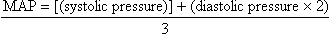Arterial Pressure-Based Cardiac Output Monitoring
PREREQUISITE NURSING KNOWLEDGE
• Knowledge of the anatomy and physiology of the cardiovascular system is necessary.
• Knowledge of the anatomy and physiology of the vasculature and adjacent structures is needed.
• Understanding of the pathophysiologic changes that occur in heart disease and affect flow dynamics is necessary.
• Understanding of aseptic technique is needed.
• Understanding of the hemodynamic effects of vasoactive medications is needed.
• Understanding of the principles involved in hemodynamic monitoring is necessary.
• Knowledge of invasive cardiac output monitoring is needed.
• Knowledge of arterial waveform interpretation is needed.
• Knowledge of definitions and norms for cardiac output, cardiac index, systemic vascular resistance, stroke volume, stroke index, preload, afterload, and contractility and stroke volume variation is necessary.
• Arterial pressure represents the forcible ejection of blood from the left ventricle into the aorta and out into the arterial system. During ventricular systole, blood is ejected into the aorta, generating a pressure wave. Because of the intermittent pumping action of the heart, this arterial pressure wave is generated in a pulsatile manner (see Fig. 62-1). The ascending limb of the aortic pressure wave (anacrotic limb) represents an increase in pressure because of left ventricular ejection. The peak of this ejection is the peak systolic pressure, which is normally 100 to 140 mm Hg in adults. After reaching this peak, the ventricular pressure declines to a level below aortic pressure and the aortic valve closes, marking the end of ventricular systole. The closure of the aortic valve produces a small rebound wave that creates a notch known as the dicrotic notch. The descending limb of the curve (diastolic downslope) represents diastole and is characterized by a long declining pressure wave, during which the aortic wall recoils and propels blood into the arterial network. The diastolic pressure is measured as the lowest point of the diastolic downslope and is normally 60 to 80 mm Hg.
• The difference between the systolic and diastolic pressures is called the pulse pressure, with a normal value of 40 mm Hg.9
• Arterial pressure is determined by the relationship between blood flow through the vessels (cardiac output), the compliance of the aorta and larger vessels and the resistance of the more peripheral vessel walls (systemic vascular resistance). The arterial pressure is therefore affected by any factors that change either cardiac output, compliance or systemic vascular resistance.
• The average arterial pressure during a cardiac cycle is called the mean arterial pressure (MAP). It is not the average of the systolic plus the diastolic pressures, because at normal heart rates, systole accounts for 1/3 of the cardiac cycle and diastole accounts for 2/3 of the cardiac cycle. The MAP is calculated automatically by most patient monitoring systems; however, it can be calculated roughly by using the following formula:
• MAP represents the driving force (perfusion pressure) for blood flow through the cardiovascular system. MAP is at its highest point in the aorta. As blood travels through the circulatory system, systolic pressure increases and diastolic pressure decreases, with an overall decline in the MAP (see Fig. 62-2).
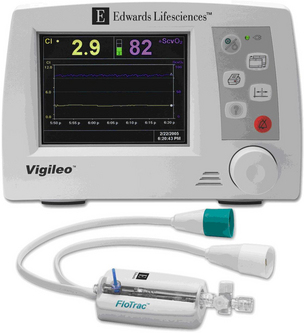
• Arterial pressure-based cardiac output (APCO) is obtained from an arterial catheter.3,5,11
• APCO technology measures the rate of flow (cardiac output).
• Stroke volume and heart rate are key determinants of cardiac output.
• Although systemic vascular resistance affects cardiac output, the location of that effect is global and not limited by location of that measurement because cardiac output is flow per minute throughout the body. Manufacturers of the arterial pressure-based cardiac output systems have factored in variance for both radial artery catheters and femoral artery catheters.3
EQUIPMENT
• Invasive arterial catheter and insertion kit
• Specialized sterile transducer and sensor kit (manufacturer-specific)
• Intravenous (IV) pole and cartridge holder (manufacturer-specific)
• Pressure transducer system, including flush solution recommended according to institution standard, a pressure bag or device, pressure tubing with transducer, and flush device
• Pressure module and cable for interface with the monitor
• Monitoring system (central and bedside monitor)
• Special monitor to interface with the bedside monitor for trending and display of hemodynamic values (manufacturer-specific)
PATIENT AND FAMILY EDUCATION
• Explain the rationale for arterial line insertion, including how the arterial pressure is displayed on the bedside monitor.  Rationale: This explanation may decrease patient and family anxiety.
Rationale: This explanation may decrease patient and family anxiety.
• Explain the standard of care to the patient and family, including insertion procedure, alarms, dressings, and length of time the catheter is expected to be in place.  Rationale: This explanation encourages the patient and family to ask questions and voice concerns about the procedure and decreases patient and family anxiety.
Rationale: This explanation encourages the patient and family to ask questions and voice concerns about the procedure and decreases patient and family anxiety.
• Explain the patient’s expected participation during the procedure.  Rationale: Patient cooperation during insertion is encouraged.
Rationale: Patient cooperation during insertion is encouraged.
• Explain the importance of keeping the affected extremity immobile.  Rationale: This explanation encourages patient cooperation to prevent catheter dislodgment and ensures a more accurate waveform.
Rationale: This explanation encourages patient cooperation to prevent catheter dislodgment and ensures a more accurate waveform.
• Instruct the patient to report any warmth, redness, pain, or wet feeling at the insertion site at any time, including after catheter removal.  Rationale: These symptoms may indicate infection, bleeding, or disconnection of the tubing or catheter.
Rationale: These symptoms may indicate infection, bleeding, or disconnection of the tubing or catheter.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Obtain the patient’s medical history, including a history of diabetes and hypertension.  Rationale: Patients with diabetes mellitus or hypertension are at higher risk for arterial or venous insufficiency.
Rationale: Patients with diabetes mellitus or hypertension are at higher risk for arterial or venous insufficiency.
• Obtain the patient’s medical history for peripheral vascular disease, vascular grafts, arteriovenous (AV) fistulas or shunts, arterial vasospasm, thrombosis, or embolism. In addition, obtain the patient’s history of coronary artery bypass graft surgery in which radial arteries were removed for use as conduits.  Rationale: Extremities with any of these problems should be avoided as sites for cannulation because of the potential for complications.
Rationale: Extremities with any of these problems should be avoided as sites for cannulation because of the potential for complications.
• Assess the neurovascular and peripheral vascular status of the extremity to be used for the arterial cannulation, including color, temperature, presence and fullness of pulses, capillary refill, presence of bruit, and motor and sensory function (as compared with the opposite extremity). Note: A modified Allen’s test should be performed before cannulation of the radial artery (see Fig. 80-3).  Rationale: This assessment identifies any neurovascular or circulatory impairment before cannulation to avoid potential complications.
Rationale: This assessment identifies any neurovascular or circulatory impairment before cannulation to avoid potential complications.
• Assess the patient’s vital signs and compliance factors (e.g., age, gender, height, weight).  Rationale: This assessment provides baseline data. The compliance factors allow for the individual variables that ultimately dictate pulse pressure and its relevance (proportionality) to stroke volume.
Rationale: This assessment provides baseline data. The compliance factors allow for the individual variables that ultimately dictate pulse pressure and its relevance (proportionality) to stroke volume.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that the patient and family understand preprocedural teaching. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Ensure that informed consent has been obtained.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
• Perform a pre-procedure verification and time out, if non-emergent.  Rationale: Ensures patient safety.
Rationale: Ensures patient safety.
• Validate the patency of the peripheral IV line.  Rationale: Access may be needed for administration of emergency medications or fluids.
Rationale: Access may be needed for administration of emergency medications or fluids.
• Place the patient’s extremity in the appropriate position with adequate lighting of the insertion site.  Rationale: This placement prepares the site for cannulation and facilitates an accurate insertion.
Rationale: This placement prepares the site for cannulation and facilitates an accurate insertion.
References
![]() 1. American Association of Critical-Care Nurses, Evaluation of the effects of heparinized and nonheparinized flush -solutions on the patency of arterial pressure monitoring lines. the AACN Thunder Project. Am J Crit Care 1993; 2:3–15.
1. American Association of Critical-Care Nurses, Evaluation of the effects of heparinized and nonheparinized flush -solutions on the patency of arterial pressure monitoring lines. the AACN Thunder Project. Am J Crit Care 1993; 2:3–15.
2. Barone, J, Madlinger, RV. Should an Allen test be performed before radial artery cannulation. J Trauma. 2006; 6:468–470.
3. Button, D, Weibel, L, Reuthebuch, O, et al. Clinical evaluation of the FloTrac/Vigileo system and two established continuous cardiac output monitoring devices in patients undergoing cardiac surgery. Br J Anaesth. 2007; 99(3):329–336.
4. Cecconi, M, Wilson, J, Rhodes, A. Pulse pressure analysis. In: Vincent JL, ed. Yearbook of intensive care and emergency medicine. Brussels: Springer-Verlag; 2006:176–184.
5. Chakravarthy, M, Patil, TA, Jayaprakash, K, et al, Comparison of simultaneous estimation of cardiac output by four techniques in patients undergoing off-pump coronary -artery bypass surgery. a prospective observational study. Ann Cardiac Anaesth. 2007; 10(2):121–126.
![]() 6. Chong, BH. Heparin-induced thrombocytopenia. Br J Haematol. 1995; 89:431–439.
6. Chong, BH. Heparin-induced thrombocytopenia. Br J Haematol. 1995; 89:431–439.
7. de Waal E, Kalkma, C, Rex, S, et al. Validation of a new -arterial pulse contour-based cardiac output device. Crit Care Med. 2007; 35(8):1904–1909.
![]() 8. Greenwood, MJ, et al, Vascular communications of the hand in patients being considered for transradial coronary angiography. is the Allen’s test accurate. J Am Coll Cardiol 2005; 46:2013–2017.
8. Greenwood, MJ, et al, Vascular communications of the hand in patients being considered for transradial coronary angiography. is the Allen’s test accurate. J Am Coll Cardiol 2005; 46:2013–2017.
9. Headley, JM. Clinically relevant monitoring using arterial pressure-based technologies and stroke volume variation to assess fluid responsiveness. NTI News Online. 2007; 20(21):1–6.
![]() 10. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections, Centers for Disease Control and Prevention. MMWR. 2002; 51(RR-10):1–36.
10. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections, Centers for Disease Control and Prevention. MMWR. 2002; 51(RR-10):1–36.
11. Prasser, C, Bele, S, Keyl, C, et al. Evaluation of a new arterial pressure-based cardiac output device requiring no external calibration. BMC Anesthesiol. 2007; 7(186):1471–2253. [7-9].
![]() 12. Randolph, AG, et al, Benefit of heparin in peripheral venous and arterial catheters. systematic review and meta-analysis of randomised controlled trials. BMJ 1998; 316:969–975.
12. Randolph, AG, et al, Benefit of heparin in peripheral venous and arterial catheters. systematic review and meta-analysis of randomised controlled trials. BMJ 1998; 316:969–975.
![]() 13. Slogoff, S, Keats, AS, Arlund, C. On the safety of radial artery cannulation. Anesthesiology. 1983; 59:42–47.
13. Slogoff, S, Keats, AS, Arlund, C. On the safety of radial artery cannulation. Anesthesiology. 1983; 59:42–47.
![]() 14. Zevola, DR, Dioso, J, Moggio, R. Comparison of heparinized solutions for maintaining patency of arterial and pulmonary artery catheters. Am J Crit Care. 1997; 6:52–55.
14. Zevola, DR, Dioso, J, Moggio, R. Comparison of heparinized solutions for maintaining patency of arterial and pulmonary artery catheters. Am J Crit Care. 1997; 6:52–55.