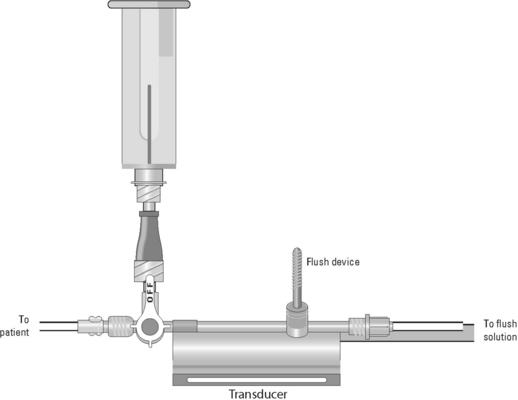
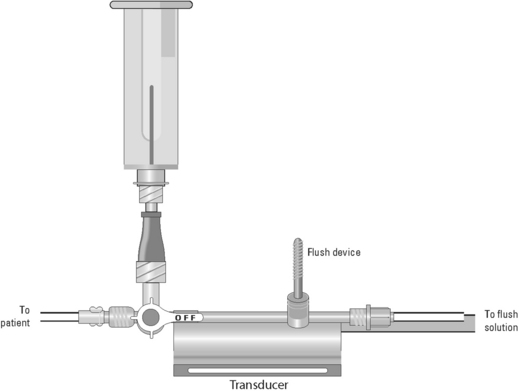
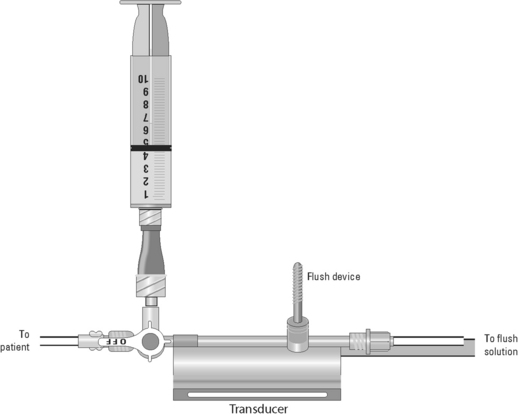
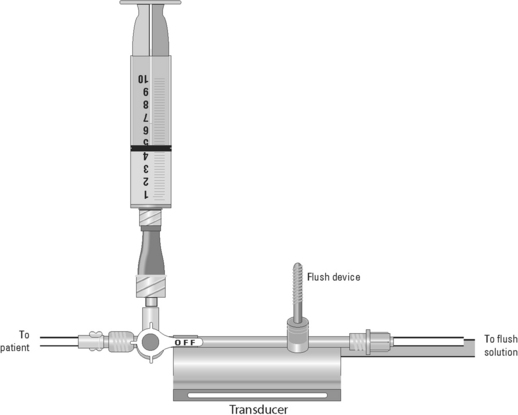
Blood Sampling from a Central Venous Catheter
PREREQUISITE NURSING KNOWLEDGE
• Knowledge of anatomy and physiology of the cardiovascular system is needed.
• Understanding of principles of sterile and aseptic technique and infection control is necessary.
• The technique for specimen collection and labeling should be understood.
• Signs and symptoms of catheter-related infection and sepsis should be known.
• Infection has been identified as a potentially life-threatening complication of central venous catheterization, with an associated estimated mortality rate of 10% to 25% for each infection.4,6,10,12
• Knowledge of strategies to prevent catheter-related infections is essential.7,8,11
• Knowledge regarding the care of patients with central venous catheters (CVCs) is needed (see Procedure 82).
• Understanding of the principles of hemodynamic monitoring is necessary.
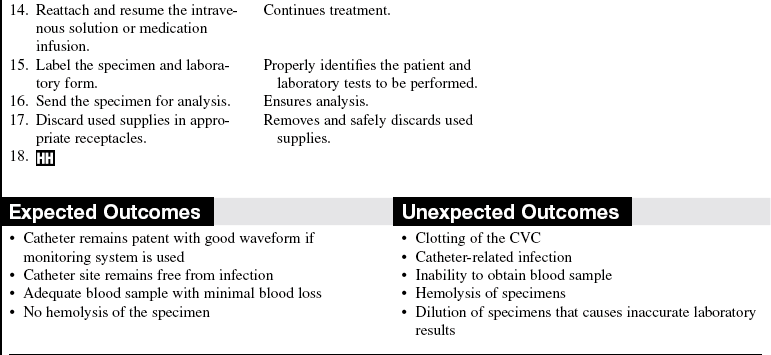
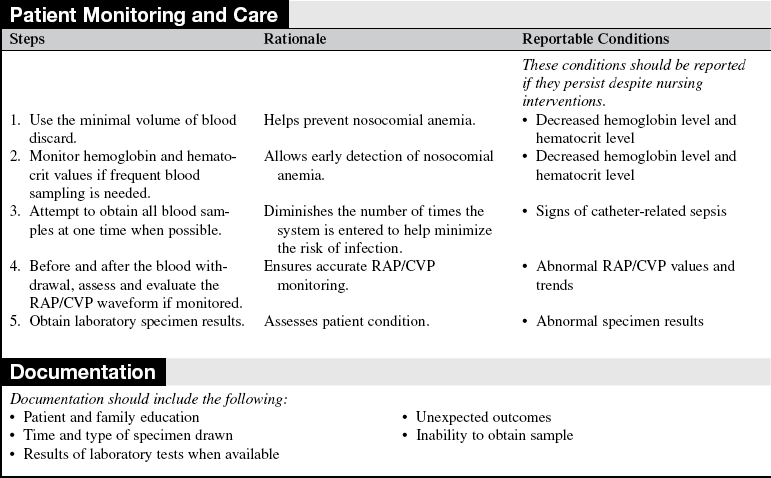
• The effect of heparin and hemolysis on various blood tests and appropriate discard volumes should be understood.
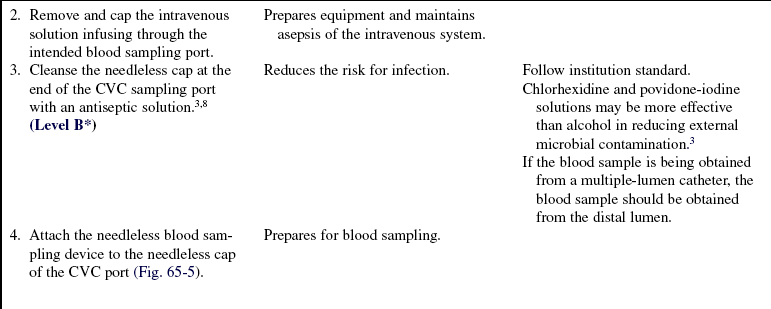
• Although blood can be withdrawn from CVCs, alternate routes of blood withdrawal (e.g., venipuncture, arterial catheters) should be considered first to minimize the risk of central venous catheter-related bloodstream infections.
• A needleless system should be used for capping and accessing CVC ports. Study results show that needleless systems not only reduce needle-stick injuries and the resultant risk for transmission of blood-borne infection to healthcare workers8 but also may reduce central venous catheter-related bloodstream infections.1,3
EQUIPMENT
• Goggles or fluid shield face mask
• Antiseptic solution (e.g., 2% chlorexidine-based solution)
• Needleless blood sampling access device
• Sterile normal saline solution for injection
• Extra blood specimen tube for discard
• Laboratory form and patient identification specimen labels
Additional equipment to have available as needed includes the following:
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
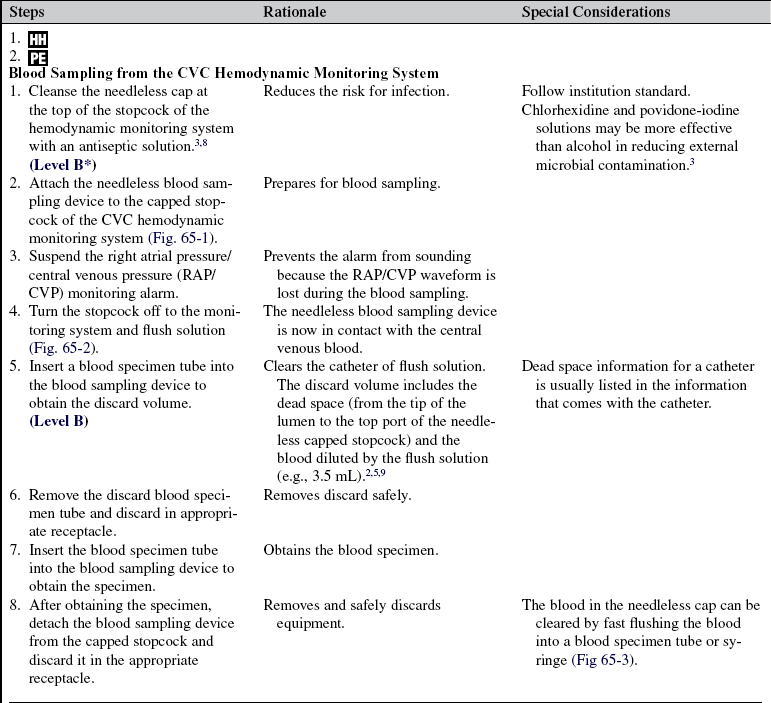
• Assess the patency of the CVC.  Rationale: This ensures a functional CVC catheter. If the CVC is clotted, blood sampling cannot be performed.
Rationale: This ensures a functional CVC catheter. If the CVC is clotted, blood sampling cannot be performed.
• Assess previous laboratory results.  Rationale: These results provide baseline data andv data for comparison.
Rationale: These results provide baseline data andv data for comparison.
• Assess whether intravenous solutions or medications are infusing through the CVC.  Rationale: Intravenous solutions and medication need to be stopped temporarily before blood sampling.
Rationale: Intravenous solutions and medication need to be stopped temporarily before blood sampling.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that the patient and family understand preprocedural teaching. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Position the patient so that the blood sampling port is exposed.  Rationale: This positioning improves the ease of obtaining the blood sample and minimizes the contamination of the stopcock.
Rationale: This positioning improves the ease of obtaining the blood sample and minimizes the contamination of the stopcock.
References
![]() 1. Bouza, E, et al, A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization. a prospective randomized study. J Hosp -Infection 2003; 54:279–287.
1. Bouza, E, et al, A needleless closed system device (CLAVE) protects from intravascular catheter tip and hub colonization. a prospective randomized study. J Hosp -Infection 2003; 54:279–287.
![]() 2. Carlson, KK, et al. Obtaining reliable plasma sodium and glucose determinations from pulmonary artery catheters. Heart Lung. 1990; 19:613–619.
2. Carlson, KK, et al. Obtaining reliable plasma sodium and glucose determinations from pulmonary artery catheters. Heart Lung. 1990; 19:613–619.
![]() 3. Casey, AL, et al. A randomized, prospective clinical trial to assess the potential infection risk associated with the PosiFlow needleless connector. J Hosp Infection. 2003; 54:288–293.
3. Casey, AL, et al. A randomized, prospective clinical trial to assess the potential infection risk associated with the PosiFlow needleless connector. J Hosp Infection. 2003; 54:288–293.
![]() 4. Kluger, DM, Maki, DG. The relative risk of intravascular device related bloodstream infections in adults [abstract]. In: In Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: American Society for Microbiology; 1999:514.
4. Kluger, DM, Maki, DG. The relative risk of intravascular device related bloodstream infections in adults [abstract]. In: In Abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: American Society for Microbiology; 1999:514.
![]() 5. Krueger, KE, et al. The reliability of laboratory data from blood samples collected through pulmonary artery catheters. Arch Pathol Lab Med. 1981; 105:343–344.
5. Krueger, KE, et al. The reliability of laboratory data from blood samples collected through pulmonary artery catheters. Arch Pathol Lab Med. 1981; 105:343–344.
![]() 6. Maki, DG. Pathogenesis, prevention and management of infections due to intravascular devices used for infusion therapy. In: Bison AL, Waldvogel F, eds. Infections -associated with indwelling medical devices. Washington, DC: American Society for Microbiology; 1989:161–177.
6. Maki, DG. Pathogenesis, prevention and management of infections due to intravascular devices used for infusion therapy. In: Bison AL, Waldvogel F, eds. Infections -associated with indwelling medical devices. Washington, DC: American Society for Microbiology; 1989:161–177.
![]() 7. Mermel, LA, Prevention of central venous catheter–related infections. what works other than impregnated or coated catheters. J Hosp Infection. 2007; 65(Supp 2):30–33.
7. Mermel, LA, Prevention of central venous catheter–related infections. what works other than impregnated or coated catheters. J Hosp Infection. 2007; 65(Supp 2):30–33.
![]() 8. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30:476–489.
8. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30:476–489.
![]() 9. Palermo, LM, Andrews, RW, Ellison, N. Avoidance of heparin contamination in coagulation studies drawn from indwelling lines. Anesth Analg. 1980; 59:222–224.
9. Palermo, LM, Andrews, RW, Ellison, N. Avoidance of heparin contamination in coagulation studies drawn from indwelling lines. Anesth Analg. 1980; 59:222–224.
![]() 10. Pittet, D, Tarara, D, Wenzel, RP, Noscomial bloodstream infection in critically ill patients. excess length of stay, extra costs and attributable mortality. JAMA 1994; 271:1598–1601.
10. Pittet, D, Tarara, D, Wenzel, RP, Noscomial bloodstream infection in critically ill patients. excess length of stay, extra costs and attributable mortality. JAMA 1994; 271:1598–1601.
![]() 11. Safdar, N, Kluger, DM, Maki, DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters. Medicine. 2002; 81:466–479.
11. Safdar, N, Kluger, DM, Maki, DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters. Medicine. 2002; 81:466–479.
![]() 12. Smith, RL, Meixler, SM, Simberkoff, MS. Excess mortality in critically ill patients with nosocomial bloodstream infections. Chest. 1991; 100:164–167.
12. Smith, RL, Meixler, SM, Simberkoff, MS. Excess mortality in critically ill patients with nosocomial bloodstream infections. Chest. 1991; 100:164–167.
Beutz, M, et al. Clinical utility of blood cultures drawn from central vein catheters and peripheral venipuncture in critically ill medical patients. Chest. 2003; 123:854–861.
Maki, DG, et al, The risk of bloodstream infection in adults with different intravascular devices. a systematic review of 200 published prospective studies. Mayo Clin Proc 2006; 81:1159–1171.
Shapey, IM, et al, Central venous catheter-related bloodstream infections. improving post-insertion catheter care. J Hosp Infection 2009; 71:117–122.

 Rationale: Teaching provides information and decreases anxiety and fear.
Rationale: Teaching provides information and decreases anxiety and fear. Rationale: This explanation increases patient cooperation and assistance.
Rationale: This explanation increases patient cooperation and assistance.