acute appendicitis, which tends to progress and ‘mature’ over hours.
 Location of pain (see Table 7.2.1), migration of pain, radiation of pain: location of pain helps to identify the area of pathology, although occasionally this may be misleading, especially if the pain is referred. Migration of pain over time gives a clue to possible underlying aetiology, e.g. pain from appendicitis typically starts at the umbilicus or epigastrium and later localizes to the right iliac fossa.
Location of pain (see Table 7.2.1), migration of pain, radiation of pain: location of pain helps to identify the area of pathology, although occasionally this may be misleading, especially if the pain is referred. Migration of pain over time gives a clue to possible underlying aetiology, e.g. pain from appendicitis typically starts at the umbilicus or epigastrium and later localizes to the right iliac fossa.
 Radiation of pain may suggest specific conditions (see Table 7.2.1), e.g. pain from acute pancreatitis and perforated peptic ulcers often radiates to the back.
Radiation of pain may suggest specific conditions (see Table 7.2.1), e.g. pain from acute pancreatitis and perforated peptic ulcers often radiates to the back.
Table 7.2.5
Temporal characteristics of abdominal pain
Sudden maximal pain at or near onset
Perforated peptic ulcer
Ruptured abdominal aortic aneurysm
Ruptured ectopic pregnancy, ruptured ovarian cyst
Ovarian/testicular torsion
Mesenteric infarction
Pulmonary embolism
Acute myocardial infarction
Progression to maximal pain within minutes
Acute pancreatitis
Renal and ureteric colic
Biliary colic
Strangulated hernia
Volvulus
Intussusception
Gradual onset (increased pain over hours)
Appendicitis
Strangulated hernia
Inflammatory bowel disease
Chronic pancreatitis
Salpingitis/prostatitis
Cystitis
From White MJ, Counselman FL. 2005 Troubleshooting acute abdominal pain. Emedmag 2002. http://www.emedmag.com/html/pre/cov/covers/011502.asp with permission.
Associated symptoms
Patients with abdominal pain often have other associated symptoms that may give a clue to the possible cause. These include:
Table 7.2.6 lists some of the historical high-yield questions in abdominal pain.
Table 7.2.6
High-yield historical questions
1. How old are you?
Advanced age means increased risk
2. Which came first – pain or vomiting?
Pain first is more likely to be caused by surgical disease
3. How long have you had the pain?
Pain for less than 48 hours is more likely to be caused by surgical disease
4. Have you ever had abdominal surgery?
Consider adhesion or obstruction in patients with previous abdominal surgery
5. Is the pain constant or intermittent?
Constant pain is more likely to be caused by surgical disease
6. Have you had this before?
A report of no prior episode is more likely to be caused by surgical disease
7. Do you have a history of cancer, diverticulosis, pancreatitis, kidney failure, gallstones, or inflammatory bowel disease?
All are suggestive of more serious disease
8. Do you have human immunodeficiency virus (HIV)?
Consider occult infection or drug-related pancreatitis
9. How much alcohol do you drink per day?
Consider pancreatitis, hepatitis, cirrhosis
10. Are you pregnant?
Test for pregnancy; consider ectopic pregnancy
11. Are you taking antibiotics or steroids?
These may mask infection
12. Did the pain start centrally and migrate to the right lower quadrant?
High specificity for appendicitis
13. Do you have a history of vascular or heart disease, hypertension or atrial fibrillation?
Consider mesenteric ischaemia and abdominal aneurysm
Adapted from Colucciello SA, Lukens TW, Morgan DL. Assessing abdominal pain in adults: a rational, cost-effective, and evidence-based strategy. Emerg Med Pract 1999;1:1 with permission.
Physical examination
A systematic, directed and thorough physical examination can help strengthen the clinical impression formed from the history or to uncover unexpected abnormalities. Physical findings help to rule in, but not rule out, the underlying diagnosis.
General
Consider the general condition and the vital signs of the patient. Patients who look drowsy or unwell or have abnormal vital signs need urgent attention. The posture of the patient may give a clue to the possible underlying disease. Patients with renal colic typically roll about in pain, whereas those with peritonitis lie still as movements aggravate the pain. Inspect for pallor, jaundice, hydration status, enlarged lymph nodes and signs of chronic liver or renal disease.
The abdomen
This is carried out with the patient lying supine and the abdomen exposed from the costal margins to the pubic symphysis. Ideally, the patient should be fairly relaxed, comfortable and cooperative. It is almost impossible to perform an abdominal examination in an uncooperative patient thrashing about in pain. Adequate pain relief should be given before examination if necessary. There is strong evidence that analgesia does not mask physical signs. Abdominal examination in an obtunded patient is unreliable and other assessment modalities such as imaging have to be considered.
 Specific abdominal signs (Table 7.2.7): distinctive signs have been described that are associated with specific diagnoses. Some of these signs have not been studied and their sensitivity and specificity remain unknown.
Specific abdominal signs (Table 7.2.7): distinctive signs have been described that are associated with specific diagnoses. Some of these signs have not been studied and their sensitivity and specificity remain unknown.
Table 7.2.7
| Sign | Description | Association |
| Murphy’s sign | Inability of patient to perform deep inspiration due to pain on palpation of right hypochondrium | Acute cholecystitis (sensitivity 97%; specificity 50%) |
| Kehr’s sign | Severe left shoulder tip pain, especially when the patient is lying supine | Haemoperitoneum, e.g. from ruptured spleen or ectopic pregnancy |
| Cullen’s sign | Ecchymoses around the periumbilical area | Retroperitoneal haemorrhage (haemorrhagic pancreatitis, abdominal aortic aneurysm rupture) |
| Grey–Turner’s sign | Ecchymoses of the flanks | Retroperitoneal haemorrhage (haemorrhagic pancreatitis, abdominal aortic aneurysm rupture) |
| McBurney’s sign | Tenderness localized to a point at two-thirds distance on a line drawn from the umbilicus to the right anterior superior iliac spine | Appendicitis |
| Iliopsoas sign | Extension of right hip causes abdominal pain | Appendicitis (sensitivity 16%; specificity 95%) |
| Obturator’s sign | Internal rotation of the flexed right hip causes abdominal pain | Appendicitis |
| Rovsing’s sign | Right lower quadrant (RLQ) pain with palpation of the left lower quadrant | Appendicitis |
| Heel-drop sign | RLQ pain on dropping heels on the ground after standing tiptoes; alternatively RLQ pain from forcefully banging the patient’s heel with the examiner’s hand | Appendicitis (sensitivity 93%) |
| Cough test | Post-tussive abdominal pain | Peritonitis (sensitivity up to 95%) |
From White MJ, Counselman FL. Troubleshooting acute abdominal pain Emedmag, 2005. http://www.emedmag.com/html/pre/cov/covers/011502.asp with permission.
Rectal examination
This is useful in cases of gastrointestinal haemorrhage, perianal or perirectal diseases, stool impaction, prostatic pathologies and rectal foreign bodies. Contrary to classic teaching, rectal examination does not provide additional input in suspected cases of appendicitis.
Examination of hernia orifices
All hernias should be examined for signs of strangulation. Hernias are most commonly present in the inguinal or femoral area, along the midline or arising from old surgical scars. Rarely, they may be present in the paramedian, lumbar or gluteal areas.
Examination of genitalia
In women, examination of the pelvic organs may yield important clues to possible gynaecological or obstetric causes of abdominal pain. Testicular pathology needs to be considered in male patients with lower abdominal pain.
Limitations of the abdominal examination
A significant proportion of patients with serious intra-abdominal conditions, such as ruptured aortic abdominal aneurysm and mesenteric ischaemia, may present with non-specific abdominal findings. The area of tenderness does not always correlate to the anatomical location of the disease. For example, up to 20% of patients with surgically proven appendicitis have no right lower quadrant tenderness. Signs of peritonism may not always be present, especially in the elderly and the immunocompromised.
Although involuntary guarding or rigidity increase the likelihood of peritonitis, rebound tenderness has been shown to have no predictive value.
Examination of extra-abdominal systems
Besides the abdomen, extra-abdominal systems, especially the cardiovascular and respiratory systems, should also be examined. Directed examination of extra-abdominal systems is important because:
Serial examination
Physical signs may often be non-specific in the early phases of the disease. Serial examinations over a period of hours can help to distinguish a surgical from a non-surgical abdomen and improve the diagnostic yield.
Clinical investigations
Although the history and physical examination may give a clue to the possible underlying pathology, many patients with abdominal pain do not present ‘classically’. Where indicated, judicious use of investigations may assist in determining diagnosis and disposition. An investigation should be ordered to answer focused clinical questions. It is also important to be aware of the test’s accuracy and limitations. Results should be interpreted in the correct clinical context. Negative test results may not fully rule out serious pathologies in patients with high pre-test probabilities. Further observation, reassessment and admission may need to be considered.
Bedside tests
Laboratory tests
Most laboratory tests do not aid in differentiating surgical from non-surgical causes of abdominal pain.
Imaging
Plain X-rays
The value of plain radiographs in the evaluation of patients with abdominal pain is limited. However, there is still a place for plain X-rays as a first-line investigation in patients with suspected bowel obstruction, bowel perforation and foreign body. A three-view series comprising upright chest, supine and upright abdominal radiographs is recommended. X-ray findings for bowel obstruction and perforation are fairly specific but not sensitive, i.e. they help to establish, but not exclude, these diagnoses.
Ultrasound
Ultrasound does not involve ionizing radiation, is rapid, non-invasive and may be performed at the bedside. This makes it the ideal evaluation tool in unstable patients or those who are pregnant. Selective use of focused ultrasound in the appropriate clinical context maximizes its diagnostic sensitivity. However, ultrasound is operator dependent and appropriate training is necessary to ensure competence. The sensitivity of ultrasound may also be reduced by technical limitations (e.g. obesity, bowel gas, subcutaneous emphysema). Focused bedside emergency ultrasound examination has significantly affected the diagnosis and management of the following life-threatening conditions:
Ultrasound may also be used for evaluating patients in the following conditions that may not be immediately life threatening:
Computed tomography
With the advent of helical and multidetector scanning technology, CT has become the imaging modality of choice for evaluation of abdominal pain in the non-obstetric patient. It has a high degree of accuracy, establishing diagnoses in more than 95% of cases in one study. In the elderly, CT resulted in changes to the management and disposition of a significant proportion of patients.
CT allows for detailed visualization of intra-, extra- and retroperitoneal structures. It identifies the exact site of disease, as well as its impact on the surrounding structures, thereby guiding further management. CT may be performed with or without intravenous and oral contrast agents. In the emergency setting, CT is useful for:
 assessment in abdominal trauma
assessment in abdominal trauma
 detection of inflammatory lesions (e.g. appendicitis, pancreatitis, diverticulitis, abscesses)
detection of inflammatory lesions (e.g. appendicitis, pancreatitis, diverticulitis, abscesses)
 detection of neoplastic lesions
detection of neoplastic lesions
 evaluation of vascular pathology (e.g. aortic aneurysm, aortic dissection, mesenteric ischaemia)
evaluation of vascular pathology (e.g. aortic aneurysm, aortic dissection, mesenteric ischaemia)
 detection of intra-abdominal and retroperitoneal bleed or abscesses
detection of intra-abdominal and retroperitoneal bleed or abscesses
The main limitations to CT are that the patient must be stable enough for transport to the scanning facility, ionizing radiation is involved, it may miss up to 20% of gallstones because the stones may be of the same radiographic density as bile and it may miss up to 10–17% of traumatic small bowel perforations.
The sensitivity of CT is not 100% for most conditions. Clinical decisions should not be based on CT results alone. If initial CT findings are negative but clinical suspicion is high, further observation, evaluation or even repeat scans may be needed.
In the patient with very high suspicion for conditions that require immediate surgical intervention (e.g. unstable patient with obvious peritonitis), use of CT may result in delay in definitive treatment. For the patient in whom the clinical suspicion for serious abdominal pathology is very low, urgent CT scan is likely to have a low yield and the cost and potential side effects of CT outweigh its benefits. In one study for ED patients with suspected urgent abdominal conditions, a diagnostic strategy with initial ultrasound examination, followed by CT when ultrasound findings were negative or inconclusive, resulted in the best CT sensitivity. This strategy also reduced CT use by up to 51%.
Magnetic resonance imaging (MRI)
With the introduction of high-speed techniques, MRI protocols for patients with acute abdominal pain can now be reduced to below 15 minutes.
MRI does not involve ionizing radiation and offers better soft tissue visualization than CT. The high intrinsic contrast resolution of images rendered by MRI may allow for contrast-free scanning in certain cases. Compared to CT, MRI is able to provide increased information for hepatobiliary disease, pancreatitis and mesenteric ischaemia. MRI has also demonstrated promising accuracy for diagnosis of appendicitis, diverticulitis, small bowel obstruction and abdominal and pelvic venous thrombosis.
Currently, the evidence for use of MRI in ED patients presenting with acute abdominal pain is still relatively limited; it is most frequently used in selected pregnant patients in whom ultrasound findings are non-diagnostic.
MRI is significantly more costly than CT, takes longer to perform and may have limited availability in some centres. MRI is contraindicated in patients with claustrophobia or implanted metallic devices.
Imaging for the pregnant patient
Ultrasound is currently still the most common initial imaging modality used to evaluate the pregnant patient presenting with acute abdominal pain, although MRI is now playing an increasingly important role. Both imaging techniques do not involve ionizing radiation and have not been shown to cause any ill effects to the fetus. The routine use of gadolinium-based MR contrast agents is currently not recommended as these agents pass through the placenta into fetal circulation and their effects on the fetus remain unknown.
CT is a valuable imaging tool in evaluation of abdominal pain in the pregnant patient, as it remains one of the most reliable imaging modality in the diagnoses of many acute abdominal conditions. The ionizing radiation doses involved in CT studies are below the doses that would lead to developmental or neurological deficits. However, radiation levels should still be kept as low as reasonably achievable as there are no known radiation limits for fetal stochastic effects, e.g. carcinogenesis.
Iodine-based CT contrast agents are also known to cross the placenta into fetal circulation. Small studies have indicated that single dose exposure to CT contrast agents does not lead to fetal teratogenesis or hypothyroidism. Similar to MR contrast agents, intravenous CT contrast agents should be avoided unless the accuracy of the imaging study is dependent on their use.
Pitfalls
The elderly
In elderly patients presenting with abdominal pain, conditions requiring surgical intervention (e.g. cholangitis, intestinal obstruction), serious vascular pathologies (e.g. AAA, aortic dissection, mesenteric ischaemia) and intra-abdominal neoplasms are more common (Table 7.2.8). Unfortunately, abdominal pain is frequently misdiagnosed in the elderly because:
 pain perception in the elderly may be blunted
pain perception in the elderly may be blunted
 vital signs may be normal in spite of serious underlying illness
vital signs may be normal in spite of serious underlying illness
Table 7.2.8
Disease spectrum in those less than 50 years old vs those over 50
| Confirmed cause of acute abdominal pain | Acute abdominal pain in patient<50 (n=6317) (%) | Acute abdominal pain in patient≥50 (n=2406) (%) |
| Biliary tract disease | 6 | 21 |
| Non-specific abdominal pain | 40 | 16 |
| Appendicitis | 32 | 15 |
| Bowel obstruction | 2 | 12 |
| Pancreatitis | 2 | 7 |
| Diverticular disease | <0.1 | 6 |
| Cancer | <0.1 | 4 |
| Hernia | <0.1 | 3 |
| Vascular | <0.1 | 2 |
Adapted from deDombal FT. Acute abdominal pain in the elderly. J Clin Gastroenterol 1994;29:331–5 with permission.
Adverse outcomes in the elderly are more common as a result of missed or delayed diagnosis than in younger patients. This is because the elderly tend to seek treatment late in the disease process and complications tend to be more common, they have reduced physiological reserve and often more co-morbidities. It has been estimated that with each decade of life, diagnostic accuracy decreases while mortality increases, such that for the octogenarian diagnostic accuracy is about 30%, although the corresponding mortality rate is 70 times that of patients under 30. Therefore, geriatric patients with abdominal pain need to be carefully evaluated and the threshold for imaging studies, surgical consultation and admission should be lowered.
The immunocompromised
Inflammatory responses are suppressed in the immunocompromised and abdominal signs from peritoneal irritation may be absent. Patients with human immunodeficiency virus (HIV) infection may develop unusual conditions, such as opportunistic viral or bacterial enterocolitis (e.g. cytomegalovirus or Mycobacterium avium intrecellulare enterocolitis), AIDS-related cholangiopathy, lymphoma or drug-induced pancreatitis. Patients on peritoneal dialysis or with advanced liver cirrhosis are at risk of developing spontaneous bacterial peritonitis.
Women of childbearing age
It is important to determine whether these patients are pregnant. Menstrual history, compliance with contraception, history of tubal ligation or claims of sexual abstinence cannot reliably exclude the possibility of pregnancy.
If the patient is pregnant, the possibility of ectopic pregnancy should be considered. In addition, pelvic conditions (e.g. pelvic inflammatory disease, ovarian pathology, pregnancy-related complications, such as threatened or missed abortion) and urinary tract infections are relatively common.
For patients in the second or third trimester of pregnancy, the gravid uterus may displace structures in the lower abdomen away from their usual position, e.g. the appendix may migrate to a higher position in the right hypochondrium or the right flank, changing symptoms and signs. Clinical signs of peritonism may be obscured due to loss of abdominal wall musculature elasticity.
Dangerous mimics (Table 7.2.9)
Misdiagnosed patients are most commonly given the labels of gastroenteritis, constipation, gastritis, urinary tract infection or pelvic inflammatory disease.
Table 7.2.9
| True diagnosis | Initial misdiagnosis |
| Appendicitis | Gastroenteritis, pelvic inflammatory disease (PID), urinary tract infection (UTI) |
| Ruptured abdominal aortic aneurysm | Renal colic, diverticulitis, lumbar strain |
| Ectopic pregnancy | PID, UTI, corpus luteum cyst |
| Diverticulitis | Constipation, gastroenteritis, non-specific abdominal pain |
| Perforated viscus | Peptic ulcer disease, pancreatitis, non-specific abdominal pain |
| Bowel obstruction | Constipation, gastroenteritis, non-specific abdominal pain |
| Mesenteric ischaemia | Constipation, gastroenteritis, ileus, small bowel obstruction |
| Incarcerated or strangulated hernia | Ileus, small bowel obstruction |
| Shock or sepsis from perforation, bleed and abdominal infection in the elderly | Urosepsis or pneumonia (in elderly) |
From Colucciello SA, Lukens TW, Morgan DL. Assessing abdominal pain in adults: a rational, cost-effective, and evidence-based strategy. Emerg Med Pract 1999;1:1 with permission.
Diagnosis versus disposition
In the ED management of patients presenting with abdominal pain, empirical management of acute conditions and proper disposition are more important than diagnostic accuracy. When the diagnosis is based only on clinical findings and basic laboratory investigations, overall diagnostic accuracy is about 50% (as high as 80% in young adults and as low as 30% in the elderly). Fortunately, the rate of inappropriate discharge from the ED is low (about 1% across all age groups), albeit slightly higher in the elderly (about 4%).
Treatment
Resuscitation
Prompt resuscitation should take precedence over diagnosis in unstable patients. Patients may be in shock as a result of blood loss, fluid loss, sepsis or from concurrent cardiovascular events. Appropriate fluid therapy and inotropic support should be instituted early to prevent further deterioration and end-organ dysfunction.
Symptom relief
Pain relief should be instituted as early as possible for patients presenting with abdominal pain. This may require intravenous opiates titrated to response. Specific treatment (e.g. non-steroidal anti-inflammatory agents in renal colic) should be used when available. The previously widely held dogma that opiates mask signs of serious pathology (in particular peritonism) has been disproved in many studies. In fact, the use of opioids in abdominal pain is not only safe, but actually aids diagnosis by facilitating physical examination and relaxing the abdominal musculature. The dosage used should be titrated to the patient’s response.
Antibiotics
Antibiotics are indicated in suspected intra-abdominal sepsis. The antibiotics used should cover Gram-negative aerobes as well as anaerobes. Additional coverage for Gram-positive aerobes is required in patients with spontaneous bacterial peritonitis. See chapters on specific conditions for more details.
Surgical review
Early involvement of surgeons should be the rule in cases of suspected surgical abdomen. Early surgical intervention is crucial in improving outcome for urgent conditions, e.g. ruptured abdominal aortic aneurysm, ruptured ectopic pregnancy, intraperitoneal haemorrhage and bowel perforation.
Disposition
Admission
The following patients need to be admitted:
 patients with specific diagnoses that require inpatient management
patients with specific diagnoses that require inpatient management
 patients who are ill, unstable or with altered mentation
patients who are ill, unstable or with altered mentation
 the elderly or the immunocompromised in whom diagnoses are unclear
the elderly or the immunocompromised in whom diagnoses are unclear
 patients in whom potentially serious conditions cannot be excluded
patients in whom potentially serious conditions cannot be excluded
 patients in whom symptoms (e.g. pain, vomiting) cannot be ameliorated
patients in whom symptoms (e.g. pain, vomiting) cannot be ameliorated
 patients who are unable to follow discharge instructions or who have poor social support.
patients who are unable to follow discharge instructions or who have poor social support.
In addition, the admission threshold should be lowered for patients returning to the ED for the same complaints, especially where the cause of the abdominal pain had not been fully elucidated.
Observation
Patients who do not meet admission criteria but have persistent pain may be observed over a period of hours, in the ED or an observation unit if appropriate. Serial examination over a period of hours has been found to improve diagnostic accuracy.
The main aims of observing the patient with abdominal pain are to improve diagnostic yield with serial examination, to monitor progress after treatment, to detect the development of signs of acute abdomen and for further diagnostic work-up if indicated.
Discharge advice
It is important to give discharge advice, as some conditions develop over time. The patient should be advised to return if:
 pain is persistent (>24 hours) or worsening
pain is persistent (>24 hours) or worsening
 they develop incessant vomiting or are unable to retain fluids
they develop incessant vomiting or are unable to retain fluids
 vague pain has become localized, e.g. to the right iliac fossa
vague pain has become localized, e.g. to the right iliac fossa
 they develop high fever or chills, or feel increasingly ill, weak or unwell
they develop high fever or chills, or feel increasingly ill, weak or unwell
 they develop fainting episodes
they develop fainting episodes
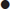 there is blood in the stools or vomitus
there is blood in the stools or vomitus
 they develop new medical problems requiring urgent consultation.
they develop new medical problems requiring urgent consultation.
Non-specific abdominal pain
A large proportion of stable patients with abdominal pain will not have a definitive diagnosis on discharge from the ED. In patients under 50 years of age, non-specific abdominal pain may be as high as 40%. This figure is lower in the elderly, at about 15% (see Table 7.2.8).
Most younger patients may be safely discharged from the ED once their symptoms have resolved with treatment and a period of observation. They should be clearly informed that the cause of their pain has not been determined and appropriate discharge advice, including to return to the ED if symptoms recur or worsen, should be given. Referral to a specialist for further evaluation may be indicated.
Non-specific abdominal pain in younger patients tends to have a benign course. However, about 10% of the elderly labelled with it are subsequently found to have an underlying malignancy. It is also important to rule out extra-abdominal causes in the elderly.
Developments in the next 5–10 years
Early recognition of acute mesenteric ischaemia greatly improves patient outcome as timely angiographic or surgical intervention is required. However, clinical diagnosis of acute mesenteric ischaemia, especially in the early stages, remains challenging. Diagnosis currently relies heavily on imaging. The search for accurate serum and urine biomarkers has shown promise and may alter the management of this subset of patients presenting to the ED with acute abdominal pain.
7.3 Bowel obstruction
Willem Landman and Kim Yates
Pathology and pathophysiology
Bowel obstruction is the interruption of the normal peristaltic progression of intestinal contents. Mechanical bowel obstruction can be caused by lesions outside or within the bowel wall or within the lumen itself. It may be partial or complete, strangulating or non-strangulating. Common causes of small bowel obstruction (SBO) include adhesions, hernias and neoplasms. Less common causes include inflammatory bowel disease, gallstones, foreign bodies, strictures, radiation, diverticulitis, endometriosis and abscesses. Common causes of large bowel obstruction (LBO) include neoplasm, diverticulitis and volvulus. Faecal impaction, inflammatory bowel disease, strictures and extraintestinal tumours are less common causes.
Paralytic ileus may mimic obstruction but there is no mechanical cause; rather, it is associated with abnormal propulsive motility. Paralytic ileus can be caused by a wide range of conditions. Metabolic causes include hypokalaemia (most common), hyponatraemia, hypomagnesaemia and hypoalbuminaemia. Drugs, such as tricyclic antidepressants, opiates, antihistamines, β-adrenergic agonists and quinidine, have also been implicated. Acute colonic pseudo-obstruction (Ogilvie syndrome) is characterized by acute large bowel dilatation without mechanical obstruction due to diffuse incoordination and reduction of colonic peristalsis. Its causes include a postoperative state, cardiorespiratory disease, trauma, infection, medications, such as opiates and antidepressants, and neurological disease.
The pathophysiology of mechanical bowel obstruction relates to rising intraluminal pressure, mucosal injury, bacterial overgrowth and inflammatory response. Bowel proximal to the obstruction distends with gas, fluid and electrolytes, then hypersecretion escalates, bowel absorptive ability decreases and progressive systemic volume losses occur. Vomiting ensues more quickly the more proximal the bowel obstruction is and worsens the dehydration and electrolyte disturbances.
If obstruction persists, then the intraluminal pressure rises and local vascular compromise can occur, especially venous stasis. As pressures rise and blood flow diminishes, the bowel can strangulate and necrosis may follow with consequent perforation and sepsis. A closed-loop obstruction implies both proximal and distal obstruction (e.g. strangulating hernia or volvulus) and, typically, leads to vascular compromise more quickly and therefore a higher risk of strangulation, necrosis and perforation.
Clinical features
History
In early bowel obstruction, abdominal pain is poorly localized and colicky, but later may become more constant and, if severe, suggests ischaemia or peritonitis. Pain from SBO tends to be more severe earlier and cramps tend to be more frequent compared to LBO where dull, lower abdominal cramps are more common. Vomiting is more common in SBO and is a late symptom in LBO. Faeculent vomiting or distension suggests a more distal SBO or a LBO. Obstipation was thought typical, but the passage of flatus and stool may continue.
The gastrointestinal and surgical history helps differentiate causes of mechanical obstruction and drug history and systems enquiry may identify potential causes of non-mechanical obstruction.
Examination
Classical examination findings are abdominal distension and absent, reduced or ‘tinkling’ bowel sounds. Abdominal tenderness, if present, is usually mild. A mass may or may not be palpable. The presence of fever, tachycardia, guarding or peritonism suggests strangulating obstruction; however, vascular compromise can occur in their absence. Signs of dehydration are often present. Abdominal distension is more commonly present in LBO or distal SBO. On auscultation, rushes or high-pitched tinkles may be heard, but are not absolute indicators of obstruction. Surgical scars suggest adhesions as a cause of obstruction and examination for hernias is essential. Rectal examination may be normal in SBO, but the presence of faecal impaction, blood or a mass may assist with diagnosis of cause. Pelvic examination may be useful if abscesses or inflammation are suspected.
A focused medical examination should also be performed to exclude causes of paralytic ileus and pseudo-obstruction and to assess anaesthetic risk.
Clinical investigations
Laboratory tests
Laboratory tests are of limited value for diagnosing bowel obstruction but help in assessment of severity and guiding resuscitation. Of all laboratory tests, only lactate and interleukin (IL)-6 levels appear to have significant predictive value for strangulating obstruction. If both are raised, the positive predictive value is 95% and the negative predictive value 97%. Haematocrit may be raised if dehydration is present. Electrolyte abnormalities, such as hyponatraemia, hypokalaemia and impaired renal function, are common. Serum amylase may be mildly raised in SBO. Blood gas analysis may show metabolic alkalosis if vomiting is severe, metabolic acidosis if shock, dehydration or ketosis is present. A blood or urine pregnancy test, where appropriate, and urine microscopy are important in excluding other causes of abdominal pain.
Imaging studies
The ideal study would define the grade of obstruction (complete, high grade, low grade), the level of the obstruction and the cause of the obstruction as well as any associated complications.
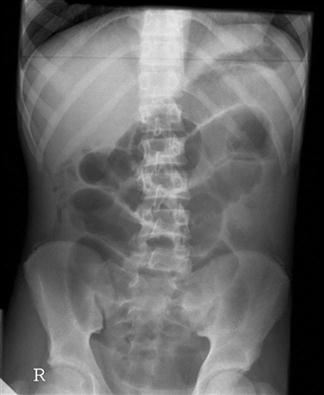
The presence of dilated loops of bowel with multiple air–fluid levels on abdominal X-rays is highly suggestive of bowel obstruction; however, the overall sensitivity of plain radiography is 30–70% and specificity is around 50% (E-Figs 7.3.1 and 7.3.2). X-rays may appear normal with closed loop or strangulated obstructions. Abdominal computed tomography (CT) is particularly useful in cases where plain radiology is non-diagnostic. The overall sensitivity and specificity of CT is around 95%. With a typical clinical picture for obstruction plain radiology and CT have similar sensitivities. On the other hand, ileus and pseudo-obstruction have a similar X-ray appearance to mechanical obstruction. CT can determine the level and cause of obstruction, as well as emergent causes of obstruction, such as volvulus and strangulation. For these reasons and its ability to do tumour staging, it is the modality of choice to facilitate decision making.
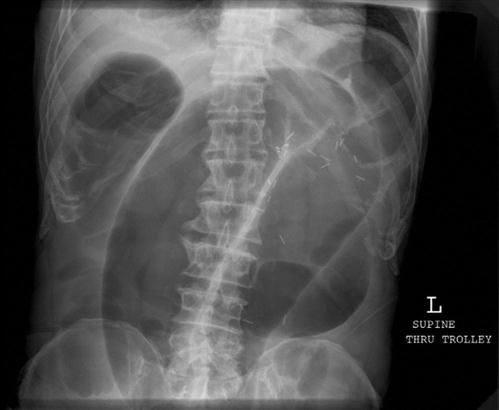
In the assessment of acute obstruction in adults, magnetic resonance imaging has improved sensitivity, specificity and accuracy compared to CT; however, limited availability, long scan times and the need for extensive oral preparation mean it is of limited use in the ED setting unless minimizing radiation exposure is paramount.
Bedside ultrasonography by a trained examiner looking for dilated small bowel has a similar sensitivity and a greater specificity than plain radiology, but is not widely used and is subject to significant operator dependency. It may have particular utility in unstable and pregnant patients.
Endoscopy
Careful sigmoidoscopy is safe in LBO and therapeutic in sigmoid volvulus when used to place a rectal tube. In some centres, endoscopy is performed acutely to decompress LBO by inserting drainage tubes or self-expanding metal stents.
Treatment and prognosis
General measures
Most patients with bowel obstruction are dehydrated, so treatment with crystalloid intravenous fluid is required and electrolyte disturbances should be corrected. Urinary catheterization and monitoring of urine output, vital signs and electrolytes should guide ongoing fluid and electrolyte therapy. Nasogastric decompression is customary, but evidence of benefit in patients without significant vomiting is weak. Analgesia is often required, with titrated increments of IV opiates the most appropriate option. Antibiotics are prescribed by some to counter bacterial translocation, but evidence of their effectiveness is sparse.
Patients with bowel obstruction associated with haemodynamic compromise, shock or sepsis require combined, ongoing management by surgical and intensive care teams. Patients with suspected strangulating bowel obstruction or perforation should have urgent surgery. Stable patients and those with partial bowel obstruction can be started on conservative therapy and monitored closely as inpatients for signs of deterioration.
Conservative therapy
Ongoing intravenous fluid therapy, electrolyte management and bowel rest are the mainstay interventions. Monitoring of vital signs, urine output and clinical state should continue and deterioration or failure to improve are indications for surgical therapy.
Where bowel obstruction due to malignancy is inoperable, octreotide appears superior to hyoscinebutyl bromide in relieving symptoms and, although corticosteroids are commonly advocated, evidence for their effectiveness is less clear.
In patients with acute colonic pseudo- obstruction unresponsive to conservative therapy, IV neostigmine 2 mg has initiated rapid colonic decompression.
A non-strangulating sigmoid volvulus can be temporarily decompressed by a rectal tube inserted via sigmoidoscope.
Endoscopic placement of self-expanding metallic stents can relieve malignant LBO, either prior to elective surgical resection or as definitive palliative therapy if the malignancy is inoperable. Reported complications of metallic stents include perforation, stent migration and reobstruction.
Surgical therapy
Bowel obstruction due to hernias and complete SBO usually requires surgery. Strangulating bowel obstruction is an indication for urgent surgery and should be suspected in the presence of severe pain and localized tenderness. Additional suggestive features include a fever, shock, mass, hernia, acidosis, marked leucocytosis, raised lactate, sepsis and confirmatory CT findings. It can, however, occur without these features. Broad-spectrum parenteral antibiotics are indicated preoperatively and if sepsis is suspected.
Mortality escalates dramatically the longer surgery is delayed in strangulating bowel obstruction or perforation (≈30% compared to 3–5% in non-strangulating bowel obstruction), so prompt surgery is vital. The surgical approach adopted will depend on the suspected pathology and operative findings. Some centres use laparoscopy to treat SBO with variable success rates (33–87%). Higher success rates have been reported in those with a history of appendicectomy only, or with band adhesions. In LBO, decompressive stomas followed by a definitive operation at a later date are sometimes useful in very sick patients; however, right-sided lesions can often be resected at laparotomy with a primary anastomosis, avoiding a stoma completely. One-stage resection/anastomosis is possible with left-sided lesions, but there is a higher risk of contamination in unprepared bowel and higher mortality rates.
7.4 Hernia
Neil A Goldie
Introduction
A hernia is defined as a protrusion of a viscus or part of a viscus through a weakness in the wall of the containing cavity. It has an aperture, coverings (usually peritoneum and abdominal wall layers) and contents, which may be any intra-abdominal organ but are usually omentum or small bowel. Surgical treatment requires reduction of the contents and closure of the aperture, with reinforcement to prevent recurrence.
There are a number of described sites for herniae. This chapter will focus on the more common of these, but the principles of assessment and treatment apply to herniae at other sites.
Aetiology, pathology and clinical features
Inguinal hernia
Inguinal herniae are extremely common and account for 75% of all abdominal wall herniae. There is a lifetime risk of occurrence of 27% for men and 3% for women and an annual incidence of 130 per 100 000 population. Up to 9% of hernia repairs are performed urgently. Emergency repairs are more common in the elderly and carry greater morbidity than elective repair.
As their name implies, direct inguinal herniae bulge directly through the posterior wall of the inguinal canal. They are caused by weak abdominal musculature, are common in the elderly and frequently bilateral. They have a large neck and hence seldom become irreducible or strangulate until they are of considerable size.
For indirect inguinal herniae, the hernial sac comes through the internal inguinal ring, travels the length of the inguinal canal and emerges from the external inguinal ring. Thus, it usually lies above and medial to the symphysis pubis. Later, the internal inguinal ring may stretch and the hernial sac and its contents may descend to and fill the scrotum, occasionally becoming very large. As the internal inguinal ring is usually narrow, irreducibility is common. Indirect inguinal herniae occur throughout life (E-Fig. 7.4.1).
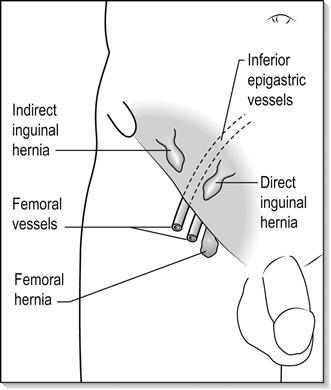
Direct and indirect inguinal herniae may be distinguishable by simple clinical tests. When an indirect hernia is reduced, finger pressure over the site of the internal ring may hold it reduced; however, a direct inguinal hernia will flop out again unless several fingers or the side of the hand props up the entire length of the inguinal canal.
Femoral hernia
Femoral herniae appear lateral and inferior to the symphysis pubis. They are formed by the peritoneal sac and contents, which occupy the potential space of the femoral canal, medial to the femoral vein. They are proportionately more common in women and rarely large. Symptoms usually occur early and complications are common.
Both femoral canal areas should be closely examined in any patient presenting with abdominal pain or signs of bowel obstruction, as femoral herniae are frequently overlooked, especially in patients who are elderly and obese. Diagnosis of a femoral hernia mandates early surgery. Morbidity from emergency femoral hernia repair increases with the presence of small bowel obstruction. Mortality with emergency surgery can be as high as 5%.
Umbilical hernia
Umbilical and periumbilical herniae protrude through and around the umbilicus. They are very common in the newborn, but most resolve by 4 years of age. As they have a broad neck, emergency complications are uncommon. They can be difficult to diagnose in very obese people. If complicated, they can present resembling abdominal wall cellulitis.
Epigastric hernia
Epigastric herniae appear in the midline above the umbilicus. A small extraperitoneal piece of fat may be stuck in this hernia, causing pain.
Other herniae
Obturator hernia
Rarely, viscera may pass through a defect in the obturator foramen and present as a small bowel obstruction. This occurs most commonly in elderly emaciated women with chronic disease. Diagnosis of this internal hernia and the hernia of the foramen of Winslow is seldom made preoperatively.
Spigelian hernia
Spigelian herniae are rare and are due to a defect in the anterolateral abdominal wall musculature. They usually present as a reducible lump in the elderly male, lateral to the rectus muscle in the lower half of the abdomen. Complications are rare.
Incisional hernia
These may occur at the site of any previous abdominal wound, such as appendicectomy or laparotomy. The wound area becomes weak, allowing the protrusion of a viscus or part of a viscus.
Sportsman’s (athlete’s) hernia
This is a term used for those who present with the painful symptoms of a hernia in the groin following exertion. It is defined as an occult hernia caused by weakness or a tear of the posterior inguinal wall without a clinically recognizable hernia. Generally, by the time of diagnosis, non-operative treatment options have failed and surgery often results in a return to sport. Ultrasound can be a useful diagnostic medium to detect herniae which are intermittently symptomatic but without clinical signs.
Complications
In the early stages, herniae are usually reducible, producing only intermittent pain in the groin, but reducible herniae may become irreducible (incarcerated). Incarcerated herniae may lead to a bowel obstruction. Strangulation and interruption of the blood supply to the contents of the hernia (usually small bowel) may supervene. In this case, there will be increasing local pain, tenderness, warmth and overlying erythema. This is accompanied by signs of bowel obstruction and a leucocytosis.
Rarely, only part of the bowel wall is caught in a hernial constricting ring. Bowel wall necrosis ensues that is not circumferential; this is termed a Richter’s hernia. In this case, there may be signs of strangulation without signs of obstruction.
Very rarely, neglected herniae can fistulate, with bowel contents appearing at the abdominal wall or through the hernial orifices.
Treatment
Reduction
It may be possible to reduce a hernia that initially appears irreducible in the emergency department, but caution must be exercised. If the skin over the hernia is already inflamed and pain is severe, the contents may be compromised and urgent surgical exploration is required. Reduction of the contents in this circumstance can be dangerous, as false reassurance can occur followed by the later development of peritonitis due to intra-abdominal perforation of the hernia contents.
As a general rule, if the hernia has been irreducible for less than 4 hours, vital signs are normal and there are no symptoms of bowel obstruction, reduction of an incarcerated hernia may be attempted. This is achieved by giving adequate analgesia to relax the patient and applying gentle pressure manipulating the hernia site for several minutes. Elevating the foot of the bed may be helpful. Successful reduction relieves pain, may prevent strangulation and reduces the urgency for surgical intervention. Notwithstanding, all herniae that have undergone a complication require surgical consultation with view to definitive treatment at the time of presentation.
Surgical repair
Inguinal hernia repair is a very common operation in general surgery. Rates of repair range from 10 per 10 000 population in the UK to 28 per 10 000 in the USA.
Timely repair of herniae reduces the incidence of complications and avoids the greater risk associated with emergency surgery. Until the introduction of synthetic mesh, inguinal hernia repair had changed little for over 100 years. Mesh is used to reinforce the repaired defect and can be placed by an open method or laparoscopically. Laparoscopic transabdominal preperitoneal hernia repair takes longer than open surgery and has a more serious complication rate with regard to visceral injuries, but is being increasingly performed as it reduces postoperative pain and significantly reduces time off work. It is also much more operator dependent, is more difficult to learn and has higher overall hospital costs.
Patients requiring emergency surgery for bowel obstruction or strangulation should be prepared with adequate fluid resuscitation and analgesia.
7.5 Gastroenteritis
Anita Liu
Introduction
Gastroenteritis is a common clinical syndrome. It poses one of the world’s major clinical and public health problems and, in developing countries with poor-quality drinking water and low levels of sanitation, it is a major cause of morbidity and mortality, especially among children and the elderly.
Gastroenteritis is caused by infection of the gastrointestinal tract by various viruses, bacteria and protozoa. Transmission is most commonly by the faecal–oral route. The syndrome consists of diarrhoea, abdominal cramping or pain, nausea and vomiting, lethargy, malaise and fever. Each of these features may be present to a varying degree and may last from 1 day to more than 3 weeks.
In developed countries, even though serious morbidity and mortality are low, gastroenteritis may be an extremely painful and unpleasant event causing disruption to daily life and significant loss of working and school days. Patients often seek emergency medical care because of the acuteness of onset of symptoms, the frequency of the diarrhoea, the severity of abdominal pain and cramps or because of concerns regarding dehydration.
Pathogenesis and pathology
Microorganisms of all descriptions are constantly entering the gastrointestinal tract through the mouth. Extremely few of these progress to cause clinical illness. The natural defences of the gastrointestinal tract against infection include gastric acid secretion, normal bowel flora, bile salt production, bowel motility, mucosal lymphoid tissue and secreted immunoglobulin A. People with disturbances in any of these defences are more prone to a clinical infection. For example, patients with achlorhydria, bowel stasis or blind loops, immunodeficiency states or recent antibiotic therapy that has disturbed bowel flora are prone to gastroenteritis. Some organisms, such as rotavirus, occur principally in children, as previous infection confers immunity.
Microbiology
A wide variety of viruses, bacteria and protozoa may cause gastroenteritis and the list is continually growing. Viral agents include rotavirus, enteric adenovirus, astrovirus, calicivirus, norovirus, coronavirus and cytomegalovirus. Bacteria include Campylobacter jejuni, Staphylococcus aureus, Bacillus cereus, Escherichia coli, Vibrio cholerae, Shigella dysenteriae, Salmonella enteriditis, Yersinia enterocolitica, Clostridium perfringens and C. difficile. Protozoa include Giardia lamblia, Cryptosporidium parvum and Entamoeba histolytica.
Microorganisms cause gastroenteritis by a number of mechanisms. They may release preformed toxins prior to ingestion, multiply and produce toxins within the gastrointestinal lumen, directly invade the bowel wall or use a combination of toxins and invasion.
Staphylococcus aureus and Bacillus cereus produce a variety of toxins in stored food that are subsequently ingested. These toxins are absorbed and, within hours, act on the central nervous system to produce an illness characterized predominantly by vomiting and mild diarrhoea.
Invasive bacteria are characterized by Salmonella, which invades the mucosa (primarily of the distal ileum) producing cell damage and excessive secretion. Shigella likewise invades the mucosa but also produces toxins that have cytotoxic, neurotoxic and enterotoxic effects.
The many strains of E. coli have been divided into five groups, depending on the pathology of the diseases they cause. These are enteropathogenic, enterotoxigenic, enteroinvasive, enteroaggregative and enterohaemorrhagic. Enterohaemorrhagic E. coli is associated with haemorrhagic colitis and the haemolytic–uraemic syndrome, whereas enterotoxigenic E. coli is associated with traveller’s diarrhoea. The protozoan Giardia lamblia adheres to the jejunum and upper ileum, causing mucosal inflammation, inhibition of disaccharidase activity and overgrowth of luminal bacteria.
Rotavirus is estimated to be the cause of 50% of gastroenteritis admission in Australia prior to the introduction of rotavirus vaccine. Rotavirus vaccine was introduced into the funded Australian National Immunization Programme in July 2007. Comparison study of gastroenteritis prior to the vaccine introduction against the 30 months following the vaccine introduction shows marked reduction in emergency department (ED) encounters, as well as hospitalization for rotavirus and non-rotavirus gastroenteritis. There also appears to be an indirect population protective effect of the vaccine as older children who were ineligible for the rotavirus vaccine have also demonstrated reduced hospitalization and positive rotavirus test.
Epidemiology
In Australia, the estimated incidence of gastroenteritis is 17.2 million cases per year. Thirty-two per cent of these cases are food borne, which is equivalent to 0.3 episodes per person per year. Altogether, food-borne gastroenteritis causes 15 000 hospitalizations and 80 deaths annually. The economic impact on the healthcare system is estimated at $30 million per year.
Norovirus, enteropathogenic E. coli, Campylobacter and Salmonella are the leading causes of gastroenteritis in Australia.
Gastroenteritis may occur in many settings. It may be a sporadic isolated event, a small outbreak either within a family or other close living group, such as in a geriatric residential facility, or part of a larger community epidemic. It may occur in a traveller, either while still overseas or on their return home. It is important to be aware of the circumstances and context in which the illness occurs, as these will often dictate the course of investigation or management.
Clinical Features
History
The clinical history and examination are directed at confirming the diagnosis of gastroenteritis, excluding other diagnoses and determining the degree of dehydration.
The principal clinical manifestation of gastroenteritis is diarrhoea. There is a lack of standardized definition of gastroenteritis. The World Health Organization syndromic definition of gastroenteritis is ‘three or more abnormally loose or fluid stools over 24 hours’. The diarrhoea of gastroenteritis is often watery and profuse in the early stages of the illness and may last for up to 3 weeks. It is important to determine the frequency, volume and characteristics of the stool. Some organisms, such as enterohaemorrhagic E. coli, Shigella, Salmonella, Campylobacter and Entamoeba histolytica, may cause acute and bloody diarrhoea, whereas others, such as Giardia, may cause loose, pale, greasy stools.
Abdominal pain is common and is most often described as a diffuse intermittent colicky pain situated centrally in the abdomen. It may occur just prior to, and be partially relieved by, a bowel action. Severe pain is often caused by Campylobacter, Yersinia and E. coli. Abdominal pain is also the hallmark of many other forms of intra- and extra-abdominal pathology. Diagnoses other than gastroenteritis should be seriously considered if the pain is well localized, constant and severe or radiates to the back or shoulder.
Vomiting may be present, particularly early in the illness, and can be variable in severity and persistence. The amount of vomiting and the ability to keep down clear fluids should be determined, as this will dictate the management of dehydration. Severe vomiting often occurs with organisms that produce preformed toxin, although it does not usually persist for longer than 24 hours. Anorexia, nausea and lethargy are common. Fever and systemic symptoms, such as headache, are prominent with organisms that invade the bowel wall and enter the systemic circulation, such as Yersinia. Lethargy may be related to the dehydration or merely the strain of constant and persistent diarrhoea from any aetiology.
Specific inquiry regarding fluid status is essential. The aim should be to determine the amount of fluids that have been taken orally and kept down over the course of the illness, along with the estimated urine output. It is also important to ascertain pre-existing or intercurrent illness, such as diabetes or immunosuppression, which may alter management.
Physical examination
Suitable infection control procedures should be instituted prior to the examination to prevent spread to the examining doctor and hence to other patients. Where possible, the patient should be in an isolation cubicle. Hand hygiene procedures before and after the consultation, the use of gloves and prompt disposal of soiled clothing and linen are important.
A careful clinical examination should be performed, concentrating on the abdomen and the circulatory state of the patient. The vital signs, temperature and urinalysis should be obtained.
In mild to moderate gastroenteritis, the clinical examination is often unremarkable. There may be some general abdominal tenderness, active bowel sounds and facial pallor, but little else. In more severe disease, the abdominal tenderness may be pronounced and signs of dehydration present. Of note, uncomplicated gastroenteritis is extremely unlikely if the abdominal examination reveals localized tenderness or signs of peritoneal irritation.
Fluid losses through diarrhoea, vomiting and fever, together with poor oral fluid intake, can lead to clinically apparent dehydration. This may be manifest as tachycardia, tachypnoea, reduced tissue turgor, delayed capillary return, reduced urine output and, in its more severe stages, hypotension, impaired conscious state and death.
Extra-abdominal signs of a primary gastroenteritis can occur. Campylobacter has been associated with reactive arthritis and Guillain–Barré syndrome. The clinical features, course and complications for various causative agents are summarized in Table 7.5.1.
Table 7.5.1
| Causative agent | Incubation period | Duration of illness | Predominant symptoms | Foods commonly implicated |
| Bacteria | ||||
| Campylobacter jejuni | 1–10 days (usually 2–5 days) | 2–5 days occasionally>10 days | Sudden onset of diarrhoea, abdominal pain, nausea, vomiting | Raw or undercooked poultry, raw milk, raw or undercooked meat, untreated water |
| E. coli enterohaemorrhagic | 2–10 days | 5–10 days | Severe colic, mild to profuse bloody diarrhoea can lead to haemolytic uraemic syndrome | Many raw foods (especially minced beef), unpasteurized milk, contaminated water |
| E. coli enteropathogenic, enterotoxigenic, enteroinvasive | 12–72 h (enterotoxigenic) | 3–14 days | Severe colic, watery to profuse diarrhoea, sometimes bloody | Many raw foods, food contaminated by faecal matter, contaminated water |
| Salmonella serovars (non-typhoid) | 6–72 h | 3–5 days | Abdominal pain, diarrhoea, chills, fever, malaise | Raw or undercooked meat and chicken, raw or undercooked eggs and egg products |
| Shigella spp. | 12–96 h | 4–7 days | Malaise, fever, vomiting, diarrhoea (blood and mucus) | Foods contaminated by infected food handlers and untreated water contaminated by human faeces |
| Yersinia enterocolitica | 3–7 days | 1–21 days | Acute diarrhoea sometimes bloody, fever, vomiting | Raw meat, especially pork, raw or undercooked poultry, milk and milk products |
| Vibrio cholerae | A few hours to 5 days | 3–4 days | Asymptomatic to profuse painless watery diarrhoea, dehydration | Raw seafood, contaminated water |
| Vibrio parahaemolyticus | 4–30 h (usually 12–24 h) | 1–7 days | Abdominal pain, diarrhoea, vomiting and sometimes fever Illness of moderate severity | Raw and lightly cooked fish, shellfish, other seafoods |
| Viruses | ||||
| Norovirus (and other viral gastroenteritis) | 24–48 h | 12–60 h | Severe vomiting, diarrhoea | Oysters, clams, foods contaminated by infected food handlers and untreated water contaminated by human faeces |
| Rotaviruses | 24–72 h | Up to 7 days | Malaise, headache, fever, vomiting, diarrhoea | Foods contaminated by infected food handlers and untreated water contaminated by human faeces |
| Parasites | ||||
| Cryptosporidium | 1–12 days | 4–21 days | Profuse watery diarrhoea, abdominal pain | Foods contaminated by infected food handlers and untreated water contaminated by human faeces |
| Giardia lamblia | 1–3 weeks | 1–2 weeks to months | Loose pale greasy stools, abdominal pain | Foods contaminated by infected food handlers and untreated water contaminated by human faeces |
| Entamoeba histolytica | 2–4 weeks | Weeks to months | Colic, mucous or bloody diarrhoea | Foods contaminated by infected food handlers and untreated water contaminated by human faeces |
| Toxin-producing bacteria | ||||
| B. cereus (toxin in food) | 1–6 h (vomiting) or 6–24 h (diarrhoea) | <24 h | Two known toxins causing nausea and vomiting or diarrhoea and cramps | Cereals, rice, meat products, soups, vegetables |
| C. perfringens (toxin in gut) | 6–24 h | 24 h | Sudden onset colic, diarrhoea | Meats, poultry, stews, gravies, (often inadequately reheated or held warm) |
| Staphylococcus aureus (toxin in food) | 30 min–8 h | 24 h | Acute vomiting, and cramps, may lead to collapse | Cold foods (much handled during preparation) milk products, salted meats |
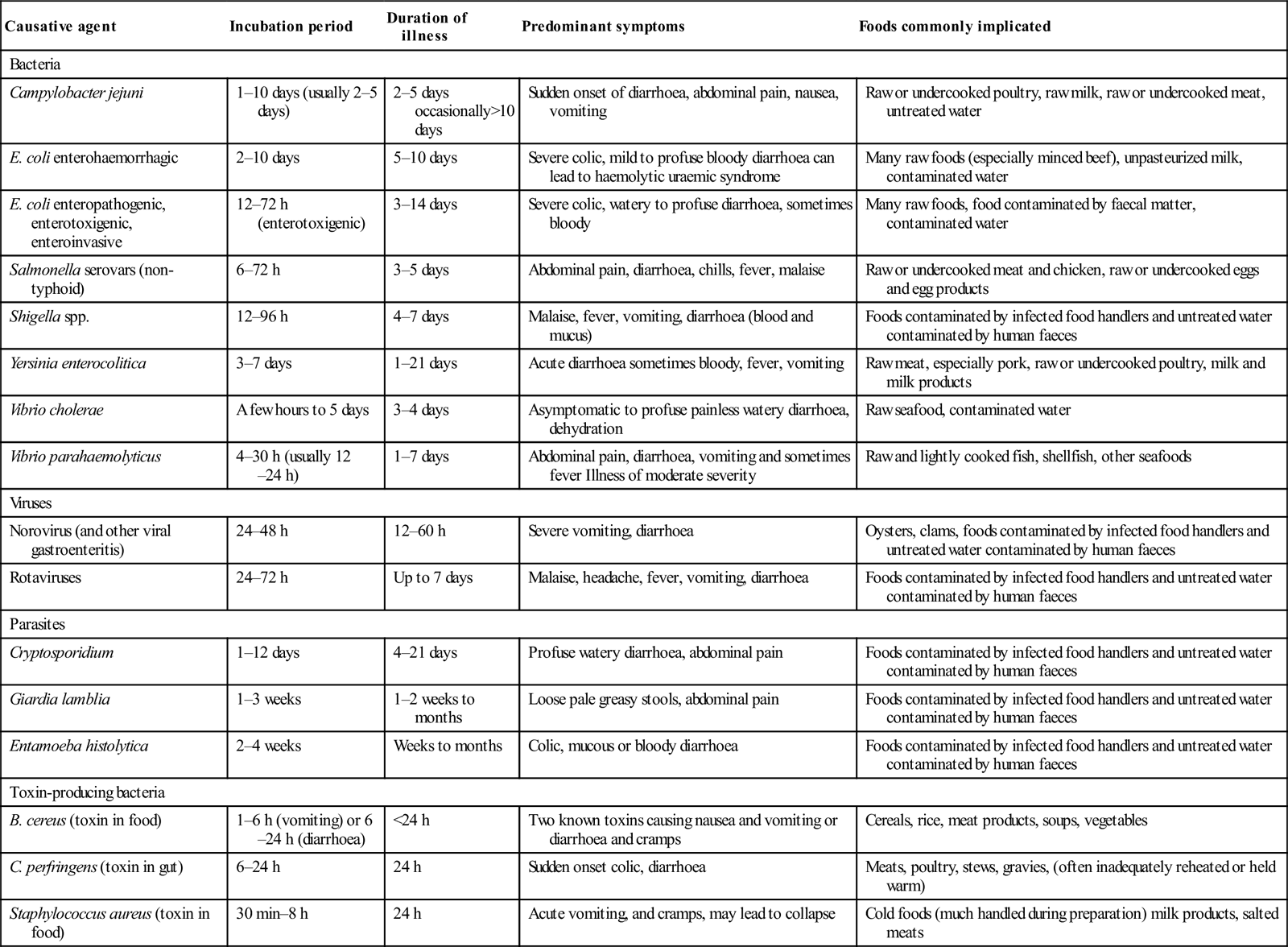
Adapted from Guidelines for the Control of Infectious Diseases – The Blue Book. Communicable Diseases Section, Public Health Group, Victorian Government Department of Human Services; 2005. (Reproduced with the kind permission of the Communicable Diseases Section, Public Health Group, Victorian Government Department of Human Services.)
Diarrhoea in certain circumstances
Traveller’s diarrhoea
Millions of travellers each year are affected by diarrhoea. Southeast Asia, the Middle East, the Mediterranean basin, Central and South America are areas of frequent occurrence. The incidence of diarrhoea in travellers to these areas is as high as 30–50%. Bacteria are the most common cause of traveller’s diarrhoea. Pathogens include enterotoxigenic E. coli, enteroaggregative E. coli, Salmonella, Shigella and Campylobacter. Protozoans, such as Giardia, Cryptosporidium and Entamoeba histolytica, account for 10% of cases. Rotavirus and norovirus are the principal viral pathogens, but account for less than 10% of traveller’s diarrhoea. Many cases do not become symptomatic until after return home. Antibiotic prophylaxis for traveller’s diarrhoea, although effective, is not usually recommended as, in most instances, the illness will be self-limiting.
The immunocompromised patient
Patients with impaired immune (AIDS, IgA deficiency, immunosuppressive therapy following organ transplantation and long-term corticosteroid usage) are not only more susceptible to the common causes of gastroenteritis, but are also vulnerable to the less common organisms, such as Cryptosporidium, Microsporidium, Isospora and Cytomegalovirus. Infections are often more severe, have a higher incidence of complications and may be more resistant to conventional therapy. Isolation of the causative organism and determination of antibiotic sensitivity are essential to guide management.
Hospital-acquired diarrhoea
Clostridium difficile is the most common cause of antibiotic-associated and nosocomial diarrhoea. It may range from a mild disease to life-threatening pseudomembranous colitis and can follow treatment with almost any antibiotic but particularly cephalosporins and clindamycin. Methods of laboratory detection include stool culture, polymerase chain reaction (PCR), cell-culture cytotoxicity assay and enzyme immunoassays. Patients should be treated empirically with oral metronidazole, reserving oral vancomycin for severe disease or subsequent recurrences.
Differential diagnosis
Many pathological conditions, especially early in their course, may present with a clinical picture similar to that of gastroenteritis. Appendicitis, mesenteric adenitis, small bowel ischaemia and inflammatory bowel disease can all present in a similar fashion. Conversely, Campylobacter may cause severe abdominal pain with little diarrhoea and may be misdiagnosed as appendicitis or inflammatory bowel disease. Medical conditions, such as toxic ingestions, diabetic ketoacidosis, hepatitis and pancreatitis, may also present with vomiting, abdominal pain, tenderness and ‘loose’ stools.
Clinical investigations
In most circumstances, no investigations are necessary in order to make the diagnosis of gastroenteritis or to manage the patient effectively.
Identification of the infective agent may be useful when there is an outbreak of gastroenteritis to ensure that adequate public health measures are instituted, in an attempt to limit spread of the disease. Additionally, in a patient who has a persistent illness or clinical features of a specific illness (such as Campylobacter, Giardia or Salmonella), identification of the organism may be helpful in directing antimicrobial therapy or identifying a carrier state. Although the history and examination may give clues as to the aetiological agent, they are unreliable as many similarities exist between the clinical syndromes produced by each organism. Laboratory identification is the only accurate method.
The infective agent may be identified by microscopy and culture of faeces, looking specifically for pathogenic bacteria, cysts, ova or parasites. A fresh specimen of faeces will assist in detection. Occasionally, multiple specimens are required, especially for organisms which may shed into the faeces only sporadically.
Rotavirus infection is detected by looking for rotavirus antigen in the stool by electron microscopy, PCR, enzyme-linked immunosorbent assay (ELISA) or latex agglutination.
If a patient is dehydrated or systemically unwell, a full blood examination, serum electrolyte determination and serum glucose are warranted. In rare cases, where there are signs suggestive of septicaemia or severe systemic illness, blood cultures and liver function tests may be indicated.
Abdominal X-rays are only useful if it is necessary to exclude a bowel obstruction or free intra-abdominal gas.
Treatment
The principles of treatment for gastroenteritis are to replace fluid and electrolyte losses, minimize symptoms if possible and, in selected cases, administer specific antimicrobial therapy. Clear fluids for 24 hours are often recommended, with the rationale that keeping the stomach empty will minimize vomiting. If the patient wishes to eat, it is allowed. Strictly withholding feeding, especially from children, is not necessary.
Replacement of fluid losses may be achieved enterally, either by mouth or via a nasogastric tube or intravenously. The method selected will depend on the cooperation of the patient, the degree of dehydration, the rate at which rehydration is desired and the presence of other diseases, such as diabetes.
Specific oral rehydration solutions are the most appropriate for oral or nasogastric use. There are a number of commercial preparations available through pharmacies without prescription. These consist of a balanced formula of glucose, sodium and potassium salts and, in worldwide trials, have been shown to be extremely effective and safe, even when used in the most primitive of conditions. Although many commonly available fluids may be used and will probably be effective in mild disease, fluids that contain large amounts of glucose, such as degassed lemonade or undiluted fruit juice, should not be encouraged in adults and are contraindicated in children. These fluids are hyperosmolar and deficient in electrolytes, thus promoting further fluid losses. Glucose-containing electrolyte solutions use the gut’s co-transport system for glucose and sodium, thereby facilitating the absorption of water as well. Milk and other lactose-containing products should be avoided during the acute phase of the illness, as viral or bacterial enteropathogens often result in transient lactose malabsorption. Caffeine-containing products should also be avoided as caffeine increases cyclic AMP levels, thereby promoting the secretion of fluid and worsening diarrhoea.
Intravenous rehydration is necessary in patients who are in shock or who are becoming progressively dehydrated despite oral or nasogastric fluids. Resuscitation should be commenced with normal saline at a rate which accounts for ongoing losses, as well as replacing the estimated fluid deficit. In severely dehydrated patients, one or two 20 mL/kg boluses of normal saline may be necessary. Patients should also be encouraged to take oral fluids, unless vomiting is prohibitive. As soon as an adequate intake is achieved, the intravenous fluids can be scaled back and ceased.
Close monitoring of the serum electrolytes is necessary during intravenous rehydration. In particular, it is important to monitor serum sodium, as the exclusive use of normal saline for rehydration can lead to hypernatraemia. Potassium should be added to the fluid as determined by the serum potassium, remembering that low serum potassium in this circumstance is indicative of low total body potassium.
In adults, parenterally administered antiemetic drugs, such as metoclopramide, prochlorperazine or ondansetron, may be useful in the management of severe vomiting. In children, an unacceptably high incidence of dystonic reactions precludes the use of prochlorperazine and metoclopramide. There is growing evidence that oral ondansetron is useful for those children who fail initial oral rehydration therapy and can avoid the need for intravenous fluids. Data in adults are lacking. While antimotility agents, such as loperamide, have been shown to reduce the number of diarrhoeal stools and the duration of the illness, they have significant side effects and should only be used if it is essential.
Even though many bacteria that cause gastroenteritis respond to antibiotics, they are rarely indicated. In the majority of these cases, the illness will be short-lived and mild. Many isolates of Campylobacter jejuni, Shigella and Salmonella are resistant to many antibiotics. Choice of antibiotics should be based on antibiotic sensitivity patterns and local therapeutic guidelines. Antibiotics may be indicated in Giardia infections, Shigella causing severe disease, Salmonella in infants, the immunosuppressed or the elderly, Campylobacter in food handlers and in traveller’s diarrhoea. Antibiotics are contraindicated in uncomplicated Salmonella infections as they may prolong the carrier state. Recommended antibiotic regimens are summarized in Table 7.5.2.
Table 7.5.2
Giardia lamblia
Tinidazole 2 g (child: 50 mg/kg up to 2 g) orally, as a single dose
OR
Metronidazole 2 g (child: 30 mg/kg up to 2 g) orally, daily for 3 days
Amoebiasis
Tinidazole 2 g (child: 50 mg/kg up to 2 g) orally, daily for 3 days
OR
Metronidazole 600 mg (child: 15 mg/kg up to 600 mg) orally, 8-hourly for 7–10 days.
PLUS
Paromycin 500 mg (child: 10 mg/kg up to 500 mg) orally 8-hourly for 7 days (to eradicate cysts and prevent relapse)
Shigellosis
Ciprofloxacin 500 mg (child 12.5 mg/kg up to 500 mg) orally, 12-hourly for 5 days
OR
Norfloxacin 400 mg (child: 10 mg/kg up to 400 mg) orally, 12-hourly for 5 days
OR
Co-trimoxazole 160/800 mg (child: 4/20 mg/kg up to 160/800 mg) orally, 12-hourly for 5 days
Campylobacter
Azithromycin 500 mg (child: 10 mg/kg up to 500 mg) orally, daily for 3 days
OR
Ciprofloxacin 500 mg (child 12.5 mg/kg up to 500 mg) orally, 12-hourly for 3 days
OR
Norfloxacin 400 mg (child: 10 mg/kg up to 400 mg) orally, 12-hourly for 5 days
Clostridium difficile
Metronidazole 400 mg (child: 10 mg/kg up to 400 mg) orally, 8-hourly for 10 days (and cease implicated antibiotic)
For severe disease: vancomycin 125 mg (child: 3 mg/kg up to 125 mg) orally 6-hourly for 10 days
After eTG complete [Internet] Melbourne: Therapeutic Guidelines Limited; 2013 July with permission.
7.6 Haematemesis and melaena
Colin A Graham
Introduction
Upper gastrointestinal bleeding (UGIB) is a common medical emergency with significant morbidity and mortality. Over the last two decades, there have been advances in drug therapy for peptic ulcer disease and varices, improvements in endoscopic techniques, interventional radiology and surgical management, in addition to advances in resuscitation and supportive care. Mortality for patients presenting with UGIB remains around 6–10%, although there is some evidence that mortality has declined in the UK and the USA. Fewer patients (approximately 2%) now require emergency surgery. Patients with UGIB are increasingly elderly and have more co-morbidity than in the past, which may explain the slow improvement in mortality despite the many technical advances in management, particularly endoscopy. Patients now rarely die of exsanguination, but more commonly of multiple organ failure secondary to pre-existing co-morbidities.
Definitions, epidemiology and pathogenesis
Upper gastrointestinal bleeding is defined as any bleeding within the gastrointestinal (GI) tract proximal to the ligament of Treitz. Any bleeding arising distal to that is a lower GI bleed. Haematemesis is the vomiting of bright red blood. ‘Coffee-ground vomiting’ is the vomiting of digested blood clot, whereas melaena is the passage of black, tarry stools as a result of bacterial degradation of haemoglobin within the gut. Melaena usually represents a source of UGIB, but it can rarely occur due to a lower gastrointestinal source of bleeding. Haematochezia is the passage of bright red blood per rectum and, in the context of UGIB, represents a briskly bleeding source of haemorrhage. Melaena of itself is not associated with poorer outcomes in UGIB, but haematochezia due to an upper GI source is associated with double the risk of death.
Peptic ulceration remains the most common cause of UGIB despite the recognition and treatment of Helicobacter pylori infection as a primary cause of peptic ulcer disease (accounting for 36% of cases in a recent UK audit). The pathogenesis of peptic ulcer disease is complex but is closely related to a variety of risk factors, including Helicobacter pylori infection, use of non-steroidal anti-inflammatory drugs (NSAIDs) including aspirin, smoking and alcohol use.
Gastroduodenal erosions and oesophagitis make up a further 15% of cases. Oesophagogastric varices, resulting from portal hypertension, are the source of 11% of episodes of UGIB and up to 20% in patients less than 60 years old. Mallory–Weiss tears, the result of repeated vomiting, reportedly account for less than 5% of cases (although patients with a typical history often do not undergo endoscopy) and usually do not require specific treatment. The remaining causes (all<2%) include vascular lesions, such as angiodysplasia, Dieulafoy’s lesion and aortoenteric fistula.
Prevention
The development of peptic ulcer disease is closely related to management of the risk factors. The effective identification and eradication of H. pylori has led to a significant reduction in the incidence of peptic ulcer disease as the cause of UGIB.
There is little doubt that restricting the prescription of NSAIDs in the elderly (the highest risk group for development of UGIB from NSAIDs and the age group with the highest risk of mortality from UGIB) would prevent a significant number of episodes of UGIB. This is particularly relevant to emergency medicine practice, where NSAIDs are often prescribed as analgesia for musculoskeletal conditions. Care should be taken to prescribe the safest drugs (ibuprofen has the lowest risk profile) for the shortest possible time at the lowest effective dose. If patients are assessed at high risk for possible UGIB, NSAIDs should be avoided or, if unavoidable, a proton pump inhibitor (PPI) or high dose famotidine (40 mg twice daily) should be prescribed with the NSAID to maximize gastric mucosal protection.
Clinical features
It is usually necessary to determine whether the blood loss is from a gastrointestinal source. Blood from the nose or oropharynx can be swallowed, resulting in haematemesis and/or melaena. If bleeding is thought to be from the upper GI tract, then a number of diagnoses need to be considered (see below).
Some historical clues and caveats must be considered:
Clues to the speed or acuity of blood loss include:
The key message is that if there is haemodynamic instability or other evidence of significant ongoing UGIB, fluid resuscitation should continue but arrangements should be made to expedite emergency upper gastrointestinal endoscopy. The accuracy of diagnosis is not important at this stage, but the identification of major ongoing bleeding is.
Severity scores
Over the last decade, several scoring systems have been introduced to assist in the assessment of the severity of UGIB. The best known of these is the Rockall score, which requires endoscopic elements for completion, and the Glasgow–Blatchford score, which utilizes clinical criteria only. The Rockall score appears to be better at determining prognosis, whereas the Glasgow–Blatchford score is very sensitive and can be used to identify patients who may be suitable for outpatient care.
Clinical investigations
Blood tests
Blood should be drawn for full blood count, coagulation studies (INR/PT, APTT and fibrinogen), electrolytes, urea, creatinine, glucose level, liver function tests and urgent cross-matching. The initial haemoglobin is of limited value, as 24–48 hours are required for the intravascular volume to equilibrate. Thrombocytopaenia and leucocytosis are associated with increasing morbidity and mortality. UGIB may also result in an elevation of the urea level (relative to the creatinine), as there is a combination of an increased protein load in the gut and intravascular hypovolaemia. Blood should be taken for blood gas analysis to assess acid–base balance in those with significant bleeds. Venous blood gas analysis is appropriate unless coexisting respiratory failure is suspected. Similarly, a serum lactate level can help to identify patients with clinically occult hypoperfusion who are at high risk of significant haemorrhage.
Imaging
A chest X-ray may be indicated where aspiration is suspected, in the elderly or in patients with cardiopulmonary co-morbidities. It should also be performed if perforation is suspected, however, perforation associated with significant UGIB is rare.
Endoscopy
Although clinical and historical features can point towards the most likely diagnosis, they are not specific. The Rockall score (without the endoscopic components) and the Glasgow–Blatchford score can help predict the need for endoscopy. There is no empirical therapy that effectively treats all causes of UGIB. As a result, a specific endoscopic diagnosis almost always needs to be made. Exceptions may include those with a classic history suggestive of a Mallory–Weiss tear with no ongoing UGIB symptoms and stable haemoglobin and haemodynamic status and the very elderly with major co-morbidity and poor health status (e.g. patients with advanced dementia). Most centres rely on endoscopy to:
Endoscopy should be performed within 24 hours of presentation. Urgent endoscopy should be performed in patients with active or recurrent bleeding, bright red blood on haematemesis, large bleeds (>2 units of blood required) and when variceal bleeding is suspected. However, there is no evidence that early endoscopy (<12 hours) is associated with reduced mortality, although it is associated with a reduced length of hospital stay. Pro-motility agents, such as erythromycin, promote gastric emptying pre-endoscopy, but have not been shown to have any benefits on mortality, need for surgery or length of stay. They are now not routinely recommended in the ED prior to endoscopy.
Treatment
Resuscitation
Continuous ECG monitoring, non-invasive blood pressure monitoring and pulse oximetry should be instituted, with frequent clinical reassessment. Urine output should also be measured and recorded hourly. Invasive arterial and central venous pressure monitoring may be necessary in massive bleeds, intubated patients and those with co-morbidities.
Oxygen should be administered to patients who are hypoxaemic (oxygen saturation<92%) or have evidence of significant ongoing bleeding. Massive ongoing bleeding may compromise the airway to the extent that endotracheal intubation may be required to secure and protect it. Intubation in these circumstances can be both difficult and hazardous and high-volume effective suction is essential. The extent of bleeding is often underestimated and, under these conditions, doses of induction agents should be dramatically reduced from normal levels.
The intravascular volume should then be optimized. The presence of shock (in most studies this was defined as a systolic blood pressure<100 mmHg) places the patient at high risk for re-bleeding, requirement for surgery and death. Note that, in the elderly, patients with autonomic neuropathies (frequently found in diabetics) and those taking β-blockers or calcium channel antagonists, the vital signs, including postural hypotension, may not be a reliable indicator of the degree of blood loss. Propranolol is a commonly used (and effective) prophylaxis for the prevention of variceal bleeding in cirrhotic patients and this may blunt the haemodynamic responses of patients with acute massive variceal bleeding.
Intravascular volume should initially be replaced with isotonic crystalloid (saline or Hartmann’s) or colloid. There is no evidence of superiority for either class of intravenous fluid in UGIB. Blood should be given promptly if there is persistent haemodynamic instability despite 2 L of crystalloid or colloid, if the initial haemoglobin level is<7 mg/dL, if there is a significant risk of re-bleeding and in those patients with co-morbidities making them unable to tolerate periods of anaemia (e.g. chronic obstructive pulmonary disease, coronary artery disease). The thresholds for transfusion have recently been questioned, as early transfusion has been associated with increased mortality in UGIB. However, a higher haemoglobin level (>10 mg/dL) is generally accepted as desirable if there is a history of severe underlying cardiorespiratory disease (e.g. ischaemic heart disease).
Correction of coagulopathy
Transfusion of fresh frozen plasma and platelets should be considered early to prevent and treat coagulopathy associated with massive haemorrhage. Fresh frozen plasma should be given when the prothrombin time is 3 seconds greater than the control or when large transfusions are required. In all patients requiring massive transfusion, attempts should be made to avoid hypothermia by using blood warmers, heating blankets and overhead heaters.
Endoscopy
Although endoscopy is diagnostic for UGIB, it is also therapeutic in the majority of cases and should be performed within 24 hours of admission. It should be carried out without delay when patients remain unstable despite initial fluid and blood product resuscitation. Although many guidelines stress the need for ‘haemodynamic stability’ prior to endoscopy, in cases where this is difficult to achieve, consideration must be given to achieving haemostasis by endoscopic means as part of the ongoing resuscitation process.
Specific therapy
Peptic ulcer disease
Bleeding ceases spontaneously in 80% of cases and the mortality rate is approximately 5–6%, significantly less than with variceal bleeding.
Drug therapy
Haemostasis is known to be a pH-dependent process, so it has been hypothesized that medications that inhibit acid secretion will also reduce the rates of re-bleeding, need for surgery and mortality. The two main drug classes are the histamine (H2) antagonists and the proton pump inhibitors.
H2 antagonists
Most data relating to the benefit of H2 antagonists in acute upper GI bleeds are unconvincing. A large meta-analysis in 2002 reported that H2 antagonists had only modest effects on bleeding gastric ulcers, reducing re-bleeding by 7.2%, surgery by 6.7% and death by 3.2%. There were no effects on bleeding duodenal ulcers. H2 antagonists are not recommended in the contemporary management of UGIB.
Proton pump inhibitors
The PPIs are the most common class of drugs used for peptic disease based on their profound and persistent acid suppression. Current guidelines recommend that PPIs should be given intravenously in high doses after endoscopy to promote ulcer healing and prevent re-bleeding, particularly in patients with high-risk stigmata at endoscopy. High dose oral PPIs may also be used when intravenous administration is not possible and is also effective. Oral doses of PPIs should be at least four times the standard oral dose. Reversible risk factors, such as H. pylori and NSAIDs, should be eliminated where possible. PPIs can be given prior to endoscopy and this recommendation is controversial. Randomized trial data do not show any effect on mortality or emergency surgery but do suggest that ulcer healing may be accelerated.
Somatostatin/octreotide
Studies have found conflicting results in the use of somatostatin and octreotide in peptic ulcer disease. A meta-analysis suggested that there may be a reduction in re-bleeding and the need for surgery in patients with bleeding ulcers, but there was no effect on mortality. Somatostatin and octreotide are no longer recommended in the acute management of peptic ulcer disease.
Endoscopy
Endoscopic therapy is the core of all modern management of UGIB. The ongoing development of new endoscopic techniques for haemostasis means that endoscopy has almost completely replaced surgery as the definitive therapy. Combination therapy using submucosal adrenaline injections combined with cautery or mechanical clips is the best option for ulcers requiring endoscopic treatment.
Surgery
Surgery is required in<2% of patients. It is indicated for continuous or recurrent active bleeding, especially in patients aged over 60, in whom early surgery produces significant benefits in terms of mortality. Other indications include massive blood transfusion, refractory shock and failure to respond to endoscopic therapy. Salvage surgery is associated with poor outcomes and therefore early surgical consultation should be considered, particularly for patients aged over 60 years, those with significant co-morbidities, those with evidence of active bleeding (active bright red haematemesis, haematochezia), when there is a significant risk of re-bleeding or when there is continuing haemodynamic instability. Trans-arterial embolization appears to offer selected patients a good alternative to open surgery when bleeding is not manageable by conventional endoscopic means, although it is rarely used at present. Success rates of up to 69% have been reported, which is comparable to the results of open surgery.
Gastro-oesophageal varices
Although haemorrhage from gastro-oesophageal varices accounts for 2–15% of all UGIB, it represents a significant therapeutic challenge. Bleeding ceases spontaneously in only 20–30%, yet as bleeding is often more severe and recurrent, mortality approaches 25–40% for each episode of variceal haemorrhage. Factors influencing mortality include the stage and rate of deterioration of the underlying liver disease, the presence of co-morbidities, variceal size and specific endoscopic criteria. Patients with known severe varices should be considered for early transfer to a specialist hepatology centre with expertise in dealing with acute massive variceal bleeding.
Drug therapy
Drugs should be used when endoscopic expertise is not available, if massive bleeding prevents immediate sclerotherapy, or as an adjunct to further treatment if continued variceal haemorrhage is suspected. However, it must be emphasized that endoscopic haemostasis procedures are still the mainstay of treatment for varices and endoscopy is required for all cases despite drug therapy.
Somatostatin, octreotide, vasopressin and terlipressin have all been used in this situation. Somatostatin and octreotide therapy produces dramatic reductions in splanchnic arterial blood flow and portal venous pressure, while preserving cardiac output and systemic blood pressure. Treatment results in the control of bleeding in 74–92% of cases, with endoscopic evidence of cessation of bleeding in 68% of patients within 15 minutes. Vasopressin increases peripheral vascular resistance and mean arterial pressure, with reduced cardiac output and coronary blood flow; it is therefore contraindicated in patients with coronary artery disease. Vasopressin results in the control of bleeding in 50–75% of cases. Terlipressin is a synthetic analogue of vasopressin. It can be given by bolus IV injection and has been shown to have a 34% relative risk reduction in mortality from acute variceal haemorrhage and a much lower incidence of side effects than vasopressin. A systematic review in the recent NICE guidelines supports the use of terlipressin as soon as variceal bleeding is suspected or confirmed. Where available, terlipressin (2 mg IV bolus) is therefore recommended for patients with known or highly suspected oesophagogastric varices with UGIB.
There is evidence that patients with oesophageal varices who have chronic liver disease have higher survival rates if given broad-spectrum antibiotics on admission. Intravenous antibiotics should be started early and local advice should be sought on the most appropriate antibiotic for the region where the patient lives. In the absence of local guidance, intravenous cephalosporins or quinolones are a reasonable initial choice.
Endoscopy
Endoscopy is essential to confirm the diagnosis of variceal haemorrhage, as in up to 81% of patients with known varices an alternative bleeding site is found. Endoscopy is also therapeutic in many cases. Endoscopic variceal ligation (EVL) has been shown to be more effective than endoscopic sclerotherapy in the control of variceal haemorrhage, with significantly fewer complications, less re-bleeding and lower mortality; it also requires fewer treatment sessions. Control of bleeding can be achieved subsequently in up to 95% of cases, with a reduction in the risk of re-bleeding. Therefore, EVL should be considered first-line therapy in the control of bleeding from oesophageal varices. Sclerotherapy remains an option if EVL is technically impossible due to massive bleeding at the time of endoscopy.
It is very difficult to perform EVL on gastric varices, so injections of cyanoacrylate are recommended. This should be combined with drug therapy.
Balloon tamponade
Compression of fundal and distal oesophageal varices by balloon tamponade results in control of bleeding in 70–90% of cases. Balloon tamponade may be used as a temporary means of controlling bleeding that is refractory to medical or endoscopic treatment or when bleeding is too massive for endoscopy to be performed successfully.
Because of the problems of pooling of secretions in the oesophagus (thereby increasing the risk of pulmonary aspiration), the standard Sengstaken–Blakemore tube has been modified to incorporate an oesophageal aspiration channel. Further modifications have been made with the Linton–Nachlas tube, which incorporates a single large (600 mL) gastric balloon for the tamponade of gastric varices. The principal use for balloon tamponade now is to act as a bridge to facilitate transfer to a specialist hepatology or endoscopy centre for ongoing care. Endoscopy at the earliest opportunity remains the treatment of choice for UGIB.
There are a number of problems with balloon tamponade:
Transjugular intrahepatic portosystemic stent
Transjugular intrahepatic portosystemic stent-shunt (TIPSS) involves the insertion of a stent under radiological guidance via the jugular vein, forming a portosystemic shunt between the hepatic and portal veins. This technique is effective, achieving control of bleeding in up to 90% of patients and is less invasive and faster to perform (range 30 minutes to 3 hours) than other surgical shunt procedures. However, it requires an experienced operator and often results in complications similar to those seen after other portosystemic shunts, particularly encephalopathy and deteriorating liver function.
The main role of TIPSS, therefore, appears to be in patients who continue to bleed in spite of EVL or sclerotherapy and who do not have hepatic encephalopathy, preterminal liver failure, portal vein thrombosis, intrahepatic sepsis or significant cardiac disease. TIPSS then acts as a bridging procedure until other definitive surgical procedures can be performed (such as liver transplantation, shunt surgery or, rarely, oesophageal transection).
Surgery
Since the advent of EVL and sclerotherapy, the role of surgery in the control of acute variceal bleeding has decreased and it is now largely confined to the small number of patients who continue to bleed despite endoscopic intervention. Shunt surgery and oesophageal transection have been shown to reduce bleeding. However, these techniques require specialist surgical skills and have not been shown to improve survival.
Disposition
The primary decision in most cases is whether the patient is to be admitted to the general ward or to an intensive care (ICU) or high-dependency unit (HDU). Ideally, patients with UGIB should be admitted under the joint care of a gastroenterologist and a surgeon in a specific gastrointestinal bleeding unit.
The main indications for ICU/HDU admission include:
 known or suspected variceal bleeding
known or suspected variceal bleeding
 significant co-morbidities, including cardiac, renal, pulmonary or hepatic dysfunction.
significant co-morbidities, including cardiac, renal, pulmonary or hepatic dysfunction.
The threshold for ICU/HDU admission should be lowered in patients over 60 years of age, owing to the high incidence of co-morbidities and poor physiological compensatory reserve. Lower-risk patients may be admitted to the general ward. The usual length of stay is 2–3 days, as the major risk of re-bleeding is during the first 24–48 hours.
Evidence for outpatient management of upper GI bleeding are less clear. Most UGIB ceases spontaneously and most patients compensate well, not requiring transfusion or surgery. Some authors have suggested outpatient management for selected patients. To minimize the risk of adverse events if the patient is managed as an outpatient, early endoscopy has been advocated. Early discharge is then suggested for those who are found to have clean-based ulcers or non-bleeding Mallory–Weiss tears. In the UK, patients with a score of zero on the Glasgow–Blatchford scoring system have been managed as outpatients, without adverse events. It would be interesting to see the approach used in this single centre study validated elsewhere to determine its applicability in other settings.
Likely developments over the next 5–10 years
7.7 Peptic ulcer disease and gastritis
Win Sen Kuan and Shirley Ooi
Introduction
Peptic (gastroduodenal) ulcers are defects in the gastrointestinal mucosa that extend through the muscularis mucosa. The term ‘gastritis’ is used to denote inflammation associated with mucosal injury. Gastropathy is defined as epithelial cell damage and regeneration without associated inflammation.
The discovery of the organism Helicobacter pylori (H. pylori) has resulted in a dramatic change in our understanding of the aetiology and pathophysiology of peptic ulcer disease. What was once a chronic disease prone to relapse and recurrence has now become eminently treatable and curable.
Patients presenting to emergency departments may do so with ‘classic’ ulcer symptoms, undifferentiated abdominal or chest pain or, more dramatically, with life-threatening complications, such as perforation or haemorrhage.
Aetiology, genetics, pathogenesis and pathology
Aetiology
Peptic ulcer disease is associated with two major factors: H. pylori infection and the consumption of non-steroidal anti-inflammatory drugs (NSAIDs). Smoking is also an important contributory element but does not appear to be a risk factor for H. pylori recurrence or ulcer relapse following eradication of H. pylori.
Gastritis is usually due to infectious agents (such as H. pylori), autoimmune and hypersensitivity reactions. In contrast, gastropathy is usually caused by irritants, such as drugs (e.g. NSAIDs and alcohol), bile reflux, hypovolaemia, ischaemia or chronic congestion.
Genetics
There seems to be a distinct familial aggregation of peptic ulcer disease in pre-H. pylori studies, suggesting a polygenic inheritance of peptic ulcer disease. It remains uncertain if genetic factors predispose to H. pylori infection or whether the genetic factors function independently.
Pathogenesis and pathology
H. pylori disrupts the mucous layer of the gastroduodenal tissue, adheres to the gastric epithelium and releases enzymes and toxins. This causes the underlying mucosa to be susceptible to acid damage and incites inflammatory response by the host.
NSAIDs cause ulcers by inhibiting the production of prostaglandins in the stomach and duodenum. The decreased synthesis of prostaglandins leads to increased amounts of gastric acid being generated, decreased bicarbonate and glutathione production and reduced blood flow to the gastric mucosa. NSAIDs are more commonly associated with gastric ulceration.
Epidemiology
H. pylori infects about 50% of the world’s population. There are significant regional differences in the prevalence of peptic ulcer disease not explained by H. pylori alone, purportedly due to dietary variations. Populations with poor hygiene and low socioeconomic status are predisposed to higher prevalence of H. pylori infection.
The vast majority of patients harbouring H. pylori are asymptomatic. Although decreasing in incidence in developed regions, H. pylori is the major cause of peptic ulceration or, at least, a major cofactor in its development. H. pylori has been isolated from 20 to 50% of patients with dyspeptic symptoms. More importantly, 90–95% of patients with duodenal ulcers and 70% of those with gastric ulcers are infected with the organism. Eradication of H. pylori has been shown to markedly reduce the recurrence rate for ulceration. At least 50% of patients taking NSAIDs will have endoscopic evidence of erythema, erosions or ulcers, even if asymptomatic.
There are several risk factors that influence gastrointestinal toxicity due to NSAIDs, the most important being a prior history of clinical ulcer disease or ulcer complications. Other risk factors are the dose and duration of therapy with NSAIDs, age above 75 years and cardiovascular disease. The risk of peptic ulcer disease is highest on commencement of NSAIDs. Combined therapy of NSAIDs with corticosteroids, anticoagulants, other NSAIDs or low-dose aspirin dramatically increases the risk of ulcer complications.
Some NSAIDs are more likely to produce ulcers than others. In general, shorter-acting agents, such as ibuprofen and diclofenac, are less likely to lead to ulcers than longer-acting agents. Even though cyclooxygenase (COX)-2 selective inhibitors (coxibs) have shown a reduction in the risk of peptic ulcers and their complications compared to traditional NSAIDs, this risk is increased compared with placebo. Studies have shown that the combination of a proton pump inhibitor (PPI) with a coxib decreases the incidence of peptic ulcers. However, there is no evidence that coxibs have advantages over other NSAIDs for patients with unhealed ulcers. Coxibs appear to inhibit healing of peptic ulcers.
The interaction between NSAIDs and H. pylori is controversial and complex but evidence from two meta-analyses of case-controlled trials identified synergism between H. pylori and NSAIDs in producing peptic ulcer and ulcer bleeding. Traditional risk factors, such as smoking, alcohol and stress, may increase the risk of ulceration and delay healing, but their relative importance as aetiological agents has fallen considerably with the discovery of H. pylori. Other causes of peptic ulceration, such as Zollinger–Ellison syndrome, are rare.
Clinical features
History
Peptic ulcers may present with a wide variety of symptoms or may be completely asymptomatic until complications, such as haemorrhage or perforation, occur. ‘Indigestion’ is the most common symptom in patients found to have peptic ulcer disease. Patients classically describe a burning or gnawing pain in the epigastrium that may radiate into the chest or straight through to the back. This may be associated with belching, early satiety, nausea and vomiting. Food may either exacerbate or relieve the pain. The pain is classically both fluctuating and periodic, with bouts of discomfort of variable severity interspersed with symptom-free periods.
The symptoms ‘indigestion’ or ‘dyspepsia’, however, have relatively poor sensitivity and specificity for diagnosing the various peptic syndromes. Less than 25% of patients with dyspepsia have peptic ulcer disease proven by gastroscopy and 20–60% of patients presenting with complications of ulcer disease report no antecedent symptoms.
Other presentations include chest or abdominal pain that need to be differentiated from conditions such as myocardial ischaemia, biliary tract disease, pancreatitis and other abdominal emergencies.
Patients also present with the two most common complications of ulcer disease, namely acute gastrointestinal haemorrhage or acute perforation. The former gives symptoms of melaena with or without haematemesis, and the latter presents with sudden, severe abdominal pain.
Examination
In uncomplicated peptic ulcer disease, abdominal findings may be limited to epigastric tenderness without peritoneal signs. If perforation has occurred, patients experience severe pain and look unwell. Abdominal findings include generalized tenderness, widespread peritonism and so-called ‘board-like’ rigidity. Those with gastrointestinal bleeding will usually have melaena on per rectal examination.
Differential diagnosis
The differential diagnosis of upper abdominal pain is broad. Functional (idiopathic, non-ulcer) dyspepsia is the commonest (up to 60%) and the diagnosis is one of exclusion. Other important differential diagnoses include gastric, oesophageal or pancreatic cancer, pancreatitis, biliary tract disease, gastro-oesophageal reflux disease, ischaemic bowel disease and metabolic diseases, such as hypercalcaemia and hyperkalaemia.
Clinical investigations
The extent of investigations depends greatly on the patient’s presentation and the degree of severity of symptoms. There are no blood tests that can reliably predict the presence of peptic ulcer disease. Pathology investigations are aimed primarily at eliminating alternative diagnoses or identifying the complications of peptic ulceration.
Full blood examination
Anaemia is most likely to represent chronic rather than acute blood loss, unless bleeding is particularly heavy and hence clinically obvious. A microcytic, hypochromic anaemia suggests chronic blood loss with iron deficiency and can be confirmed with iron studies. Unexplained anaemia warrants a detailed evaluation and may raise concern for an underlying malignancy.
Blood cross-match
Patients with active bleeding may need replacement with blood products. Several units of blood may be required.
Clotting studies
These are indicated in patients taking anticoagulants and those with massive bleeding and/or a history of liver disease or alcoholism.
Liver function tests/amylase/lipase
Biliary tract disease and pancreatitis are important differential diagnoses in patients presenting with upper abdominal pain. Pancreatitis may also be the consequence of ulcer penetration through the posterior wall of the stomach.
Radiology
Radiological imaging has a very limited place in the diagnosis of uncomplicated peptic ulcer disease. However, an erect chest X-ray (CXR) is an important investigation when perforation is being considered. Gas is usually visible under the diaphragm, but its absence does not rule out perforation with sensitivity of erect CXR for detection of pneumoperitoneum ranging from 70 to 80%. Upright lateral CXR has been shown to be more sensitive than posterior-anterior CXR in detecting pneumoperitoneum. Lateral decubitus abdominal X-rays may be needed to demonstrate free gas in those unable to sit erect. Computed tomography (CT) scans of the abdomen are regarded as the criterion standard in detecting small pneumoperitonea.
Contrast studies are no longer considered first-line investigations in the assessment of patients with dyspeptic symptoms. Abdominal X-ray and ultrasound studies are useful to exclude alternative diagnoses, as indicated.
Criteria for diagnosis
Endoscopy
Endoscopy is the investigation of choice. It allows direct visualization of the mucosa of the oesophagus, stomach and proximal duodenum. It provides a definitive diagnosis which forms the basis of drug therapy and allows biopsies to be taken to exclude malignant disease and to isolate H. pylori. Endoscopic intervention may also be therapeutic in some cases of upper gastrointestinal haemorrhage (E-Fig. 7.7.1).
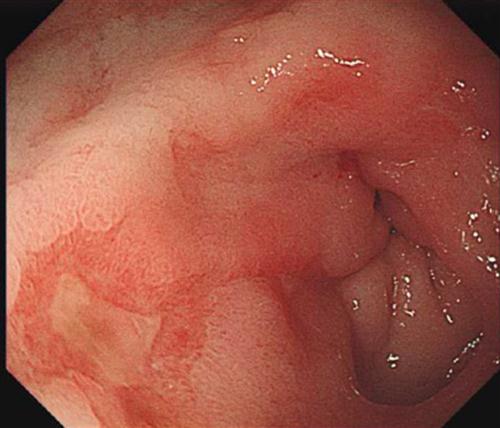
H. pylori status
Currently, there are a number of tests available, both invasive (endoscopic) and non-invasive, though their exact role in the emergency department (ED) setting has not been defined. It should be remembered that the majority of patients infected with H. pylori do not in fact have peptic ulcer disease and that the identification of H. pylori infection often bears little relation to presenting symptoms. Non-invasive tests can only make a diagnosis of H. pylori infection, not of peptic ulcer disease. A negative test in a patient not taking NSAIDs makes the likelihood of peptic ulcer disease low.
The invasive tests for H. pylori include haematoxylin and eosin staining of mucosal biopsies and rapid urease tests (e.g. CLO test). The non-invasive tests include urea breath tests and IgG serology. Urea breath tests are highly sensitive and specific for the presence of H. pylori. They are most useful in assessing H. pylori eradication without the need for further gastroscopy. The urea breath test should be done early as an important limitation is its decreased sensitivity with prolonged antisecretory therapy.
A number of IgG serology tests are available with varying specificities and sensitivities. They are inexpensive, non-invasive and well suited to primary care and, potentially, emergency medicine practice. Large studies have found uniformly high sensitivity (90–100%), but variable specificity (76–96%); the accuracy has ranged from 83 to 98%.
Treatment
The treatment of peptic ulcer disease depends on the underlying cause and clinical presentation. Traditional management of patients with dyspeptic symptoms requires the exclusion of other diseases, the removal of known precipitants, such as NSAIDs, alcohol and cigarettes, the institution of simple treatment measures aimed at symptomatic relief and referral for further investigation and management.
Cost-effectiveness analysis and consensus statements support the treatment of H. pylori-positive dyspeptic patients with antimicrobial and antisecretory therapy, followed by endoscopic study only in those with persistent symptoms, so it would also be reasonable to begin symptomatic therapy, order serological testing for H. pylori and refer for early follow-up with a primary care provider for initiation of antibacterial therapy if the test results are positive.
The choice of approach is open to debate. Early treatment prior to endoscopy may cure some patients without the need for expensive invasive procedures. However, this plan of action may hinder subsequent H. pylori isolation and delay definitive diagnosis, including the diagnosis of malignant disease.
It should be noted that the prevalence of H. pylori is lower in patients with complicated duodenal ulcers (those complicated by bleeding or perforation) than in those with uncomplicated disease. Patients with H. pylori-negative ulcers appear to have a significantly worse outcome, especially if treated empirically for infection. Thus, documenting infection is important prior to initiating antimicrobial therapy.
For patients with mild symptoms of recent onset, empirical treatment with antacids and/or histamine receptor antagonists aimed at symptomatic relief is reasonable. Review of the literature concluded that for patients with non-ulcer dyspepsia, H2-receptor blockers were significantly more effective than placebo at reducing symptoms, whereas proton pump inhibitors and bismuth salts were only marginally so. Antacids and sucralfate were not statistically superior to placebo.
Given the poor correlation between dyspeptic symptoms and gastro-oesophageal disease, gastroscopy should be considered, particularly if symptoms are not controlled or promptly recur. Early endoscopy has been advocated in patients above 45 years of age presenting with alarm symptoms, such as dysphagia, recurrent vomiting, weight loss or bleeding.
Antacids
‘Antacids’ containing combinations of calcium, magnesium, local anaesthetics and alginates are useful in providing symptomatic relief for patients with relatively mild symptoms. In many instances, patients have already tried these agents prior to presentation. Relief of symptoms with antacids are, however, not a diagnostic indicator.
Histamine-receptor antagonists
The H2-receptor antagonists, such as cimetidine, ranitidine, famotidine and nizatidine, all have similar efficacies with regard to ulcer healing. All are well absorbed orally, but their absorption may be reduced when used with antacids but not by food. Eighty to 90% of duodenal ulcers will be healed in 4–8 weeks and 70% of gastric ulcers within 8 weeks. Relapse rates of 80% over the course of 1 year are to be expected if H. pylori eradication is not also undertaken in appropriate cases. H2 antagonists are also useful in the treatment of gastro-oesophageal reflux disease and management of dyspepsia. Due to renal excretion, dosage adjustments must be made in patients with renal dysfunction.
Proton pump inhibitors
The PPIs, omeprazole, lansoprazole, rabeprazole, pantoprazole, dexlansoprazole and esomeprazole, effectively block acid secretion by irreversibly binding to and inhibiting the H+/K+ATPase pump of the gastric parietal cells, thereby inhibiting the cells’ proton pump. Acidic compartments within the stimulated parietal cell are essential for activation of a PPI. Thus, PPIs work poorly in fasting patients or those with simultaneous dosing with other antisecretory agents (H2-receptor antagonists, anticholinergic agents or somatostatin). PPIs are most effective when taken with or shortly before meals. Compared to H2-receptor antagonists, these agents result in more rapid ulcer healing and pain relief over 2–4 weeks, although differences at 8 weeks are not significant. Again, relapse rates are high, particularly if H. pylori is present and eradication therapy is not used.
Cytoprotectants
Cytoprotective agents include colloidal bismuth subcitrate (De-Nol) and sucralfate. Both act by binding to or chelating with proteins in the base of the ulcer. Bismuth compounds also suppress H. pylori. A 6–8-week course is recommended and relapse rates are high. Bismuth compounds lead to the formation of black stools that may be confused with melaena. The primary concern with bismuth is bismuth intoxication. Sucralfate should not be taken with antacids as it requires an acid environment to achieve its optimal effects. Sucralfate has minimal adverse effects other than possible aluminium toxicity.
Prostaglandin analogues
Misoprostol (a synthetic analogue of PGE1) interferes with histamine-dependent gastric acid secretion as well as being cytoprotective. It is particularly useful in the prevention of NSAID-induced ulcers, although it is probably no better than the other agents in actually treating such ulcers.
Misoprostol significantly reduces the risk of endoscopic ulcers. Standard doses of H2-blockers were effective at reducing the risk of duodenal but not gastric ulcers. Double-dose H2-blockers and proton pump inhibitors were effective at reducing the risk of both duodenal and gastric ulcers and were better tolerated than misoprostol.
H. pylori eradication
All patients with duodenal ulcers associated with H. pylori infection should undergo therapy to eradicate the organism. This recommendation is based on overwhelming data showing that cure of H. pylori infection reduces ulcer recurrence and complications, such as bleeding. A number of eradication therapies have been postulated, all with very high eradication (>80%) and low relapse rates (<5%). The development of resistance to metronidazole has resulted in amoxicillin and clarithromycin being recommended as the antibiotics of choice. These are usually combined with a proton pump inhibitor or colloidal bismuth subcitrate for 1 week. Several single-prescription packages are now available. It is generally accepted that acid suppression therapy be continued for 6–8 weeks after cessation of antibiotic therapy.
H. pylori eradication therapy in patients with non-ulcer dyspepsia may have a small yet statistically significant effect on symptoms.
Treatment of NSAID-induced ulcers
The American College of Gastroenterology issued a guideline in 2009 for the prevention of NSAID-related ulcer complications. It recommends that all patients who are to commence long-term NSAID therapy should first be tested for H. pylori. Those tested positive for H. pylori should discontinue NSAID use where clinically feasible and undergo H. pylori eradication therapy. Patients who are at moderate risk of peptic ulcer complications and high risk of cardiovascular disease should avoid NSAIDs or COX-2 inhibitors entirely and receive alternative therapy. Treatment of NSAID-induced ulcers should consist of a 4–8 week course of an H2-receptor antagonist or PPI.
Surgical management
With the success of medical treatment for peptic ulcer disease, surgical intervention has been restricted to the management of complications rather than of the primary disease.
Complications
Haemorrhage
Peptic ulceration is a common cause of upper GI bleeding, occurring in 10–20% of ulcer patients and accounting for approximately 50% of all upper GI bleeds. Urgent endoscopy is usually indicated. Surgical intervention may be required in a small proportion of patients. A meta-analysis concluded that the use of acid-reducing agents was associated with a statistically significant decrease in re-bleeding, but not mortality. Assessment and management of haematemesis and melaena is discussed in detail in Chapter 7.6.
Perforation
Perforation occurs in approximately 5% of ulcers, with duodenal, antral and gastric body ulcers accounting for 60%, 20% and 20% of perforations, respectively. One-third to one-half of perforated ulcers are associated with NSAID use; these usually occur in elderly patients. Chemical peritonitis develops suddenly, with acute severe generalized abdominal pain. Examination reveals a sick patient with a rigid, quiet abdomen and rebound tenderness. Delay in presentation and treatment, which may occur in the elderly and debilitated, sees the rapid development of bacterial peritonitis and subsequent sepsis and shock. The overall mortality rate is about 5%.
Rapid diagnosis is essential as the prognosis is excellent if treated within the first 6 hours, but deteriorates to probable death after more than a 12-hour delay. Diagnosis should be confirmed with an erect chest X-ray, bearing in mind its sensitivity of 70–80%. If free air is found, no other diagnostic studies are necessary. If there is diagnostic uncertainty, CT or ultrasound can be useful to detect small amounts of free air or fluid.
Vigorous fluid resuscitation should be instituted and renal function (via urine output) should be closely monitored. Initial empiric antibiotic therapy consisting of a combination of beta-lactam/beta-lactamase inhibitor (e.g. ampicillin–sulbactam) or third-generation cephalosporin (e.g. ceftriaxone) and metronidazole should be given, along with adequate analgesia. Cardiac and respiratory support may be needed in some cases.
As the standard of care, patients with perforation should undergo surgery for decontamination and repair (e.g. Graham patch) after resuscitation. Non-operative management, including intravenous fluids, nasogastric suction, antibiotics and antisecretory drugs, may be successful in some patients in whom the leak seals quickly in response to medical management. It may also be considered in patients who have severe co-morbidities precluding surgery and those with delayed presentations. There is some evidence that an initial period of non-operative treatment with careful observation is safe in younger patients (age under 60 years), but this is not regarded as standard practice.
Penetration
Posterior ulcers may perforate the gastric or duodenal wall and continue to erode into adjacent structures, most commonly the pancreas, without free perforation and leakage of luminal contents into the peritoneal cavity. Patients may describe their pain as becoming more severe and constant, radiating to the back and no longer eased by antacids and food. There is also loss of cyclicity of pain with meals. The serum amylase level may be mildly raised but clinical pancreatitis is not common. Endoscopy may reveal ulceration, but ‘penetration’ is difficult to confirm.
Gastric outlet obstruction
This is the least frequent complication and may occur in up to 2% of patients with ulcer disease. It may arise acutely secondary to inflammation and oedema of the pylorus or duodenal bulb or, more commonly, as a consequence of scarring due to chronic disease.
Prognosis
The prognosis for peptic ulcer disease is excellent when the underlying cause is identified and treated. The mortality rate is approximately 1 death per 100 000 cases, which is a modest decrease from a few decades ago, contributed mainly by an improved mortality rate from bleeding peptic ulcers using intravenous PPIs after endoscopic therapy.
Poor prognostic factors for peptic ulcer perforation include shock at the time of admission, presence of renal impairment, delayed presentation for more than 12 hours, age over 70 years, liver cirrhosis, immunocompromised state and perforated gastric ulcer (twice the mortality of perforated duodenal ulcer).
Disposition
Patients without complications can usually be managed as outpatients.
Likely developments over the next 5–10 years
7.8 Biliary tract disease
Stacy Turner and Andrew Walby
Introduction
Biliary tract disease is common and the vast majority of disease is related to gallstones. Stones may cause acute or chronic cholecystitis, acute biliary pain (biliary colic), pancreatitis, cholangitis or obstructive jaundice. Acute biliary pain is the most common presentation, caused by a gallstone impacting in the cystic duct. The second most common presentation is acute cholecystitis, caused by distension of the gallbladder with subsequent necrosis and ischaemia of the mucosal wall. Other diseases of the biliary tree include tumours and acalculous cholecystitis, which occurs in the absence of gallstones and often complicates critical illness.
Gallbladder disease is diagnosed by a combination of clinical features, laboratory investigations and imaging.
Gallstones and acute biliary pain
Aetiology, genetics, pathogenesis and pathology
Most biliary pathology is secondary to gallstones. Eighty per cent of gallstones in the Western world are composed primarily of cholesterol, but stones may also be formed from bile pigment (due to haemolysis) or may be of mixed origin. These components precipitate out to form crystals when bile is concentrated in the gallbladder. The crystals, if trapped in the gallbladder mucus, can grow, producing gallbladder sludge then stones. Symptoms occur when the gallbladder contracts, often after a meal, resulting in occlusion of the cystic duct by a stone, causing visceral pain (biliary colic). On relaxation of the gallbladder, the stone falls back into the gallbladder and symptoms subside. More prolonged gallbladder outlet obstruction leads to acute cholecystitis. Gallbladder distension and increased intraluminal pressure lead to inflammation, ischaemia and subsequent necrosis of the mucosal wall. Infection is not thought to play an initial part in the development of acute cholecystitis, but secondary infection may occur in up to 50% of cases. The main difference between acute cholecystitis and biliary colic is the inflammatory component, leading to ongoing pain, fever, localized peritonism and raised white cell count (WCC). Secondary bacterial infection is usually caused by aerobic bowel flora (such as Escherichia coli, Klebsiella species and, less commonly, Enterococcus faecalis). Anaerobes are found infrequently, usually in the presence of obstruction.
Cholangitis requires the presence of two factors: biliary obstruction and infection.
Epidemiology
Around 10–15% of Western adults have gallstones (cholelithiasis). Stones are less common in African and Asian populations. In young adults, four times more females are affected than males, but the disparity narrows with age. The lifetime risk of gallstones is 35% in women and 20% in men. In women, the risk is increased further during and after pregnancy and with oral contraceptive use. This is likely to be due to endogenous sex hormones that enhance cholesterol secretion and increase bile cholesterol saturation.
Other risk factors for the development of gallstones include increasing age, diabetes, obesity, rapid weight loss, drugs (most notably exogenous oestrogens, octreotide, clofibrate and ceftriaxone), genetic predisposition, diseases of the terminal ileum and abnormal lipid profile.
Two-thirds of gallstones are asymptomatic. Gallstones may be present for decades before symptoms develop. Asymptomatic patients become symptomatic at a rate of 1–4% per year but the risk decreases with time. Risk factors for stones becoming symptomatic are smoking, pregnancy and obesity. Stones may cause acute or chronic cholecystitis, acute biliary pain (biliary colic), pancreatitis or obstructive jaundice. Biliary colic is the most common presentation (56%), followed by acute cholecystitis (36%), obstructive pancreatitis and cholangitis. Less common presentations include empyema, perforation, fistula formation, gallstone ileus, hydrops or mucocele of the gallbladder and carcinoma of the gallbladder.
Prevention
Many of the risk factors for gallstones, such as age and gender, are fixed. There is limited evidence to support preventative strategies but maintaining a healthy weight and following a low-fat, high-fibre diet may reduce the risk. Those on long-term statins also appear to be protected from gallstones. Ursodeoxycholic acid is useful in preventing high-risk patients (e.g. morbidly obese patients undergoing rapid weight loss following bariatric surgery) from developing gallstones. However, ursodeoxycholic acid has no effect on reduction of symptoms once stones have formed.
Clinical features
History
The pain of biliary colic characteristically starts suddenly in the epigastrium or right upper quadrant (RUQ) and may radiate round to the interscapular region of the back. Despite the use of the term biliary colic, pain is usually constant. Pain develops in the hours after a meal, most commonly starting at night, waking the patient from sleep and usually lasting from 1 to 5 hours, subsiding spontaneously or with analgesics. Ongoing pain suggests cholecystitis. Nausea and vomiting are often present. Complaints of fevers and chills may be indicative of either cholecystitis or cholangitis. Rigors are suggestive of cholangitis.
Examination
RUQ tenderness is the most common examination finding. Patients with biliary colic have relatively normal vital signs. Significant fever is uncommon.
Jaundice is usually absent. Its presence suggests cholangitis or obstruction of the common bile duct (CBD). The presence of pain, jaundice and high fever with rigors (Charcot’s triad) is indicative of cholangitis.
Differential diagnosis
The differential diagnosis of RUQ pain includes:
 peptic ulcer disease, including perforation
peptic ulcer disease, including perforation
 coronary ischaemia, especially involving the inferior myocardial surface
coronary ischaemia, especially involving the inferior myocardial surface
 appendicitis, especially retrocaecal or in pregnancy
appendicitis, especially retrocaecal or in pregnancy
 renal disease, including renal colic and pyelonephritis
renal disease, including renal colic and pyelonephritis
 colonic pathology, such as irritable bowel syndrome
colonic pathology, such as irritable bowel syndrome
Clinical investigations
Investigations in biliary pain are aimed at confirming the diagnosis, establishing the presence of gallstones and the detection of complications.
Imaging
Ultrasound is the investigation of choice and can be used to confirm the presence of gallstones, measure the thickness of the gallbladder wall and the diameter of the CBD and detect the presence of any local fluid collection. On ultrasonography, gallstones appear as echogenic foci that cast an acoustic shadow, are usually mobile and gravitationally dependent. Ultrasound has high sensitivity and specificity (84% and 99%, respectively) for the detection of gallstones, is non-invasive and requires little preparation of the patient. However, ultrasound is not as good at visualizing stones in the CBD, identifying about half. Also, it is operator dependent, but bedside ultrasound examination has satisfactory diagnostic capability.
Abdominal X-ray. In the majority of cases, plain radiographs are not helpful in the diagnosis of gallbladder disease. On occasion, they may be useful to rule out other potential diagnoses but only 10% of biliary calculi are visible on plain radiographs.
Computed tomography (CT) should not be used as a first-line test. It is not sensitive for detecting gallstones, but is useful in diagnosing acute cholecystitis and in patients with complicated disease. CT may better demonstrate dilatation of the bile duct and pneumobilia, gangrene and perforation. In non-specific abdominal pain, it can detect acute cholecystitis and identify extrabiliary disorders.
Blood tests
In acute biliary pain, blood tests are non-specific. Bilirubin and alkaline phosphatase levels should be normal. In acute cholecystitis, mild derangement may be seen in around 25% of cases. Amylase/lipase assays are useful to evaluate the presence of pancreatitis. Amylase may also be elevated mildly in cholecystitis. Full blood examination shows a leucocytosis and left shift in the majority of cases of cholecystitis and cholangitis; however, up to 40% of patients do not have a leucocytosis at the time of presentation.
Complications