Ventricular Assist Devices
Ventricular Assist Devices
Ventricular assist devices are used for cardiogenic shock and postcardiotomy support to allow for myocardial recovery, for bridge to cardiac transplantation, and for destination therapy (permanent implantation) in patients with New York Heart Association class IIIB or IV heart failure who are on optimal medical therapy and are not eligible for cardiac transplant.3–6,9,14
PREREQUISITE NURSING KNOWLEDGE
• Understanding of the normal anatomy and physiology of the cardiovascular, peripheral vascular, and pulmonary systems is important.
• Understanding of the management of heart failure is essential.
• Knowledge of the principles of hemodynamic monitoring, cardiopulmonary bypass, electrophysiology and dysrhythmias, and coagulation is needed.
• Clinical and technical competence related to use of ventricular assist devices (VADs) is necessary.
• Advanced cardiac life support knowledge and skills are needed.
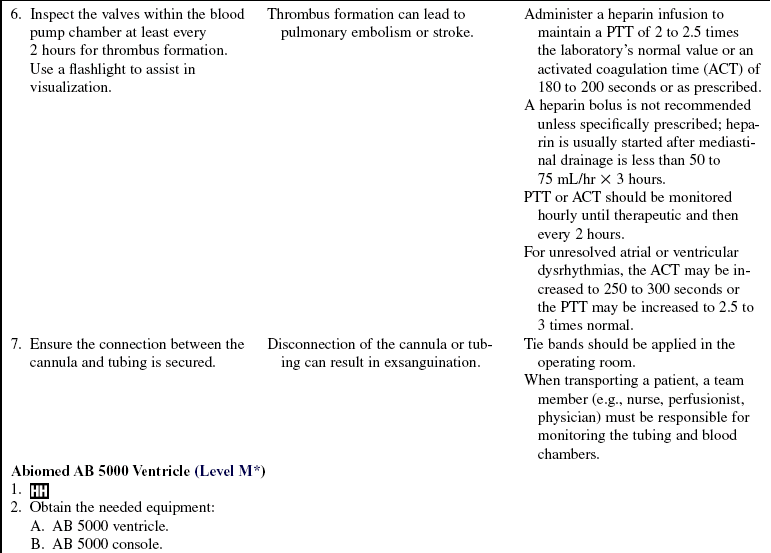
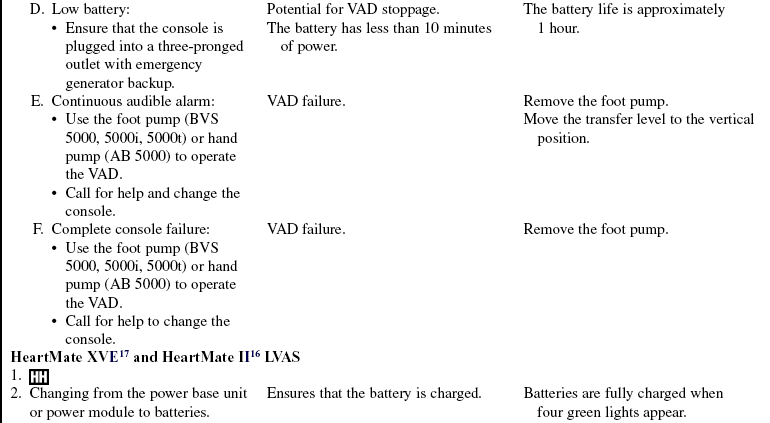
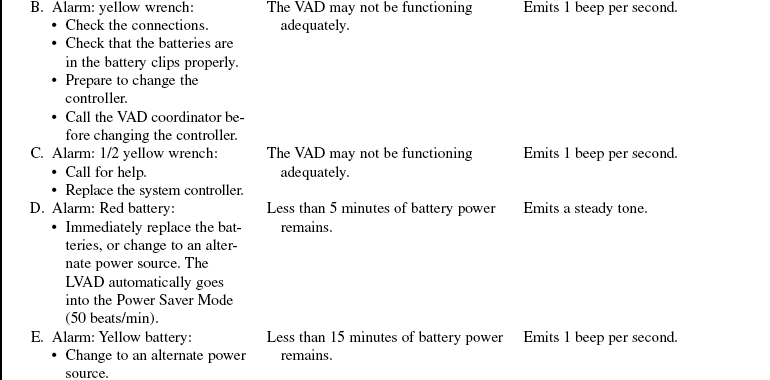
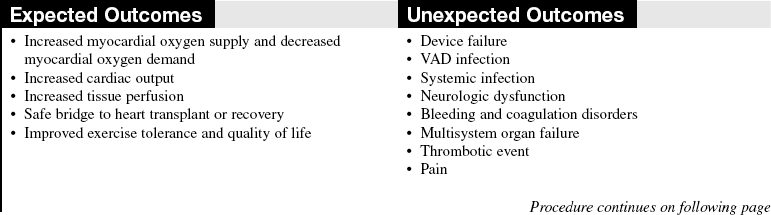
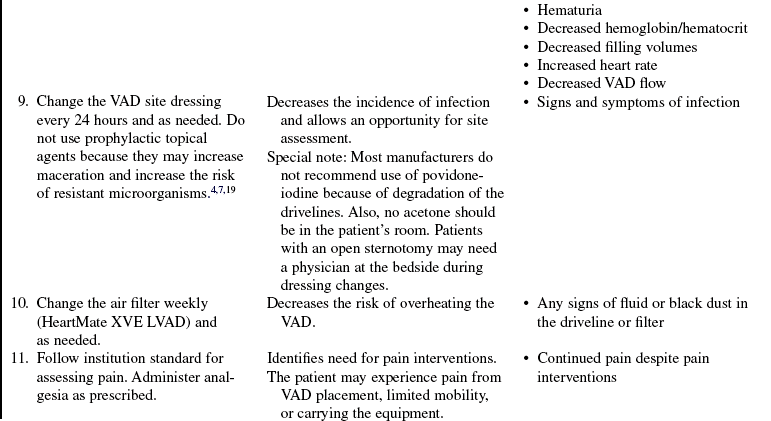
• Complications of VAD therapy include, but are not limited to, bleeding, cardiac tamponade, right ventricular failure with univentricular support, hepatic dysfunction, pulmonary dysfunction, renal dysfunction, infection, cerebral infarcts, thrombosis, embolism, and VAD malfunction.3,6
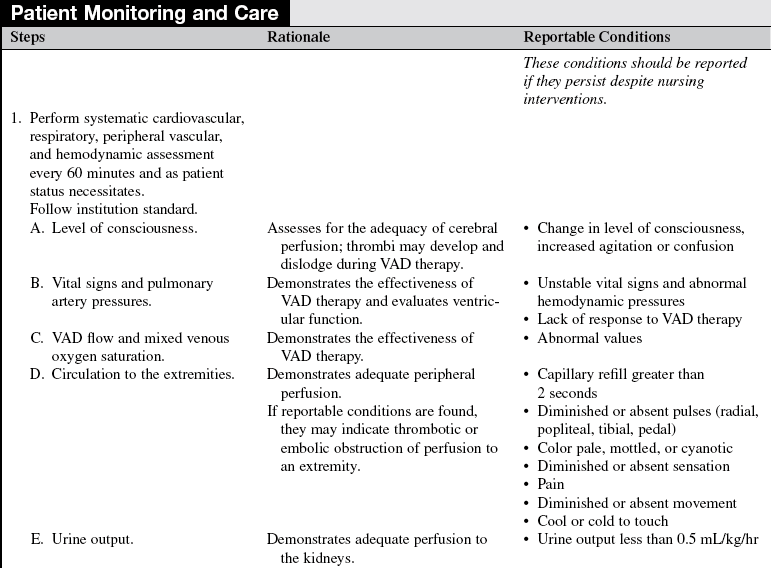
• Effective cardiac assistance with the VAD is affected greatly by preload, afterload, right ventricular failure, cardiac tamponade, and cardiac dysrhythmias; the interaction between the patient and the device requires close monitoring.
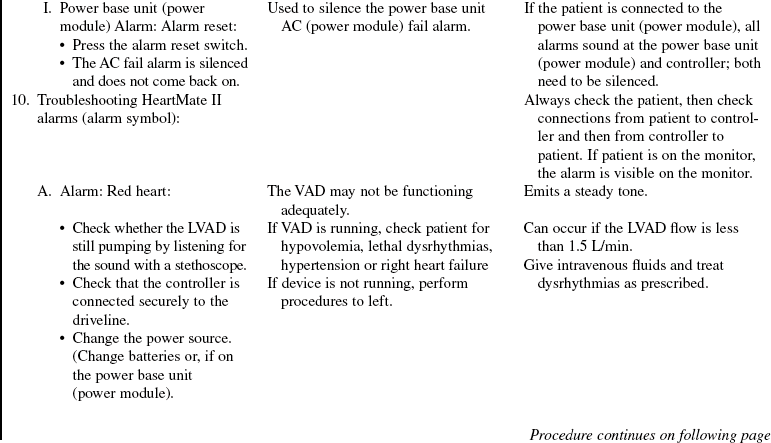
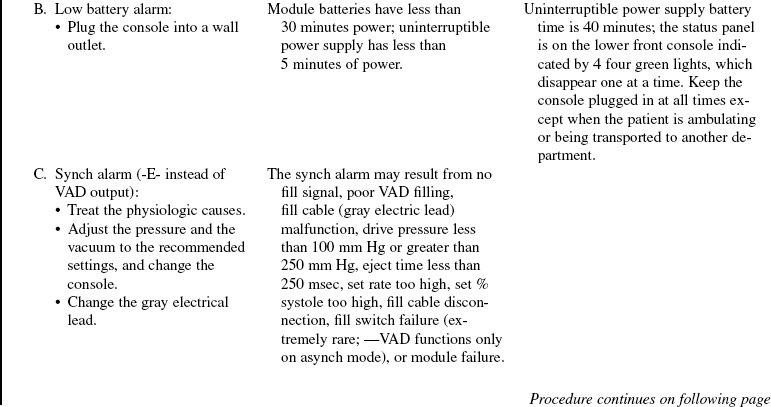
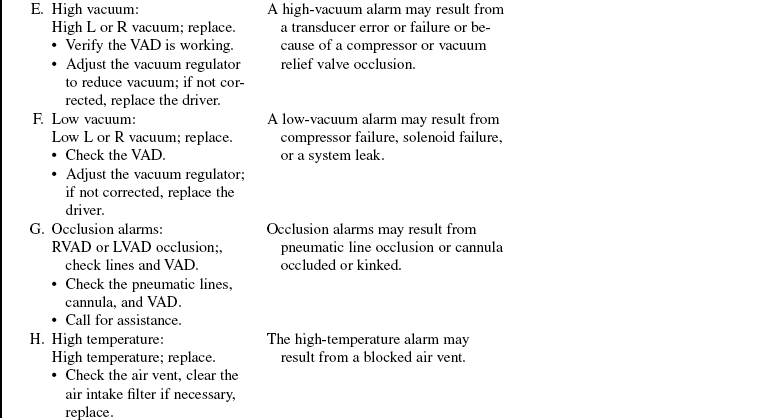
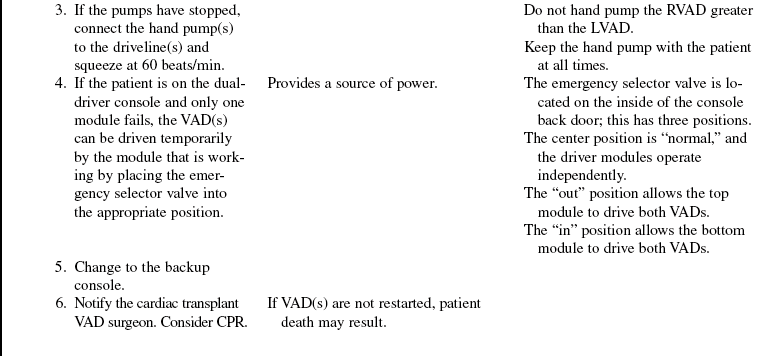
• The device is implanted surgically in the operating room. Specific information concerning controls, alarms, troubleshooting, and safety features is available from each manufacturer and should be read thoroughly by the nurse before use of the equipment. Please refer to the operator’s manual for all systems for more detail.
• Indications for VAD therapy include the following4,6, 9,10, 14:
 Inability to wean from cardiopulmonary bypass
Inability to wean from cardiopulmonary bypass
 New York Heart Association class IIIB or IV status in a patient whose condition does not respond to optimal medical therapy and who is not a transplant candidate
New York Heart Association class IIIB or IV status in a patient whose condition does not respond to optimal medical therapy and who is not a transplant candidate
• Relative contraindications of VAD therapy include the following:
 Body surface area (BSA) less than 1.3 m2 (ABIOMED BVS 5000 or AB 5000 ventricle; Abiomed Inc, Danvers, MA)1–3
Body surface area (BSA) less than 1.3 m2 (ABIOMED BVS 5000 or AB 5000 ventricle; Abiomed Inc, Danvers, MA)1–3
 BSA less than 1.5 m2 (HeartMate XVE left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
BSA less than 1.5 m2 (HeartMate XVE left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
 BSA less than 1.2 m2 (HeartMate II left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
BSA less than 1.2 m2 (HeartMate II left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
 Renal or liver failure unrelated to cardiac incident
Renal or liver failure unrelated to cardiac incident
 Comorbidity that limits life expectancy to less than 3 years
Comorbidity that limits life expectancy to less than 3 years
• Psychosocial and cognitive conditions may limit the use of a VAD except in bridge to recovery because the patient needs to have the cognitive skills to manage the VAD.
• Ventricular assist devices are pulsatile or nonpulsatile.
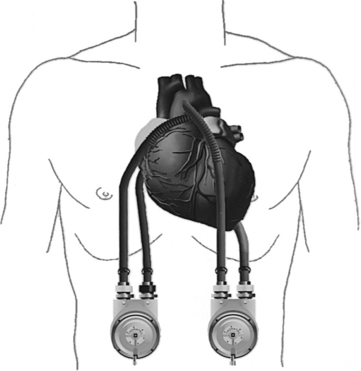
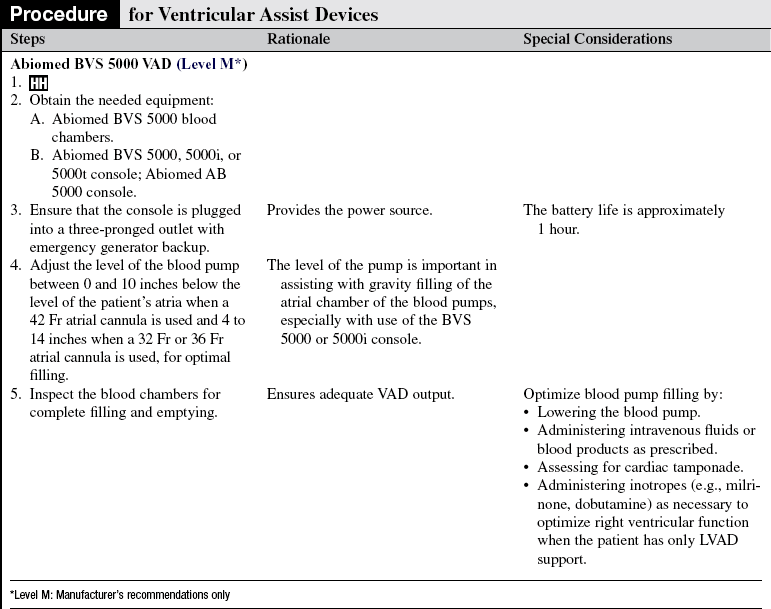
• ABIOMED BVS 5000 circulatory support system (Fig. 56-1)2:
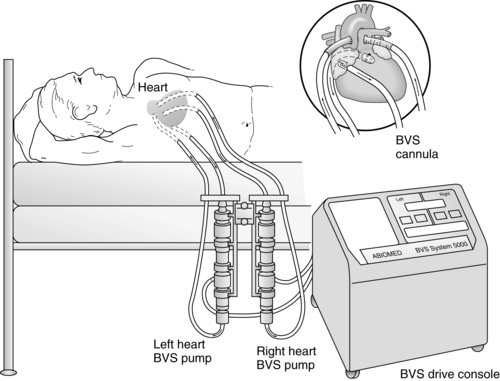
Figure 56-1 ABIOMED BVS 5000 System. (From Dixon JF, Farris DD: The ABIOMED BVS 5000 system, AACN Clin Issues Crit Care Nurs 2:552-561, 1991.)
 The ABIOMED BVS 5000 is an extracorporeal, pneumatically driven pump capable of delivering short-term (less than 3 weeks) left, right, or biventricular support.
The ABIOMED BVS 5000 is an extracorporeal, pneumatically driven pump capable of delivering short-term (less than 3 weeks) left, right, or biventricular support.
 The drive console controls systole by delivering air into the lower rigid plastic pumping chamber, displacing blood from the blood sac. Blood drains passively from the patient’s atrium into the atrial chamber of the blood pump. When the atrial chamber of the blood pump is full and the pressure inside the atrial chamber exceeds the pressure inside the ventricular chamber, the trileaflet valve opens, allowing blood to flow into the ventricular chamber of the blood pump. Blood pump diastole is completed as soon as the ventricular chamber is filled with 100 mL. The diastolic filling time is adjusted automatically to changes in the patient’s preload to ensure the ventricular chamber is filled to capacity (100 mL).
The drive console controls systole by delivering air into the lower rigid plastic pumping chamber, displacing blood from the blood sac. Blood drains passively from the patient’s atrium into the atrial chamber of the blood pump. When the atrial chamber of the blood pump is full and the pressure inside the atrial chamber exceeds the pressure inside the ventricular chamber, the trileaflet valve opens, allowing blood to flow into the ventricular chamber of the blood pump. Blood pump diastole is completed as soon as the ventricular chamber is filled with 100 mL. The diastolic filling time is adjusted automatically to changes in the patient’s preload to ensure the ventricular chamber is filled to capacity (100 mL).
 The vertically aligned pneumatic blood pumps are adjusted to optimize flow. The blood flow from the patient to the blood pump depends on the console used. The BVS 5000t (transport) console and the AB 5000 console allow for vacuum-assisted filling of the chamber. Filling of the pumps depends solely on gravity when the BVS 5000 or BVS 5000i (high-flow) consoles are used. The top of the blood pump should be between 0 and 10 inches below the level of the patient’s atria when a 42 Fr atrial cannula is used and 4 to 14 inches when a 32 Fr or 36 Fr atrial cannula is used (see Fig. 56-1). Moving the pump above or below this level can affect flow. Adjusting the height of the blood pump alters filling of the blood chambers. It is important to allow 2 minutes for the system to adjust before making additional changes.
The vertically aligned pneumatic blood pumps are adjusted to optimize flow. The blood flow from the patient to the blood pump depends on the console used. The BVS 5000t (transport) console and the AB 5000 console allow for vacuum-assisted filling of the chamber. Filling of the pumps depends solely on gravity when the BVS 5000 or BVS 5000i (high-flow) consoles are used. The top of the blood pump should be between 0 and 10 inches below the level of the patient’s atria when a 42 Fr atrial cannula is used and 4 to 14 inches when a 32 Fr or 36 Fr atrial cannula is used (see Fig. 56-1). Moving the pump above or below this level can affect flow. Adjusting the height of the blood pump alters filling of the blood chambers. It is important to allow 2 minutes for the system to adjust before making additional changes.
 Outflow from the BVS is used in place of cardiac output for calculations such as systemic vascular resistance, pulmonary vascular resistance, and cardiac index.
Outflow from the BVS is used in place of cardiac output for calculations such as systemic vascular resistance, pulmonary vascular resistance, and cardiac index.
 External heat is not applied to the blood pump, tubing, or cannulas.
External heat is not applied to the blood pump, tubing, or cannulas.
 ABIOMED tubing insulators are used to retain heat in the tubing.
ABIOMED tubing insulators are used to retain heat in the tubing.
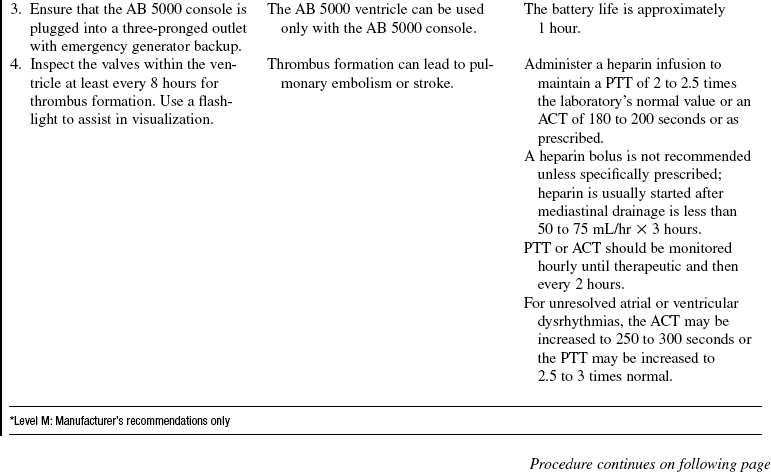
• ABIOMED AB 5000 ventricle1 (Fig. 56-2):
 The AB 5000 ventricle is a pulsatile, pneumatically driven blood pump approved for short-term (less than 3 weeks) support to allow time for myocardial recovery. It can provide support for one or both ventricles and must be used in conjunction with the AB 5000 circulatory support system console. The ventricle holds approximately 100 mL of blood. Cannulas exit the skin, and the ventricle lies on the patient’s abdomen. Filling of the ventricle is facilitated with a vacuum within the console and is not affected by height. The ventricle is made partially of aluminum and plastic and has inflow and outflow valves to ensure unidirectional blood flow.
The AB 5000 ventricle is a pulsatile, pneumatically driven blood pump approved for short-term (less than 3 weeks) support to allow time for myocardial recovery. It can provide support for one or both ventricles and must be used in conjunction with the AB 5000 circulatory support system console. The ventricle holds approximately 100 mL of blood. Cannulas exit the skin, and the ventricle lies on the patient’s abdomen. Filling of the ventricle is facilitated with a vacuum within the console and is not affected by height. The ventricle is made partially of aluminum and plastic and has inflow and outflow valves to ensure unidirectional blood flow.
 The following items must never come into contact with the ventricle because they could damage the plastic within the ventricle: ketones, such as acetone; aromatic hydrocarbons, such as gasoline; halogenated hydrocarbon–based anesthetic agents; other hydrocarbonated hydrocarbons, such as chloroform; and highly alkaline chemicals, such as sodium hydroxide.
The following items must never come into contact with the ventricle because they could damage the plastic within the ventricle: ketones, such as acetone; aromatic hydrocarbons, such as gasoline; halogenated hydrocarbon–based anesthetic agents; other hydrocarbonated hydrocarbons, such as chloroform; and highly alkaline chemicals, such as sodium hydroxide.
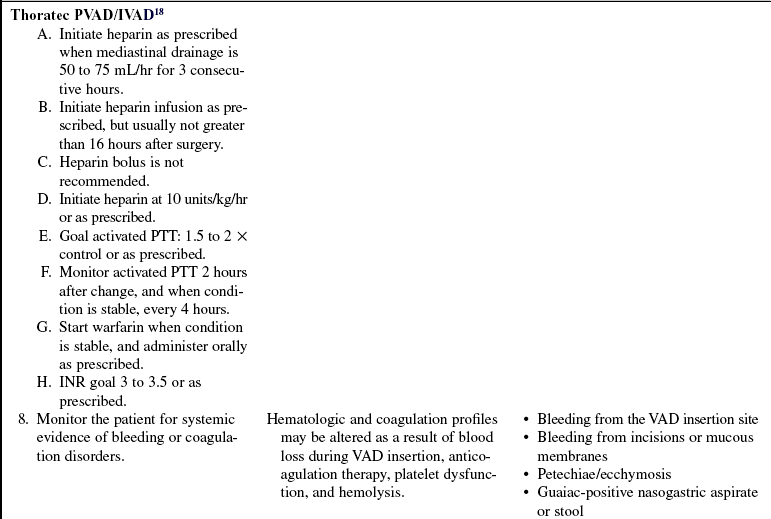
 Anticoagulation therapy with heparin initially followed by warfarin (Coumadin) is necessary.
Anticoagulation therapy with heparin initially followed by warfarin (Coumadin) is necessary.
• HeartMate XVE (extended lead vented electric) left ventricular assist system (LVAS):17
 Thoratec Corporation offers an implantable VAD for left ventricular assistance, the HeartMate XVE LVAD.
Thoratec Corporation offers an implantable VAD for left ventricular assistance, the HeartMate XVE LVAD.
 This VAD is used for long-term support.
This VAD is used for long-term support.
 The LVAD may be implanted intra-abdominally or preperitoneally in a rectus muscle pocket.
The LVAD may be implanted intra-abdominally or preperitoneally in a rectus muscle pocket.
 The HeartMate XVE LVAD is made of titanium, holds 83 mL of blood, and weighs approximately 3 lb. Blood flows through a small tube placed in the left ventricular apex through a porcine inflow valve into the pump. When the pump fills with blood, a sensor inside the device starts the electric motor. Blood is pumped through a second porcine outflow valve through a graft into the aorta. The LVAD is placed in the left upper quadrant of the abdomen.
The HeartMate XVE LVAD is made of titanium, holds 83 mL of blood, and weighs approximately 3 lb. Blood flows through a small tube placed in the left ventricular apex through a porcine inflow valve into the pump. When the pump fills with blood, a sensor inside the device starts the electric motor. Blood is pumped through a second porcine outflow valve through a graft into the aorta. The LVAD is placed in the left upper quadrant of the abdomen.
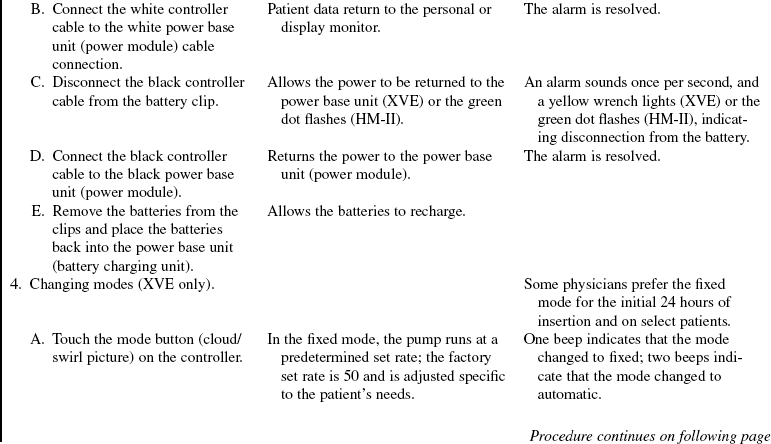
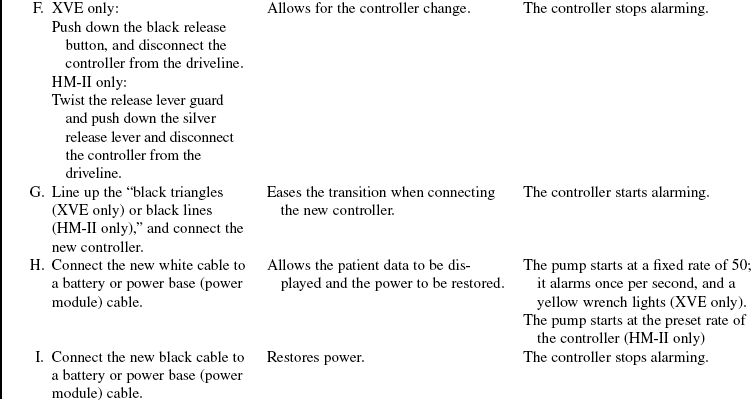
 A driveline is passed underneath the skin and exits the right upper quadrant of the abdomen. The driveline connects the LVAD to a controller and a power source (batteries or a power base unit) and vents the electric motor that is within the LVAD. The LVAD can run on the fixed rate mode (set rate) or on the automatic mode, which responds to changes in preload. On the automatic mode, the pump rate varies between 50 and 120 beats/min to meet the physiologic needs of the patients. Anticoagulation therapy with heparin or warfarin (Coumadin) is not necessary with this LVAD. Most patients receive aspirin, 81 mg or 325 mg daily, however.
A driveline is passed underneath the skin and exits the right upper quadrant of the abdomen. The driveline connects the LVAD to a controller and a power source (batteries or a power base unit) and vents the electric motor that is within the LVAD. The LVAD can run on the fixed rate mode (set rate) or on the automatic mode, which responds to changes in preload. On the automatic mode, the pump rate varies between 50 and 120 beats/min to meet the physiologic needs of the patients. Anticoagulation therapy with heparin or warfarin (Coumadin) is not necessary with this LVAD. Most patients receive aspirin, 81 mg or 325 mg daily, however.
 The device can be operated with the HeartMate IP console if the electric motor fails. Due to the increased risk of thrombosis, this mode is only used for short duration until the device can be replaced. The patient will also be anticoagulated with heparin or warfarin during this mode of operation.
The device can be operated with the HeartMate IP console if the electric motor fails. Due to the increased risk of thrombosis, this mode is only used for short duration until the device can be replaced. The patient will also be anticoagulated with heparin or warfarin during this mode of operation.
• Thoratec Paracoporeal Ventricular Assist Device (PVAD):18
 The Thoratec Paracorporeal VAD (PVAD) is made of polyurethane and is pneumatically driven. A flexible polyurethane diaphragm divides the blood chamber. An influx of pressurized air (through the pneumatic tubing and into the VAD) drives the flexible diaphragm against the blood chamber, pushing blood from the VAD to the patient, and controls the duration of systole. Mechanical valves provide unidirectional blood flow. When the pneumatic drive is used, the pump can only be run in a fixed mode (asynchronous) or auto mode (volume) for right, left, or biventricular support
The Thoratec Paracorporeal VAD (PVAD) is made of polyurethane and is pneumatically driven. A flexible polyurethane diaphragm divides the blood chamber. An influx of pressurized air (through the pneumatic tubing and into the VAD) drives the flexible diaphragm against the blood chamber, pushing blood from the VAD to the patient, and controls the duration of systole. Mechanical valves provide unidirectional blood flow. When the pneumatic drive is used, the pump can only be run in a fixed mode (asynchronous) or auto mode (volume) for right, left, or biventricular support
 The Thoratec PVAD is approved for use as a bridge to transplant and post-cardiotomy recovery.
The Thoratec PVAD is approved for use as a bridge to transplant and post-cardiotomy recovery.
 The Thoratec PVAD is connected via the driveline and a cable to the dual-drive console (DDC), which controls pump function. The console is plugged into an electrical outlet when the patient is not ambulating. This VAD is primarily for short-term and intermediate use. The VADs may also be connected to the smaller TLC-II driver giving the patient 2 hours of battery life and a smaller 20 pound driver to push rather than the 500-pound dual-drive hospital driver (DDC).
The Thoratec PVAD is connected via the driveline and a cable to the dual-drive console (DDC), which controls pump function. The console is plugged into an electrical outlet when the patient is not ambulating. This VAD is primarily for short-term and intermediate use. The VADs may also be connected to the smaller TLC-II driver giving the patient 2 hours of battery life and a smaller 20 pound driver to push rather than the 500-pound dual-drive hospital driver (DDC).
 The Thoratec PVAD may be used in smaller patients.
The Thoratec PVAD may be used in smaller patients.
 The three major components of the system are the blood pump, cannulas, and drive console. The smooth, seamless blood sac is enclosed within a rigid case. Small bubbles in a silicone oil lubricant may be seen during use. A small magnetic switch (called the Hall effect switch) is mounted in the upper case. When the PVAD is full of blood, a switch is tripped sending a signal to the console to eject blood.
The three major components of the system are the blood pump, cannulas, and drive console. The smooth, seamless blood sac is enclosed within a rigid case. Small bubbles in a silicone oil lubricant may be seen during use. A small magnetic switch (called the Hall effect switch) is mounted in the upper case. When the PVAD is full of blood, a switch is tripped sending a signal to the console to eject blood.
 The Thoratec IVAD (Thoratec Corporation) is an implantable pneumatic system approved for postcardiotomy use and as a bridge to transplantation. It can provide univentricular or biventricular support. It is the only device approved for long-term (greater than 3 weeks if necessary) biventricular support. It is also the device of choice in patients who need ventricular support with a BSA less than 1.5 m2. The three major components of the system are the blood pump, cannulas, and drive console. The smooth, seamless blood sac is enclosed within a rigid case. Two mechanical valves provide unidirectional blood flow. A fill switch with the VAD signals the console to eject the blood when the VAD is filled with blood. Either the dual-drive console or the TLC-II portable driver can operate the VAD (Fig. 56-3).
The Thoratec IVAD (Thoratec Corporation) is an implantable pneumatic system approved for postcardiotomy use and as a bridge to transplantation. It can provide univentricular or biventricular support. It is the only device approved for long-term (greater than 3 weeks if necessary) biventricular support. It is also the device of choice in patients who need ventricular support with a BSA less than 1.5 m2. The three major components of the system are the blood pump, cannulas, and drive console. The smooth, seamless blood sac is enclosed within a rigid case. Two mechanical valves provide unidirectional blood flow. A fill switch with the VAD signals the console to eject the blood when the VAD is filled with blood. Either the dual-drive console or the TLC-II portable driver can operate the VAD (Fig. 56-3).
 The Thoratec PVAD and IVADs18 are both driven by a dual-drive console, which contains two independent drive modules for left and right ventricular support. Patients with biventricular assist devices (BiVADs) need both modules, and patients with a right ventricular assist device (RVAD) or a LVAD need one module. When only one module is being used, the other module can serve as a backup if pump failure occurs. The console supplies air pressure to eject blood from the pump into the arterial system and vacuum to assist pump filling. A full 65-mL stroke volume is possible from 20 to 110 beats/min, providing cardiac outputs of 1.2 to 7.2 L/min.
The Thoratec PVAD and IVADs18 are both driven by a dual-drive console, which contains two independent drive modules for left and right ventricular support. Patients with biventricular assist devices (BiVADs) need both modules, and patients with a right ventricular assist device (RVAD) or a LVAD need one module. When only one module is being used, the other module can serve as a backup if pump failure occurs. The console supplies air pressure to eject blood from the pump into the arterial system and vacuum to assist pump filling. A full 65-mL stroke volume is possible from 20 to 110 beats/min, providing cardiac outputs of 1.2 to 7.2 L/min.
 The recommended control modes for operation include asynchronous/fixed or volume/automatic. A fixed rate allows the operator to choose a VAD rate that is asynchronous with the patient’s intrinsic heart rate. The automatic mode also is asynchronous to the patient’s intrinsic heart rate but responds to changes in physiologic conditions.
The recommended control modes for operation include asynchronous/fixed or volume/automatic. A fixed rate allows the operator to choose a VAD rate that is asynchronous with the patient’s intrinsic heart rate. The automatic mode also is asynchronous to the patient’s intrinsic heart rate but responds to changes in physiologic conditions.
 Anticoagulation therapy with heparin initially followed by warfarin is needed.
Anticoagulation therapy with heparin initially followed by warfarin is needed.
 The HeartMate II left ventricular assist system (Thoratec Corp) HeartMate II LVAS (left ventricular assist system)16 is a continuous flow pump and is approved as a bridge to transplant and destination therapy in patients with advanced heart failure.
The HeartMate II left ventricular assist system (Thoratec Corp) HeartMate II LVAS (left ventricular assist system)16 is a continuous flow pump and is approved as a bridge to transplant and destination therapy in patients with advanced heart failure.
 The LVAD may be implanted intra-abdominally or preperitoneally in a rectus muscle pocket.
The LVAD may be implanted intra-abdominally or preperitoneally in a rectus muscle pocket.
 The HeartMate II is made of titanium and weighs approximately 1.5 lb. Blood flows from the left ventricle through the pump and back to the patient’s circulation via the outflow graft.
The HeartMate II is made of titanium and weighs approximately 1.5 lb. Blood flows from the left ventricle through the pump and back to the patient’s circulation via the outflow graft.
 Continuous flow is generated by a small rotor inside the pump. The speed of the pump is set by the LVAD team and does not change in response to preload.
Continuous flow is generated by a small rotor inside the pump. The speed of the pump is set by the LVAD team and does not change in response to preload.
 The LVAD is placed in the left upper quadrant of the abdomen.
The LVAD is placed in the left upper quadrant of the abdomen.
 A driveline is passed underneath the skin and exits the right or left upper quadrant of the abdomen. The driveline connects the LVAD to a controller and a power source (batteries or a power base unit or power module).
A driveline is passed underneath the skin and exits the right or left upper quadrant of the abdomen. The driveline connects the LVAD to a controller and a power source (batteries or a power base unit or power module).
 Anticoagulation therapy with heparin or warfarin is necessary with this LVAD with a goal INR range of 1.8 to 2.5. Most patients receive aspirin, 81 mg or 325 mg daily in addition to warfarin.
Anticoagulation therapy with heparin or warfarin is necessary with this LVAD with a goal INR range of 1.8 to 2.5. Most patients receive aspirin, 81 mg or 325 mg daily in addition to warfarin.
• The Impella LP 2.5 and LP 5.0:
 The Impella LP 2.5 and 5.0 systems (Abiomed Inc) are nonpulsatile microaxial flow devices that deliver 2.5 L (Impella LP 2.5) or 5.0 L (Impella LP 5.0) of blood flow.10
The Impella LP 2.5 and 5.0 systems (Abiomed Inc) are nonpulsatile microaxial flow devices that deliver 2.5 L (Impella LP 2.5) or 5.0 L (Impella LP 5.0) of blood flow.10
 The pumps are implanted percutaneously in the cardiac catheterization laboratory. A surgical cut down to expose the femoral artery is necessary for the Impella LP 5.0.
The pumps are implanted percutaneously in the cardiac catheterization laboratory. A surgical cut down to expose the femoral artery is necessary for the Impella LP 5.0.
 The Impella 5.0 can also be implanted directly via sternotomy.
The Impella 5.0 can also be implanted directly via sternotomy.
 When positioned properly, the Impella sits across the aortic valve, with the inlet area in the left ventricle and the outlet area in the ascending aorta.
When positioned properly, the Impella sits across the aortic valve, with the inlet area in the left ventricle and the outlet area in the ascending aorta.
 Transesophageal echocardiography is needed to confirm proper placement.
Transesophageal echocardiography is needed to confirm proper placement.
 The console continuously monitors pump placement and alerts the operator to catheter displacement and other alarm states.
The console continuously monitors pump placement and alerts the operator to catheter displacement and other alarm states.
• The TandemHeart (CardiacAssist, Pittsburgh, PA):11,12
 The TandemHeart is a continuous centrifugal flow device that delivers up to 4 L of blood flow.11,12 This is a left atrial to femoral bypass system for short-term use.
The TandemHeart is a continuous centrifugal flow device that delivers up to 4 L of blood flow.11,12 This is a left atrial to femoral bypass system for short-term use.
 The pumps are implanted via a percutaneous approach in the cardiac catheterization laboratory.
The pumps are implanted via a percutaneous approach in the cardiac catheterization laboratory.
 There is a 21 Fr cannula that is inserted to the left atrium via a transseptal cannulation from the right atrium and blood is ejected to the centrifugal pump and returned via the femoral artery.
There is a 21 Fr cannula that is inserted to the left atrium via a transseptal cannulation from the right atrium and blood is ejected to the centrifugal pump and returned via the femoral artery.
 The TandemHeart operates via an electromagnetic rotor that operates at a range of 3000 to 7500 rpm.11,12
The TandemHeart operates via an electromagnetic rotor that operates at a range of 3000 to 7500 rpm.11,12
 The pump is driven by a microprocessor controller
The pump is driven by a microprocessor controller
 The TandemHeart has a dual-chamber pump. The upper housing allows for the movement of blood. The lower housing communicates with the controller and contains a continuous flow of saline with heparin to decrease the risk of thrombus formation and provide lubrication.11,12
The TandemHeart has a dual-chamber pump. The upper housing allows for the movement of blood. The lower housing communicates with the controller and contains a continuous flow of saline with heparin to decrease the risk of thrombus formation and provide lubrication.11,12
EQUIPMENT
• VAD drive console/unit or monitor (Table 56-1)
• Connection cables (specific to device; see Table 56-1)
• Backup drive console/unit/monitor, batteries, and controller (see Table 56-1)
• Emergency pump device (hand crank, foot pump, hand pump or bulb, depending on device; see Table 56-1)
PATIENT AND FAMILY EDUCATION
• Assess patient and family understanding of VAD therapy and the reason for its use.  Rationale: Clarification or reinforcement of information is an expressed patient and family need during times of stress and anxiety.
Rationale: Clarification or reinforcement of information is an expressed patient and family need during times of stress and anxiety.
• Explain the environment and planned care to the patient and family, including the frequency of assessment, sounds and function of equipment, placement of the device, explanation of alarms, dressings and therapy, decreased or assisted mobility, and parameters for discontinuation of therapy. Before surgery, a meeting with another patient on a VAD may be helpful for the patient and family, if both patients are agreeable.  Rationale: This communication provides information and encourages the patient and family to ask questions or voice concerns or fears related to the therapy. Meeting with another patient with a VAD provides social support.
Rationale: This communication provides information and encourages the patient and family to ask questions or voice concerns or fears related to the therapy. Meeting with another patient with a VAD provides social support.
• If appropriate, begin discharge teaching to include operation of VAD, dressing changes, battery changes, placement of self on and off of the battery and the power base unit or monitor, changing of the controller, and appropriate bathing techniques with use of shower equipment.  Rationale: This teaching provides information and ensures that the patient will be safe at home. It also allows the patient and family to ask questions as needed.
Rationale: This teaching provides information and ensures that the patient will be safe at home. It also allows the patient and family to ask questions as needed.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess the patient’s medical history, history of heart failure, height, weight, body surface area (BSA) specifically related to the competency of the aortic/pulmonic valves, competency of the mitral/tricuspid valves, pulmonary hypertension, right ventricular function, left ventricular function, and peripheral vascular disease.  Rationale: This assessment provides baseline data regarding cardiac functioning and facilitates decision making regarding insertion of the appropriate device and postoperative management.
Rationale: This assessment provides baseline data regarding cardiac functioning and facilitates decision making regarding insertion of the appropriate device and postoperative management.
• Perform cardiovascular, hemodynamic, peripheral vascular, neurovascular, and psychosocial assessment and assessment of body mass index and BSA.  Rationale: These assessments provide baseline data and help with determination of the type of device to use.
Rationale: These assessments provide baseline data and help with determination of the type of device to use.
• Assess the current laboratory profile, including the complete blood cell count, platelet count, prothrombin time, partial thromboplastin time (PTT), international normalized ratio (INR), blood chemistry, liver profile, protein, and albumin levels.  Rationale: This assessment provides baseline data and may indicate end-organ dysfunction related to low-flow state. It also may be used to predict the patient’s risk of bleeding.
Rationale: This assessment provides baseline data and may indicate end-organ dysfunction related to low-flow state. It also may be used to predict the patient’s risk of bleeding.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that the patient and family understand preoperative teaching. Answer questions as they arise, and reinforce information as needed.  Rationale: This communication evaluates and reinforces understanding of previously taught information.
Rationale: This communication evaluates and reinforces understanding of previously taught information.
• Ensure that informed consent has been signed (if it is known before surgery that the VAD will be placed).  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient and family.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient and family.
• Perform a pre-procedure verification and time out, if non-emergent.  Rationale: Ensures patient safety.
Rationale: Ensures patient safety.
• Provide emotional support to the patient and family.  Rationale: The patient and family are under an extreme amount of stress.
Rationale: The patient and family are under an extreme amount of stress.
References
![]() 1. ABIOMED. IncAB 5000 ventricle training guide. Danvers, MA: Abiomed; 2003.
1. ABIOMED. IncAB 5000 ventricle training guide. Danvers, MA: Abiomed; 2003.
![]() 2. ABIOMED. Inc Clinical reference manual. Danvers, MA: Abiomed; 2003.
2. ABIOMED. Inc Clinical reference manual. Danvers, MA: Abiomed; 2003.
![]() 3. Chillocott, S, Atkins, P, Adamson, R. Left ventricular assist as a viable alternative for cardiac transplantation. Crit Care Nurs Q. 1998; 20:64–69.
3. Chillocott, S, Atkins, P, Adamson, R. Left ventricular assist as a viable alternative for cardiac transplantation. Crit Care Nurs Q. 1998; 20:64–69.
4. Drews, T, Jurmann, M, Michael, D, et al. Differences in pulsatile and non-pulsatile mechanical circulatory support in long-term use. J Heart Lung Transplantation. 2008; 27:1096–1101.
![]() 5. Frazier, OH, Prologue. ventricular assist devices and total artificial heartsa historical perspective. Cardiol Clin 2003; 21:1–13.
5. Frazier, OH, Prologue. ventricular assist devices and total artificial heartsa historical perspective. Cardiol Clin 2003; 21:1–13.
![]() 6. Holmes, EA. Outpatient management of long-term assist devices. Cardiol Clin. 2003; 21:93–99.
6. Holmes, EA. Outpatient management of long-term assist devices. Cardiol Clin. 2003; 21:93–99.
![]() 7. Hravnak, M, George, E, Kormos, RL. Management of chronic left ventricular assist device percutaneous lead insertion sites. J Heart Lung Transplant. 1993; 12(5):856–863.
7. Hravnak, M, George, E, Kormos, RL. Management of chronic left ventricular assist device percutaneous lead insertion sites. J Heart Lung Transplant. 1993; 12(5):856–863.
![]() 8. Konstam, MA, Czerska, B, Bohm, M, et al, Continuous aortic flow augmentation. a pilot study of hemodynamic and renal responses to a novel percutaneous intervention in decompensated heart failure. Circulation 2002; 112:3107.
8. Konstam, MA, Czerska, B, Bohm, M, et al, Continuous aortic flow augmentation. a pilot study of hemodynamic and renal responses to a novel percutaneous intervention in decompensated heart failure. Circulation 2002; 112:3107.
![]() 9. Kukuy, EL, et al. Devices as destination therapy. Cardiol Clin. 2003; 21:67–73.
9. Kukuy, EL, et al. Devices as destination therapy. Cardiol Clin. 2003; 21:67–73.
10. LaRocca, GM, Shimbo, D, Rodriguez, CJ, et al, The Impella Recover LP 5. 0 left ventricular assist device. a bridge to coronary artery bypass grafting and cardiac transplantation. J Am Soc Echocardiogr 2006; 19:468. [e5-7].
11. Lee, MS, Makkar, RR. Percutaneous left ventricular assist devices. Cardiol Clini. 2006; 24:265–275.
![]() 12. Lemos, PA, Cummins P, et alLee, CH. Usefulness of percutaneous left ventricular assistance to support high-risk percutaneous coronary interventions. Am J Cardiol. 2003; 91:479–481.
12. Lemos, PA, Cummins P, et alLee, CH. Usefulness of percutaneous left ventricular assistance to support high-risk percutaneous coronary interventions. Am J Cardiol. 2003; 91:479–481.
13. Long. Improving outcomes with long-term destination therapy using left ventricular assist devices. J Thorac Cardiovasc Surg. 2008; 135:1353–1361.
![]() 14. Rose, EA, et al. Long-term mechanical left ventricular assistance for end stage heart failure. N Engl J Med. 2001; 345:1435–1443.
14. Rose, EA, et al. Long-term mechanical left ventricular assistance for end stage heart failure. N Engl J Med. 2001; 345:1435–1443.
![]() 15. Thiele, H, Lauer, B, Hambrecht, et al. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral atrial bypass assistance. Circulation. 2001; 104:2917–2922.
15. Thiele, H, Lauer, B, Hambrecht, et al. Reversal of cardiogenic shock by percutaneous left atrial-to-femoral atrial bypass assistance. Circulation. 2001; 104:2917–2922.
16. Thoratec Corporation. Thoratec HeartMate II LVAS clinical operation and patient management. Thoratec, Pleasanton, CA, 2008.
![]() 17. Thoratec Corporation. HeartMate XVE LVAS operating manual. Thoratec, Pleasanton, CA, 2003.
17. Thoratec Corporation. HeartMate XVE LVAS operating manual. Thoratec, Pleasanton, CA, 2003.
![]() 18. Thoratec Corporation. Thoratec VAD and IVAD clinical operation and patient management. Thoratec, Pleasanton, CA, 2004.
18. Thoratec Corporation. Thoratec VAD and IVAD clinical operation and patient management. Thoratec, Pleasanton, CA, 2004.
Arabia, F, et al. Biventricular cannulation for the Thoratec ventricular assist device. Ann Thorac Surg. 1998; 66:2119–2120.
![]() Bond, AE, et al. The left ventricular assist device. Am J Nurs. 2003; 103:32–41.
Bond, AE, et al. The left ventricular assist device. Am J Nurs. 2003; 103:32–41.
![]() DeRose, J, et al. Implantable left ventricular assist devices provide an excellent outpatient bridge to transplantation and recovery. J Am Coll Cardiol. 1997; 30:1773–1777.
DeRose, J, et al. Implantable left ventricular assist devices provide an excellent outpatient bridge to transplantation and recovery. J Am Coll Cardiol. 1997; 30:1773–1777.
![]() Dixon, JF, Farris, DD. The ABIOMED BVS 5000 system. AACN Clin Issues Crit Care Nurs. 1991; 2:552–561.
Dixon, JF, Farris, DD. The ABIOMED BVS 5000 system. AACN Clin Issues Crit Care Nurs. 1991; 2:552–561.
![]() Goldstein, DJ, Oz, MC. Cardiac assist devices. New York: Futura Publishing; 2000.
Goldstein, DJ, Oz, MC. Cardiac assist devices. New York: Futura Publishing; 2000.
John, R, et al. Low thromboembolic risk for patients with the Heartmate II left ventricular assist device. J Thorac Cardiovasc Surg. 2008; 136:1318–1323.
![]() Livinston, E, et al. Increased activation of the coagulation and fibrinolytic systems leads to hemorrhagic complications during left ventricular assist implantation. Circulation. 1996; 94(Suppl II):II227–II234.
Livinston, E, et al. Increased activation of the coagulation and fibrinolytic systems leads to hemorrhagic complications during left ventricular assist implantation. Circulation. 1996; 94(Suppl II):II227–II234.
![]() McCarthy, P, et al, One hundred patients with the HeartMate left ventricular assist device. evolving concepts and technology. J Thorac Cardiovasc Surg 1998; 115:904–912.
McCarthy, P, et al, One hundred patients with the HeartMate left ventricular assist device. evolving concepts and technology. J Thorac Cardiovasc Surg 1998; 115:904–912.
McGee E Ci Jr, McCarthy P Mi, Moazami N i. Temporary Mechanical Circulatory Support. In: Cohn Lh, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:507–534.
Miller, L, Pagani, F, Russell, S, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007; 357:885–896.
![]() Mussivand, T, et al. Critical anatomic dimensions for intrathoracic circulatory assist devices. Artif Organs. 1992; 16:281–285.
Mussivand, T, et al. Critical anatomic dimensions for intrathoracic circulatory assist devices. Artif Organs. 1992; 16:281–285.
Pitsis, AA, Visouli, AN. Update on ventricular assist device management in the ICU. Curr Opin Crit Care. 2008; 14(5):569–578.
![]() Schakenbach, LH, Care of the patient with a ventricular assist device. InProtocols for practice. American Association of Critical-Care Nurses, Aliso Viejo, CA, 2002.
Schakenbach, LH, Care of the patient with a ventricular assist device. InProtocols for practice. American Association of Critical-Care Nurses, Aliso Viejo, CA, 2002.
Wiegand, DL, Kalowes, PG. Withdrawal of cardiac medications and devices. AACN Adv Crit Care. 2007; 18(4):415–425.
Windecker, S. Percutaneous left ventricular assist devices for treatment of patients with cardiogenic shock. Curr Opin Crit Care. 2007; 13:521–527.