CHAPTER 56 Cancer
THE ROLE OF PSYCHIATRY IN THE CARE OF CANCER PATIENTS
The seriousness of the diagnosis of cancer challenges the capacity to survive, to set a course in life, and to fulfill hopes and dreams. Over the twentieth century, even as cancer treatments improved and some patients were cured, psychiatrists in the tradition of humane psychiatry used their skills to stand by patients who were overwhelmed, to help them to speak in their own voices, to make complex treatment choices, and to shape the rest of their life or the end of life. Psychiatrists have offered expert diagnosis and management of co-morbid psychiatric syndromes and collaborated with oncologists so that treatable psychiatric illness does not stand in the way of technical oncological care. Specific cancer-related or cancer-treatment–related neuropsychiatric syndromes (Table 56-1)1–5 can be recognized and treated. Psychiatrists can help patients to cope with physical symptoms, developmental losses, changes in relationships, and the effects of cancer on families.
| HORMONES1 |
| Anti-estrogens Tamoxifen, toremifene: hot flashes, insomnia, mood disturbance; at high doses tamoxifen can cause confusion Anastrazole (Arimidex), letrozole (Femara), exemestane (Aromacin): hot flashes, fatigue, mood swings, and irritability; cognitive effects are not known Raloxifene (Evista): no cognitive side effects noted Leuprolide (Lupron), goserelin (Zoladex): hot flashes, fatigue, and mood disturbance |
| Androgen Blockade Leuprolide (Lupron), goserelin (Zoladex): hot flashes, fatigue, and mood disturbance Flutamide (Eulexin), bicalutamide (Casodex), nilutamide (Nilandron): as above |
| Glucocorticoids Dose-related, variable psychiatric side effects including insomnia, hyperactivity, hyperphagia, depression, hypomania, irritability, and psychosis Treated by ad hoc antipsychotics easily with cancer patients Other drugs with benefit: lithium, valproate, lamotrigine, and mifepristone Dexamethasone (Decadron) 9 mg equals 60 mg of prednisone; psychiatric side effects are associated with this dose level Steroids used as part of an antiemetic treatment with chemotherapy infusion, with lymphoma protocols as high as prednisone 100 mg for 5 days, with nervous system radiation treatment to reduce swelling, with taxanes to reduce side effects |
| BIOLOGICALS |
| Interferon-alpha2,3 Depression, cognitive impairment, hypomania, psychosis, fatigue, and malaise Responsive to antidepressants, hypnotics, antipsychotics, stimulants, and antianxiety agents Associated with autoimmune thyroiditis that may increase or decrease thyroxine; check thyroid function May inhibit metabolism of some antidepressants by P450 enzymes CYP1A2, CYP2C19, CYP2D6 Interferon-beta has less neurotoxicity |
| Interleukin-2 Delirium, flu-like syndrome, dose-dependent neurotoxicity, and hypothyroidism |
| CHEMOTHERAPY |
| Vincristine (Oncovin), vinblastine (Velban), vinorelbine (Navelbine) Neurotoxicity is dose-related and usually reversible. Fatigue and malaise are noted. Seizure and SIADH are uncommon. Postural hypotension may be an aspect of autonomic neuropathy. Less toxicity is noted with vinblastine and vinorelbine. |
| Procarbazine (Matulane) Mild reversible delirium, depression, and encephalopathy A weak MAO inhibitor Antidepressant use must consider the timing of procarbazine or risk serious interactions Disulfiram-like effect; avoid alcohol |
| Asparaginase (Elspar) Depression, lethargy, and delirium with treatment |
| Cytarabine (ARA-cell, Alexin) High-dose IV treatment (over 18 g/m2/course) can cause confusion, obtundation, seizures and coma, cerebellar dysfunction, and leukoencephalopathy. Older patients with multiple treatments are more susceptible. Delirium and somnolence can be seen 2-5 days into treatment. Those with renal impairment are more vulnerable. |
| Fludarabine (Fludara) Rare somnolence, delirium, and rare progressive leukoencephalopathy |
| 5-Fluorouracil (5-FU) The primary neurotoxicity is cerebellar, but encephalopathy with headache, confusion, disorientation, lethargy, and seizures has also been seen. Rare deficiency of enzyme that metabolizes dihydropyrimidine dehydrogenase (DPD) is associated with greater exposure and more toxicity. Fatigue is the most common side effect. Cerebellar syndrome and rarely seizure or confusion or parkinsonism may be noted. High-dose IV thymidine may be an antidote for toxicity. |
| Capecitabine (Xeloda) Related to 5-FU, but with less neurotoxicity |
| Methotrexate Causes neurotoxicity particularly when the route is intrathecal or high-dose IV (usually over 1 g/m2). The toxicity, which is usually reversible, is related to peak level and duration of exposure. Leptomeningeal disease or other conditions that break the blood-brain barrier may impair drug clearance. Prolonged exposure allows the drug to pass through the ependyma of the ventricles to cause leukoencephalopathy. The risk is greater in patients also exposed to cranial radiation. Intrathecal methotrexate may also cause seizures, motor dysfunction, chemical arachnoiditis, and coma. Serum levels are followed closely; folinic acid (leucovorin) rescue is an antidote. Alkalinization may lower the serum level. There is a dose- and route-related risk of delirium. |
| Pemetrexed (Alimta) An antifolate given with supplements of folate, intramuscular vitamin B12, and dexamethasone. It is associated with a 10% rate of depression and fatigue. |
| Gemcitabine (Gemsar) Fatigue, flu-like syndrome, and a rare autonomic neuropathy |
| Etoposide (Eposin) Postural hypotension and rare disorientation |
| Carmustine (BCNU) Delirium, only at high dose, rare leukoencephalopathy |
| Thiotepa Rare leukoencephalopathy |
| Ifosfamide (Ifex) Transient delirium, lethargy, seizures, drunkenness, parkinsonism, and cerebellar signs that improve within days of treatment Risk factors: liver and kidney impairment Hyponatremia Leukoencephalopathy Thiamine or methylene blue may be antidotes |
| Cisplatin Rare reversible posterior leukoencephalopathy, parietal, occipital, frontal with cortical blindness. Peripheral neuropathy, poor proprioception, and rarely autonomic Hypomagnesemia secondary to renal wasting Vitamin E (300 mg), amifostine may limit peripheral toxicity Hearing is decreased due to dose-related sensorineural hearing loss |
| Carboplatin Neurotoxicity only at high doses |
| Oxaliplatin (Eloxatin) Acute dysesthesias of hands, feet, perioral region, jaw tightness, and pharyngo-laryngodysesthesias |
| Paclitaxel (Taxol) Sensory peripheral neuropathy not worse with continued treatment Rarely seizures and transient encephalopathy, and motor neuropathy Given with steroids |
| Docetaxel (Taxotere) Like paclitaxel but less neurotoxicity |
| INHIBITORS OF KINASE SIGNALING ENZYMES4,5 |
| The newest class of medications, specific inhibitors of kinase signaling enzymes, do not typically cause major behavioral side effects. However, their toxicity related to overlapping effects on several kinase pathways has not been fully defined. Hypertension has been an important side effect related to inhibition of the vascular endothelial growth factor (VEGF). Asthenia or feelings of weakness are commonly reported. |
| Imatinib (Gleevec) Can cause fluid retention and fatigue, rarely low phosphate; confusion and papilledema |
| Sunitinib (Sutent) Hypothyroidism, TSH should be checked every 3 months |
| Sorafenib (Nexavar) Fatigue and asthenia and rarely hypophosphatemia |
| Bevacizumab (Avastin) A monoclonal antibody that blocks VEGF-binding, causes fatigue, and rarely causes reversible posterior leukoencephalopathy |
| Thalidomide Drowsiness and somnolence improve over 2-3 weeks, dose-related, associated with dizziness, orthostatic hypotension, tremor, loss of libido, hypothyroidism, and rarely confusion |
| Bortezomib (Velcade) Postural hypotension and asthenia; confusion, psychosis, and suicidal thoughts have been reported |
| Rituximab (Rituxan) Headache and dizziness |
| Trastuzumab (Herceptin) Headache, insomnia, and dizziness |
IV, Intravenous; MAO, monoamine oxidase; SIADH, syndrome of inappropriate antidiuretic hormone; TSH, thyroid-stimulating hormone.
Since Weisman and co-workers explored how to help patients cope with cancer when they are demoralized,6–8 psychiatrists have tried to understand who the patient was before the diagnosis and the nature of the existential predicament. The psychiatric interview can assess the personal past, present plight, anticipated future, regrets, salient concerns, physical symptoms, disabilities, coping strategies, and psychiatric vulnerability (Table 56-2).7
Table 56-2 Concerns of Patients with Specific Cancer Types
| Cancer Type | Likely Concerns |
|---|---|
| Prostate cancer | Significance of serum prostate-specific antigen (PSA) test results: anxiety |
| Once diagnosed, the initial choices are watchful waiting, surgery, or radiation treatment | |
| Side effects of surgery or radiation: incontinence or erectile dysfunction | |
| Sexual function and dysfunction | |
| Androgen blockade and its effects on fatigue and loss of sexual interest | |
| Breast cancer | Body image related to mastectomy or to reconstruction |
| Adjuvant chemotherapy and its side effects: alopecia, weight gain, fatigue, and impaired concentration | |
| Menopausal symptoms: insomnia, sexual dysfunction, and hot flashes related to adjuvant treatment, antiestrogens, or aromatase inhibitors | |
| The question of prophylactic mastectomy | |
| Sexuality and fertility (or infertility) | |
| Colon cancer | Adjustment to surgery or an ostomy |
| Body image and sexual function | |
| Bowel dysfunction | |
| Lung cancer | Physical limitations of reduced lung capacity |
| Postthoracotomy neuralgia | |
| Cough | |
| Guilt about nicotine addiction (past and present) | |
| Ovarian cancer | Anxiety about the tumor marker CA125 |
| Sexual dysfunction and infertility | |
| Pain and recurrent bowel obstruction | |
| Pancreatic cancer | Maintenance of adequate nutrition |
| Poor appetite | |
| Bowel function (and the need for pancreatic enzymes and laxatives) | |
| Pain | |
| Diabetes | |
| Depressed mood | |
| Head and neck cancer | Facial deformity |
| Dry mouth | |
| Poor nutrition | |
| A weak voice and difficulty with communication | |
| Posttreatment hypothyroidism | |
| Alcohol and nicotine dependency | |
| Lymphoma | Corticosteroid-induced mood changes |
| The need for recurrent chemotherapy and its effects | |
| Hodgkin’s disease | Posttreatment hypothyroidism |
| Fatigue | |
| Osteosarcoma | Amputation/prosthesis vs. bone graft |
| Impaired mobility | |
| Postthoracotomy neuralgia |
Denial and “Middle Knowledge”
Patients often seem to know and want to know about the gravity of their illness, yet they often talk as if they do not know and do not want to be reminded about their cancer.9 Weisman used the expression “middle knowledge” for the space between open acknowledgement of death and its utter repudiation. Patients may deny facets, implications, or mortal threat of an illness.9 Middle knowledge is most apparent at transition points (such as a recurrence of cancer). However, denial is an unstable state, almost impossible to maintain against even the reluctant patient’s inner perceptions. To preserve a relationship, patients often deny their knowledge of impending death to different people at different times.10 Tactful discussion of mortality allows patients to be responsive to those most close as long as possible.9
Hope and the Doctor-Patient Relationship
Physicians convey respect by exploring the patient’s capacity to cope. That respect allows the patient to nurture courage and resiliency.11 Trust between the patient and the physician is borne out of mutual respect. Patients regain a sense of control as they appraise and reappraise what choices to make. Presenting the facts about an illness does not break trust between a patient and a doctor. Furthermore, hope is not merely related to prognosis. The patient’s capacity to hope is also related to an ego ideal and to the conviction of one’s influence on the world. As the physician sustains the patient’s self-esteem, a sense of purpose adds value to life regardless of the time frame. The psychiatrist’s capacity to listen to a patient in a nonjudgmental way allows patients to express doubts and weaknesses, to accept who they are and why they see things as they do. The physician’s presence there protects patients from abandonment and offers a place where they can explore what is meaningful.11,12
Medical Choices
The psychiatrist also clarifies with the patient the medical understanding of what choices are feasible. Unfazed by personal shock, anxiety, and denial, and armed with a medical education, the psychiatrist is in an excellent position to understand (better than the patient) the individualized medical plan. Diagnosis, treatment, and prognostic decisions are complex as set forth by medical experts. As the psychiatrist learns how the patient thinks, and if necessary, adds appropriate psychopharmacological treatment for symptoms or Axis I diagnoses, the psychiatrist can maintain a focus on necessary anticancer treatments that are most likely to give the best outcome. Focusing on problems, setting priorities, making clear what the patient is doing and not doing about a problem, and exploring strategies are key elements of care. This technique allows patients to make the decisions that are most critical to them. Meanwhile, the psychiatrist, in collaboration with oncology staff, sorts through differential diagnoses as new psychological symptoms develop and the medical condition and treatment progress. The psychiatric assessment includes evaluation of physical symptoms, psy chiatric diagnosis, and the differential diagnosis. The work includes education about how to support significant others and how to allow help or to relinquish control to those who have shown themselves trustworthy. The goal of honest communication is to support acceptance, to reduce bitterness, and to replace denial with the courage to confront what cannot be changed.10
Distress
In the study of newly diagnosed cancer patients, Weisman and associates found that the peak of distress varied from 1 or 2 days to 3 months, but that the intense distress lessened over time.13 Those more depressed and anxious, who had less financial and social support, more alcohol abuse, more troubled relationships, and more burden from illness, were more distressed. The researchers learned that high-risk patients were unable to generate a number of alternate coping strategies.7 Vulnerable patients tended to overuse strategies that were ineffective for finding relief and resolution. Weisman and associates defined a treatment to reduce distress, to correct deficits in coping, to reclaim personal control, and to improve morale and self-esteem. Patients were asked to examine their plight in relation to cancer (their current concerns); to articulate their understanding of what might interfere with good coping; and by looking beyond, to use options that were feasible for finding satisfactory solutions. Staff took the view that change was possible and that patients could be helped to take steps on their own behalf, as problems were broken down into manageable proportions. They focused on coping and adaptation rather than on psychopathology; they conveyed an expectation of positive change, a sense that options and alternatives are seldom completely exhausted, and an awareness that flexibility in perceiving problems helps to attain additional information and support. They compared a brief psychodynamic and behavioral technique; both were effective in reducing distress and denial.7
Screening
Weisman and Worden defined a screening instrument and a concise interview to identify patients at high risk for psychosocial vulnerability and ineffective coping (because those individuals were most apt to benefit from psychosocial intervention). To make screening more efficient, Zabora and co-workers validated the Brief Symptom Inventory–1814 to the Index of Current Concerns (now a Brief Symptom Inventory–11)15 to identify the more anxious and depressed patients with more somatic symptoms and distress. To call attention of oncology staff to distressed cancer patients, Holland and colleagues in the National Comprehensive Cancer Network (NCCN) guidelines operationalized a visual nomogram (a distress thermometer score ≥ 5) with a variety of needs assessed.16
Psychosocial Interventions
Weisman’s work foreshadowed major studies of preventive psychosocial interventions for cancer patients. Fawzy and colleagues17–20 described a 6-week structured group intervention for patients with melanoma Stage I and II; it included health education, stress management, coping skills, and supportive group psychotherapy. They also taught simple relaxation exercises (e.g., progressive muscle relaxation, guided imagery or self-hypnosis, as well as problem-solving and coping methods). The interaction of the patients within the group provided a source of emotional support.17–20 The group with 6 weeks of treatment had a survival benefit at 5 to 6 years, but this comparative benefit was not as evident in the tenth year.
Spiegel and others developed a group therapy supportive-expressive intervention for women with metastatic breast cancer. In two randomized multisite studies, the benefit for survival has not been found, but the ability of this intervention to reduce distress, to offer patients social support and safe conduct, and to increase their ability to confront difficult challenges has been documented.21–24
Chochinov25 focused on conserving dignity at the end of life by asking patients what they feel is most important and what they want their loved ones to remember. Informed by the work of Frankl,26 Greenstein and Breitbart27 and Breitbart and colleagues28 reported on a group intervention for advanced cancer patients who focus on faith and meaning.
Combinations of interventions augment patients’ coping skills.29 Teaching about relaxation has had a benefit for cancer patients,29,30 and a variety of educational interventions, tailored to disease type and phase (e.g., stress management, cognitive therapy, and behavioral training), have improved coping and decreased distress.31 As parents with cancer worry about their children, Rauch and Muriel32 have offered guidance.
ANXIETY SYNDROMES
Claustrophobia becomes clinically important when magnetic resonance imaging (MRI) is required for careful physical evaluation or when patients are trapped in bed by orthopedic care (e.g., a repair of a leg with osteogenic sarcoma). Needle phobias, which can be problematic, may be treated with rapid desensitization.33
Some patients live on tenterhooks waiting for the results of the 3-month scans to assess disease recurrence. They develop great anxiety in the week or so before each reassessment. The roller coaster ride of life-threatening experiences and uncertainty associated with cancer treatment parallels the unpredictable aversive stimuli that provoke conditioned helplessness and depression.34 For some patients, the challenge of cancer initiates chronic anxiety, but many patients have arrived with a predisposition to generalized anxiety disorder (GAD), phobias, or panic disorder. Patients anticipate the results of cancer markers (e.g., women with ovarian cancer who await the report of the CA125); their mood rises and falls with the results of tumor markers or progressive disease.35 Most patients are alert to physical symptoms after treatment and worry that such symptoms signify recurrent disease. A visit to the doctor can be reassuring for most; but for some, the alarm of danger does not turn off. They remain preoccupied with fear, as every symptom signals cancer recurrence. Fortunately, antidepressant medications suppress the chronic state of alarm and reduce chronic anxiety. Benzodiazepines are best used for specific anxiety-provoking procedures. Tools of cognitive-behavioral therapy (CBT) also improve coping.
Posttraumatic stress disorder (PTSD) is uncommon (occurring in 3% to 10%) in patients treated for cancer36; however, Pitman and colleagues37 have shown that women with breast cancer have a physiological response 2 years after hearing a narrative of the two most stressful experiences during their cancer. Leukemia survivors who developed anticipatory nausea with treatment are also more apt to become nauseated in response to reminders of their treatment.38 The numbness and tingling of peripheral neuropathy can be a reminder of treatment that triggers intrusive thoughts and avoidant behaviors of PTSD.39 The symptoms of PTSD are often associated with co-morbid depression.37
Panic disorder and other anxiety disorders occur among cancer patients at about the same rate as they do in the general population.40 However, specific cancer-related symptoms (e.g., embarrassment related to unexpected diarrhea) can contribute to anticipatory anxiety and agoraphobia. Disability and poor quality of life have been associated with co-morbid anxiety disorders and physical conditions.41
Nausea and Vomiting
Many chemotherapy agents and radiation treatments cause nausea and vomiting. Even in this era of advanced anti-emetics, highly and moderately emetogenic chemotherapy is associated with nausea in 60% of patients and vomiting in 36%. Delayed nausea, which comes after the first days of chemotherapy, significantly compromises quality of life even more than vomiting does.42 As a result of vomiting during chemotherapy, patients can develop conditioned nausea and anxiety associated with the smells and sights linked with treatment. They may have nausea and vomit even before arriving at the hospital for treatment.43 Conditioned nausea morphs into anticipatory anxiety, insomnia, and aversion to treatment. Hypnosis, cognitive-behavioral techniques, and antianxiety agents (e.g., alprazolam or lorazepam) can reduce phobic responses, as well as anticipatory nausea and vomiting (both during and after chemotherapy).44
The best antiemetic drugs make conditioned vomiting less likely, but patients still vomit after doxorubicin, cisplatin, or carboplatin (even when a 5-hydroxytryptamine-3 [5-HT3] receptor antagonist and dexamethasone are employed).45 Some patients who develop anticipatory anxiety avoid the hospital and its routine smells long after cancer treatment has ended. Anticipatory nausea and vomiting are more likely to occur in younger patients, in those who have had more emetic treatments, and in those who have trait anxiety.43 Nausea may also be a symptom of anxiety that persists as part of major depressive disorder (MDD).
DEPRESSION
In people with cancer, MDD is associated with poor quality of life, worse adherence to treatment, longer hospital stays, greater desire for death, and an increased suicide and mortality rate.46–49 Reports of its prevalence (10% to 25%) in people with cancer have varied widely.50 People with histories of MDD are more likely to develop MDD after the diagnosis of cancer, but about half of the cases occur in people without a history of MDD.51
As in people without cancer, the diagnosis of MDD is made with DSM-IV criteria. However, the diagnosis can be complicated by symptoms that overlap with cancer and cancer treatments. To address this issue, alternative criteria have been proposed, such as the Endicott criteria, which substitute psychological symptoms for physical symptoms in the DSM criteria. While the inclusion of physical symptoms that could be related to cancer or cancer treatments in the diagnosis of MDD remains controversial, alternative criteria have not yielded markedly different results.52
Similar to the evaluation of other medically ill patients who appear depressed, it is critical to consider possible medical contributions in the differential diagnosis. Untreated pain, hypothyroidism, and medications such as corticosteroids and chemotherapies (e.g., alpha interferon, pemetrexed, and procarbazine) may contribute to MDD. Delirium, especially the hypoactive subtype, can be mistaken for depression. Although mood symptoms occur as part of delirium, key features include a generalized impairment of attention and cognition, a waxing and waning severity of symptoms, and a sleep-wake disturbance. Fatigue is a common cancer-related symptom that is prevalent and that can be difficult to tease apart from MDD. Anhedonia may be the best distinguishing factor for MDD.53 Apathy (e.g., that results from a lesion in the frontal lobes) can also be reminiscent of MDD. With apathy there are delayed responses, cognitive impairment, and a loss of spontaneous action or speech.
While antidepressants are commonly used to treat MDD that is co-morbid with cancer, few placebo-controlled trials of their efficacy in cancer exist, and there is some evidence that depression in patients with medical co-morbidity is more resistant to antidepressant therapy.50,54 Potential side effects may have greater weight in the choice of an antidepressant medication in a person with cancer. Patients may already be experiencing constipation or nausea from their cancer medications, and antidepressants should be chosen that would not exacerbate these symptoms. Many times antidepressant medications are chosen for their side-effect profile (such as sedation or increased appetite), which may be desirable in some cases. Some of the selective serotonin reuptake inhibitors (SSRIs) (e.g., fluoxetine, fluvoxamine, and paroxetine) and bupropion can interfere because of their effects on cytochrome P450 2D6 system with the metabolism of commonly used medications in oncology.55 Stimulant medications (such as methylphenidate and dextroamphetamine) may also be beneficial (i.e., may lift mood, increase appetite, and improve fatigue) for MDD in medically ill patients, but there is little evidence to support this practice. When response to stimulants develops, it is usually seen within 1 week.
Although little research has been conducted on medications for MDD in people with cancer, there are a bevy of studies that confirm the efficacy of psychosocial interventions.50 The severity of cancer-related symptoms (such as fatigue and nausea) and the demanding schedules for anticancer treatments may limit a patient’s ability to participate in the traditional weekly 50-minute psychotherapy visit. Therefore, psychotherapy visits need to be flexible and include shorter visits, meet during chemotherapy treatments, and involve more phone contact.
FATIGUE
Fatigue (the most commonly reported symptom in people with cancer and the symptom that causes the most functional impairment)56 in people with cancer can often be confused with MDD.57 Although fatigue is not a psychiatric disorder, psychiatric contributions can cause fatigue; therefore, fatigue could be considered a psychosomatic illness.
Prevalence
The prevalence (estimated at 60% to 90% of patients)58,59 of fatigue in people affected by cancer varies widely because of differing measures of fatigue and heterogeneous populations studied.
Diagnosis
Fatigue can arise before the diagnosis of cancer, during active cancer treatment, and into the survivorship years. The diagnosis is made primarily by asking questions about the presence and severity of the symptoms. While there are validated instruments for the measurement of fatigue57 (e.g., the Functional Assessment of Chronic Illness Therapy–Fatigue scale [FACIT-F]), administration of these questionnaires may not be feasible in busy clinical settings. The National Comprehensive Cancer Network (NCCN) recommends screening for fatigue at visits with a one-item, 0-to-10 scale, similar to that used for screening of pain, with 0 being “no fatigue” and 10 being “the most severe fatigue.” Scores of 4 or greater should prompt further evaluation. (The full set of guidelines that also includes a review of the literature can be viewed at www.nccn.org.)
Treatment
Stimulants
Stimulants (such as methylphenidate, dextroamphetamine, and modafinil) are commonly used in the treatment of fatigue. However, there has been mixed support for this treatment in clinical trials. Open-label trials have suggested benefit, but response rates for placebos have also been remarkably high in the few randomized, placebo-controlled trials.60 In addition to reducing fatigue, stimulants also improve mood, concentration, and appetite. However, stimulants can raise blood pressure and heart rate and should be used with caution in patients with cardiac disease. Common side effects include constipation, sleep disturbance, anxiety, and (at higher doses) anorexia.
Exercise
Abundant evidence exists for exercise as a treatment of fatigue, with several studies demonstrating the benefit of exercise for fatigue in people with cancer.61 Mental health clinicians can encourage exercise through motivational interviewing and through behavioral changes. Because patients can have serious physical morbidities, such as large bone metastases that could lead to fracture, consultation with the oncologist is recommended before initiating exercise. A physical therapist can assist in designing an exercise program that contains both strength and aerobic training, and that is appropriate for a person with physical limitations from cancer or cancer treatments. For more medically complicated patients, exercise might best be done in a cardiovascular or pulmonary rehabilitation center.
Behavioral Interventions
Behavioral interventions (such as cognitive-behavioral therapy [CBT] and energy conservation) may be beneficial as both primary and adjunctive treatments for fatigue in cancer patients. CBT emphasizes management of fatigue, rather than a cure for it.62 Energy conservation is similar to that with CBT in some respects; it focuses on prioritizing activities and delegating, problem-solving around difficulties caused by the fatigue, and improving organizational skills.63
CONFUSION AND COGNITIVE IMPAIRMENT
Infections, metabolic abnormalities, and recent surgery account for abnormal mental status in most hospitalized cancer patients, but drugs (especially opiates and benzodiazepines) are the most common culprits causing delirium in cancer patients. Older patients, as well as patients with structural brain disease, vascular disease, or lung, kidney, or liver impairment, are predisposed to delirium.64 Specific cancer-related syndromes of cognitive impairment that may cause psychiatric symptoms in oncology patients are listed below.
Hyponatremia
Hyponatremia, a common in-hospital metabolic abnormality, often results from the syndrome of inappropriate antidiuretic hormone (SIADH) that occurs as a paraneoplastic syndrome (especially from small cell carcinoma, but also from non–small cell lung cancer, mesothelioma, pancreatic cancer, duodenal cancer, lymphoma, endometrial cancer, and leukemia). SIADH is associated with lung infection, cerebral tumors, brain injury, and complications of many psychotropic medications (e.g., phenothiazines, SSRIs, carbamazepine, and tricyclic antidepressants [TCAs]). If the sodium is less than 125 mmol/L or if it falls rapidly, it may cause agitation, confusion, and hallucinations; chronic hyponatremia is associated with falls and inattention in the elderly.65
Brain Tumors
Some cancer patients with confusion have brain metastases. The incidence of primary brain tumors is 6.6 per 1,000,000, but the rate of metastatic brain tumors is higher (ranging from 8 to 11 per 100,000). While the majority of brain metastases originate in the lung, melanoma has the greatest tendency to metastasize to the central nervous system (CNS). The incidence of brain metastases is 20% for lung cancer, 7% for melanoma, 6.5% for renal cancer, 5% for breast cancer, and 2% for colorectal cancer.66 Brain metastases also occur (but less commonly) in sarcoma, thyroid, pancreatic, ovarian, uterine, prostate, testicular, and bladder cancers. In patients with small cell lung cancer, brain metastases are anticipated, and prophylactic whole brain radiation is often recommended.67 Isolated brain metastases from non–small cell lung cancer may be treated surgically, and modern radiation techniques target small areas of the brain.
Patients with primary brain tumors, particularly low-grade tumors, present problems for cancer diagnosis and chronic brain injury. Tumors (particularly of the frontal, temporal, and limbic lobes) that affect the hardwiring of motivation, attention, mood stability, and memory come to psychiatric attention. Temporal lobe epilepsy can be associated with psychological symptoms (e.g., memory dysfunction, anxiety, hypergraphia, and viscosity). For these patients, neuropsychiatric consultation, testing, and cognitive rehabilitation may be critically important to define and to treat the specific loss. Multimodal interventions for attention, memory, word-retrieval, and problem-solving abilities may be appropriate.68 Loved ones can understand more clearly the basis of some limitations, and the patient can acknowledge his or her deficits and take whatever control is possible. Psychotropic medications, used appropriately, are adjuncts to anticonvulsants and to anticancer treatment.
Leptomeningeal Disease
In the syndrome of carcinomatous meningitis or leptomeningeal disease, cancer cells infiltrate the meninges or obstruct CSF flow. If there are no focal signs or findings on neuroimaging, the associated malaise may be thought of as psychiatric. Cranial nerves (particularly III, IV, VI, and VII) may be affected. Mental changes, headache, difficulty with walking, limb weakness, or seizures are common. Dizziness and sensorineural deafness have also been noted. The tumors most often implicated are breast cancer, lung cancer, and melanoma; the histology most often is adenocarcinoma. Leptomeningeal disease also occurs with non-Hodgkin’s lymphoma, leukemia, and cancers of the head-neck, cervix, ovary, kidney, and bladder. Malignant cells in CSF confirm the diagnosis, but a large enough fluid sample improves the chance for a positive diagnosis; half to 70% may be falsely negative. A brain MRI may be unremarkable in 20%, but more likely will show hydrocephalus, brain metastases, or contrast enhancement of the sulci or cisterns.69,70
Delirium in Stem Cell Transplantation
In patients who receive a peripheral stem cell transplantation, delirium is common, due to metabolic abnormalities, infection, and subdural or intraparenchymal brain hemorrhage. Wernicke’s encephalopathy has occurred rarely. An engraftment syndrome may cause delirium with fever, headache, and rash. Usually this syndrome occurs when the neutrophil count is greater than 500/mm3 and with a cytokine effect of the hematopoietic colony-stimulating factors.71,72
In the transplant setting, immunosuppressive drugs (e.g., cyclosporin, tacrolimus, or the antifungal drug amphotericin B) can cause delirium. Hypertension, use of steroids, uremia, and previous radiation to the brain are risk factors.73 Drug serum levels may facilitate diagnosis; tremor occurs in 40%, and paresthesias in 11%. Hypomagnesemia increases the risk of seizures. Immunosuppressants are rarely associated with a syndrome of reversible posterior leukoencephalopathy that causes headache, visual loss or blurring, visual hallucinations, and confusion.74 Parkinsonism, ataxia, or dystonia can also occur. White matter edema is documented in the parieto-occipital area on fluid-attenuated inversion recover sequences (FLAIR). If this syndrome has occurred with one immunosuppressant, either tacrolimus or cyclosporin, the other may be used.75
Hyperviscosity Syndrome
Hyperviscosity syndrome in myeloma or lymphoma patients results from paraproteins that impair blood circulation in the brain. A serum viscosity level above 4.0 centipoise (1.56 to 1.68 cp) has been associated with symptoms.76,77 The clinical signs are bleeding, visual signs and symptoms, and delirium.
Cushing’s Syndrome
Cushing’s syndrome may cause delirium, psychosis, and muscle weakness in adrenocortical carcinoma or tumors with paraneoplastic production of adrenocorticotropic hormone (ACTH). Psychiatric disorders are a feature of the syndrome of ectopic ACTH production in 53% of patients. Specific treatments for this syndrome include steroidogenesis inhibitors or glucocorticoid receptor antagonists (e.g., ketoconazole, metyrapone, aminoglutethimide, opDDD, etomidate, or mifepristone).78
Paraneoplastic Neurological Disorders
Paraneoplastic neurological disorders include a myasthenic syndrome, cerebellar degeneration, or diffuse encephalomyelitis.79 These syndromes may manifest before the cancer is diagnosed. An afflicted patient may develop an immune reaction against neural tissue when exposed to a tumor antigen that reacts against normal nervous system in the setting of dying tumor cells. The exact mechanism of the immune reaction has not been established. Typically there are more T and B cells and plasma cells in these tumors. Small cell lung cancer is the tumor most commonly associated with this syndrome, and anti-Hu antibody syndromes are often noted.80
Limbic Encephalitis
Limbic encephalitis is a specific autoimmune encephalopathy that causes memory difficulties, anxiety, depression, agitation, confusion, hallucinations, and complex partial seizures. This syndrome is most likely to occur in the context of small cell carcinoma of the lung and more likely related to anti-Hu antibody, but it also occurs with Hodgkin’s lymphoma, thymoma, and cancers of the testes, breasts, ovaries, stomach, uterus, kidney, thyroid, and colon.79,81 Changes in mood and personality occur over weeks. Recent memory is more affected than remote memory. In one-third of patients the neurological findings are limited to the limbic system. MRI findings, if present, include abnormal hyperintensity on the T2-weighted image, sometimes with contrast. Anticonvulsants and empirical use of psychotropic medications may be the only aid as the tumor is diagnosed and treated. The prognosis is limited.
Toxic Leukoencephalopathy
White matter injury can be an early, a temporary, or a late consequence of cancer treatment. Whole brain radiation and certain anticancer drugs can injure the projection fibers, association fibers, and commissural tracts that affect cognition and emotion. These drugs include methotrexate, carmustine, cisplatin, levamisole, fludarabine, thiotepa, ifosfamide, cytarabine, and fluorouracil. Acutely, confusion is related to patchy, reversible edema and later to widespread edema and demyelination. More severe delayed consequences result from loss of myelin and axons related to vascular necrosis and thrombosis. Risk of leukoencephalopathy is related to patient age, total dose of radiation, fraction size, and timing of chemotherapy. In most cancer protocols, the doses and timing have been adjusted to minimize these adverse effects. However, sometimes patients with vulnerable brains or delayed metabolism of the drug may be unexpectedly affected. Neurobehavioral sequelae occur in 28% of patients acutely and are a consideration in cancer survivors.82
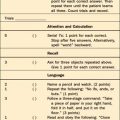
The frontal lobes, the lobes with the most white matter tracts, are the ones most likely to be injured. Patients with mild leukoencephalopathy may complain of difficulty with concentration and vigilance and problems with attention. Apathy, anxiety, irritability, depression, or changes in personality may be seen with memory loss, slowed thinking, and failure of executive oversight. In more severe cases, dementia, abulia, stupor, and coma occur with necrotic areas and diffuse hyperintensity of white matter on MRI. This injury, unlike that seen in Alzheimer’s disease, spares language, praxis, perceptions, and procedural memory. The key bedside tests on mental status examination are the elements that test attention (e.g., digit span, serial sevens, three-word delayed recall, and clock drawing for visuospatial skills and alternating motor sequences for frontal function). The contribution of white matter injury should be documented on T2-weighted MRI.83
This toxicity is seen in patients who have had high-dose methotrexate and radiation treatment for childhood acute lymphoblastic leukemia (ALL) or primary CNS lymphoma. In the latter, a rapidly progressive subcortical dementia with psychomotor slowing, executive and memory dysfunction, behavioral changes, ataxia, and incontinence can be seen. Diffuse white matter disease and cortical-subcortical atrophy are also noted.84
Cognition and Sleep Changes Related to Chemotherapy or Hormonal Change
Patients receiving adjuvant chemotherapy for breast cancer complain about troubles with working memory and concentration. Despite the reputation of “chemo brain,” subjective complaints often do not match deficits seen on neuropsychiatric testing. At the very least, fatigue, catabolic treatment effects, and hormonal change combine to cause impairment that seems to improve gradually. Of the published studies that test breast cancer survivors in the neuropsychological domain, half have found memory affected.85 Patients in greater distress report more cognitive failures, but not specific neuropsychiatric deficits.86
Patients who receive adjuvant treatment for breast cancer often develop menopausal symptoms because of a direct effect on the ovary that decreases hormone levels and hastens menopause. Menopause is a goal of treatment for those with estrogen receptor–positive tumors.87 Estrogen therapy, however, may selectively improve executive function during verbal recall tasks, and women may notice the loss of estrogen.88 Estrogen treatment during perimenopause has been associated with regional benefits, for instance in the posterior cingulum.89 Raloxifene, a selective estrogen-receptor modulator (at 20 mg each day), tended to slow cognitive decline over 3 years in postmenopausal women (regarding verbal memory and attention).90
Tamoxifen is a mixed agonist/antagonist of estrogen that is associated with hot flashes and insomnia. Aromatase inhibitors reduce estradiol to barely detectable concentrations. There seem to be no distinctions in quality-of-life measures in women taking tamoxifen or an aromatase inhibitor (e.g., anastrozole). Vasomotor symptoms are frequent with both drugs. Endocrine-related vaginal symptoms are worse at 3 months but improve slightly or stabilize thereafter. About 10% report mood swings and irritability.91,92
SSRIs reduce the frequency and severity of hot flashes and improve mood, sleep, and anxiety, and quality of life in menopausal women and women who undergo estrogen deprivation therapy for breast cancer. The benefit for hot flashes may be related to the affinity of the norepinephrine transporter as the benefit is not consistently seen with citalopram or fluoxetine.93–96 Venlafaxine (extended-release 75 mg/day) and paroxetine (20 mg/day) have shown benefit. Gabapentin (300 mg tid) has also been beneficial.97 A pilot study suggested that bupropion did not have a similar benefit.98 Hot flashes are also a problem for men with prostate cancer treated with pituitary antagonists (e.g., leuprolide).99
Treatment of Cognitive Deficits
Some causes of impaired cognition are treatable. Sobriety can lead to reversal of atrophy and to improved cognitive function in alcoholic dementia, a condition with disproportionate white matter damage, particularly in the frontal lobes. Oxygen treatment, at night or all day, may improve the cognition of lung cancer survivors who are chronically hypoxic. Stimulants may improve attentional deficits. Erythropoietin and an intervention with four visits and three phone calls, education about possible problems, stress management, arousal management, and compensatory strategies for memory and attention improves cognitive function and energy in patients with hemoglobin levels below 12.100
Survivors of Childhood Cancer
The most common consultation questions include evaluation of the child with anticipatory anxiety, sleep disturbances, behavioral problems, or mood changes. Anticipatory anxiety still affects many children despite advances in antiemetic medication. The child may feel nauseated or vomit on approaching the hospital, clinic, or phlebotomist. Behavioral modification, visualization and relaxation, and medication are helpful. Sleep disturbances may be related to children’s worries about the illness or to medications such as steroids. Child psychiatrists are also consulted for behavior problems in younger children who become more aggressive or difficult to manage. The emphasis in treatment of depression or withdrawn states is on the child’s ability to enjoy life. Overall, children tend to be quite resilient. Although children and parents are very distressed at the time of diagnosis, the prevalence of psychological problems among survivors of childhood cancer is similar to that in the community.101
Children treated for ALL or for a brain tumor with cranial irradiation are at risk for cognitive decline.102 Cognitive decline is progressive over at least 10 years. A radiation-induced progressive microvasculopathy may account for this progression. Babies are particularly prone to radiotoxicity in the first 2 years of life because of the rapid growth of the brain and white matter development in those years. Cognitive defects have been seen in verbal comprehension, perceptual organization, distractibility, and memory in survivors of ALL. Visuomotor integration, sequential memory, fine motor coordination, processing speed, and math abilities may also be affected. Risk factors include young age, female gender, and time since radiation. The volume and dose of radiation are also thought to be factors. The combination of radiation treatment and intrathecal methotrexate increases the risk of cognitive impairment. Children exposed to cranial irradiation may also suffer hypothyroidism and growth hormone deficiency. Children who have been treated for leukemia or brain tumors should be monitored for cognitive and endocrine dysfunction.
1 Sul JK, DeAngelis LM. Neurologic complications of cancer chemotherapy. Semin Oncol. 2006;33(3):324-332.
2 Kirkwood JM, Bender C, Agarwala S, et al. Mechanisms and management of toxicities associated with high-dose interferon alfa-2b therapy. J Clin Oncol. 2002;20:3703-3718.
3 Islam M, Frye RF, Richards TJ, et al. Differential effect of IFN alpha 2b on the cytochrome P450 enzyme system: a potential basis of IFN toxicity and its modulation by other drugs. Clin Cancer Res. 2002;8:2480-2487.
4 Desai J, Yassa L, Marqusee E, et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann Intern Med. 2006;145:660-664.
5 Casarett D. Editorial: terminal ballistics of kinase inhibitors: there are no magic bullets. Ann Intern Med. 2006;145:702-703.
6 Weisman AD, Worden JW: Coping and vulnerability in cancer patients: report of results, National Cancer Institute grant no R18-CA-14104, Project Omega, 1976.
7 Weisman AD, Worden JW, Sobel HJ: Psychosocial screening and intervention with cancer patients, National Cancer Institute grant no 19797 (1977-1980), Project Omega, 1980.
8 Weisman AD. The coping capacity: on the nature of being mortal. New York: Human Sciences Press, 1984.
9 Weisman AD. On denying and dying. New York: Behavioral Publications, 1972.
10 Weisman AD. The realization of death: a guide for the psychological autopsy. New York: Jason Aronson, 1974.
11 Weisman AD. The vulnerable self: conquering the ultimate questions. New York: Plenum Press, 1993.
12 Weisman AD. The existential core of psychoanalysis: reality sense and responsibility. Boston: Little, Brown, 1965.
13 Weisman AD, Worden JW. The existential plight in cancer: significance of the first 100 days. Int J Psychiatry Med. 1976;7:1-15.
14 Zabora JR, Smith Wilson R, et al. An efficient method for psychosocial screening of cancer patients. Psychosomatics. 1990;31:192-196.
15 Zabora J, Brintzenhofeszoc K, Jacobsen P, et al. A new psychosocial screening instrument for use with cancer patients. Psychosomatics. 2001;42:241-246.
16 NCCN practice guidelines for the management of psychosocial distress. National Comprehensive Cancer Network. Oncology (Huntington). 1999;12:1133-1476.
17 Fawzy FI, Cousins N, Fawzy N, et al. A structured psychiatric intervention for cancer patients. I. Changes over time in methods of coping and affective disturbance. Arch Gen Psychiatry. 1990;47:720-725.
18 Fawzy FI, Fawzy NW, Hyun CS, et al. Malignant melanoma: effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival six years later. Arch Gen Psychiatry. 1993;50:681-689.
19 Fawzy FI, Kemeny ME, Fawzy N, et al. A structured psychiatric intervention for cancer patients. II. Changes over time in immunological measures. Arch Gen Psychiatry. 1990;47:729-735.
20 Fawzy FI, Canada AL, Fawzy NW. Effects of a brief, structured psychiatric intervention on survival and recurrence at 10-year follow-up. Arch Gen Psychiatry. 2003;60:100-103.
21 Kogon MM, Biswas A, Pearl D, et al. Effects of medical and psychotherapeutic treatment on the survival of women with metastatic breast carcinoma. Cancer. 1997;28:225-230.
22 Spiegel D. Mind matters: group therapy and survival in breast cancer. N Engl J Med. 2001;345:1767-1768.
23 Classen PJ, Butler LD, Koopman C, et al. Supportive-expressive group therapy and distress in patients with metastatic breast cancer: a randomized clinical intervention trial. Arch Gen Psychiatry. 2001;58:494-501.
24 Goodwin PJ, Leszcz M, Ennis M, et al. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719-1726.
25 Chochinov HM. Dignity-conserving care—a new model for palliative care: helping the patient feel valued. JAMA. 2002;287:2253-2260.
26 Frankl V: The doctor and the soul: an introduction to logotherapy (translated by R Winston and C Winston), New York, 1957, Alfred A Knopf.
27 Greenstein M, Breitbart W. Cancer and the experience of meaning: a group psychotherapy program for people with cancer. Am J Psychother. 2000;54:486-500.
28 Breitbart W, Gibson C, Poppito SR, Berg X. Psychotherapeutic interventions at the end of life: a focus on meaning and spirituality. Can J Psychiatry. 2004;49:366-372.
29 Fawzy FI. Psychosocial interventions for patients with cancer: what works and what doesn’t. Eur J Cancer. 1999;35:1559-1564.
30 Fawzy FI, Fawzy NW, Arndt LA, et al. Critical review of psychosocial interventions in cancer care. Arch Gen Psychiatry. 1995;52:100-113.
31 Leubbert K, Dahme B, Hasenbring M. The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytic review. Psycho-oncology. 2001;10:490-502.
32 Rauch PK, Muriel AC. Raising an emotionally healthy child when a parent is sick. New York: McGraw-Hill, 2006.
33 Fernandes PP. Rapid desensitization for needle phobia. Psychosomatics. 2003;44:253-254.
34 Seligman MEP. Helplessness: on depression, development, and death. San Francisco: WH Freeman, 1975.
35 Fertig DL, Hayes DF. Psychological responses to tumor markers. In: Holland JC, editor. Psycho-oncology. New York: Oxford University Press, 1998.
36 Cordova MJ, Andrykowski MA, Kenady DE, et al. Frequency and correlates of posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. J Consult Clin Psychol. 1995;63:981-986.
37 Pitman RK, Lanes DM, Williston SK, et al. Psychophysiologic assessment of post-traumatic stress disorder in breast cancer patients. Psychosomatics. 2001;42:133-140.
38 Greenberg DB, Kornblith AB, Herndon JE, et al. Quality of life for adult leukemia survivors treated on clinical trials of Cancer and Leukemia Group B during the period 1971-1988; predictors for later psychologic distress. Cancer. 1997;80:1936-1944.
39 Kornblith AB, Herndon EJII, Weiss RB, et alfor the Cancer and Leukemia Group B [CALGB], Chicago, Ill. Long-term adjustment of survivors of early stage breast cancer 20 years after adjuvant chemotherapy. Cancer. 2003;98:679-689.
40 Stark D, Kiely M, Smith A, et al. Anxiety disorders in cancer patients: their nature, associations, and relation to quality of life. J Clin Oncol. 2002;20:3137-3148.
41 Sareen J, Jacobi F, Cox BJ, et al. Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med. 2006;166:2109-2116.
42 Bloechl-Daum B, Beuson RR, Mavros P, et al. Delayed nausea and vomiting continue to reduce patients’ quality of life after highly and moderately emetogenic chemotherapy despite antiemetic treatment. J Clin Oncol. 2006;24:4472-4478.
43 Greenberg DB, Surman OS, Clarke J, et al. Alprazolam for phobic nausea and vomiting related to cancer chemotherapy. Cancer Treatment Rep. 1987;71:549-550.
44 Andrykowski MA. The role of anxiety in the development of anticipatory nausea in cancer chemotherapy: a review and synthesis. Psychosom Med. 1990;54:458-475.
45 Hickok JT, Roscoe JA, Morrow GR, et al. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics. Cancer. 2003;97:2880-2886.
46 Richardson J, Sheldon D, Krailo M, et al. The effect of compliance with treatment on survival among patients with hematologic malignancies. J Clin Oncol. 1990;8:356-364.
47 Spiegel D, Kato PM. Psychosocial influences on cancer incidence and progression. Harv Rev Psychiatry. 1996;4:10-26.
48 Herrmann C, Brand-Driehorst S, Kaminsky B, et al. Diagnostic groups and depressed mood as predictors of 22-month mortality in medical inpatients. Psychosom Med. 1988;60:570-577.
49 Breitbart W, Rosenfeld B, Pessin H, et al. Depression, hopelessness, and desire for hastened death in terminally ill patients with cancer. JAMA. 2000;284:2907-2911.
50 Pirl WF. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. JNCI Monograph. 2004;32:32-39.
51 Kadan-Lottick NS, Vanderwalker LC, Block SD, et al. Psychiatric disorders and mental health service use in patients with advanced cancer: a report from the coping with cancer study. Cancer. 2005;104:2872-2881.
52 Kathol RG, Mutgi A, Williams J, et al. Diagnosis of major depression in cancer patients according to four sets of criteria. Am J Psychiatry. 1990;147:1021-1024.
53 Reuter K, Harter M. Fatigue and/or depression? Examination of the construct validity of CRF. Psycho-oncology. 2003;12(suppl):S259-S260.
54 Iosifescu DV, Bankier B, Fava M. Impact of medical comorbid disease on antidepressant treatment of major depressive disorder. Curr Psychiatry Rep. 2004;6:193-201.
55 Kalash GR. Psychotropic drug metabolism in the cancer patient: clinical aspects of management of potential drug interactions. Psycho-oncology. 1998;7:307-320.
56 Curt CA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353-360.
57 Pirl W. Fatigue. In: Holland JC, Greenberg DB, Hughes MK, editors. Quick reference for oncology clinicians: the psychiatric and psychological dimensions of cancer symptom management. Charlottesville, VA: American Psychosocial Oncology Society, 2006.
58 Vainio A. Prevalence of symptoms among patients with advanced cancer: an international collaborative study. Symptom Prevalence Group. J Pain Symptom Manage. 1996;12:3-10.
59 Lawrence DP, Kupelmick B, Miller K, et al. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. JNCI Monograph. 2004;32:40-50.
60 Bruera E, Valero V, Driver L, et al. Patient-controlled methylphenidate for cancer fatigue: a double-blind, randomized, placebo-controlled trial. J Clin Oncol. 2006;24:2073-2078.
61 Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362:640-650.
62 Gielissen MF, Verhagen S, Witjes F, Bleijenberg G. Effects of cognitive behavioral therapy in severely fatigued disease-free cancer patients compared with patients waiting for cognitive behavioral therapy: a randomized-controlled trial. J Clin Oncol. 2006;42:4882-4887.
63 Barsevick AM, Dudley W, Beck S, et al. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer. 2004;100:1302-1310.
64 Tuma R, DeAngelis LM. Altered mental status in patients with cancer. Arch Neurol. 2000;57:1727-1731.
65 Smith DM, Mckenna K, Thompson CJ. Hyponatraemia. Clin Endocrinol. 2000;52:667-678.
66 Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973-2001) in the metropolitan Detroit cancer surveillance system. J Clin Oncol. 2004;22:2865-2872.
67 Auperin A, Arriagada R, Pignon J, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476-484.
68 Cicerone KD, Dahlberg C, Kalmar K, et al. Evidence-based cognitive rehabilitation: recommendations for clinical practice. Arch Phys Med Rehabil. 2000;81:1596-1615.
69 Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol. 1996;53:626-632.
70 DeAngelis LM. Current diagnosis and treatment of leptomeningeal metastasis. J Neuro-oncology. 1998;38:245-252.
71 Rosenfeld MR, Pruitt A. Neurologic complications of bone marrow, stem cell, and organ transplantation in patients with cancer. Semin Oncol. 2006;33:352-361.
72 Spitzer TR. Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;27:893-898.
73 Reece DE, Frei-Lahr DA, Shepherd JD, et al. Neurologic complications in allogeneic bone marrow transplant patients receiving cyclosporin. Bone Marrow Transplant. 1991;8:393-401.
74 Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494-500.
75 Walter RW, Brochstein JA. Neurologic complications of immunosuppressive agents. Neurol Clin. 1988;6:261-278.
76 Stern TA, Purcell JJ, Murray GB. Complex partial seizures associated with Waldenstom’s macroglobulinemia. Psychosomatics. 1985;26:890-892.
77 Crawford J, Cox EB, Cohen HJ. Evaluation of hyperviscosity in monoclonal gammopathies. Am J Med. 1985;79:13-22.
78 Ilias I, Torpy DJ, Pacak K, et al. Cushing’s syndrome due to ectopic corticotrophin secretion: twenty years’ experience at the National Institutes of Health. J Clin Endocrinol Metab. 2005;90:4955-4962.
79 Darnell RB, Posner JB. Paraneoplastic syndromes affecting the nervous system. Semin Oncol. 2006;33:270-298.
80 Graus F, Keime-Guibert F, Rene R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124:1138-1148.
81 Kung S, Mueller PS, Geda YE, et al. Delirium resulting from paraneoplastic limbic encephalitis caused by Hodgkin’s disease. Psychosomatics. 2002;43:498-501.
82 Filley CM. Neurobehavioral aspects of cerebral white matter disorders. Psychiatr Clin North Am. 2005;28:685-700.
83 Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;6:1215-1228.
84 Omuro AMP, Ben-Porat LS, Panageas KS, et al. Delayed neurotoxicity in primary central nervous system lymphoma. Arch Neurol. 2005;62:1595-1600.
85 Silverman DHS, Dy CJ, Castellon S, et al: Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant treated breast cancer survivors 5-10 years after chemotherapy, Breast Cancer Res Treat 2006 Sep 29 [Epub ahead of print]. DOI = 10.1007/s10549-006-9380-z.
86 Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94:828-834.
87 Joffee H, Hall JE, Gruber S, et al. Estrogen therapy selectively enhances prefrontal cognitive processes: a randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;12:411-422.
88 Ragson NL, Silverman D, Siddarth P, et al. Estrogen use and brain metabolic change in postmenopausal women. Neurobiol Aging. 2005;26:229-235.
89 Yaffe K, Krueger K, Cummings SR, et al. Effect of raloxifene on prevention for dementia and cognitive impairment in older women: the Multiple Outcomes of Raloxifene Evaluation (MORE) randomized trial. Am J Psychiatry. 2005;162:683-690.
90 Walsh JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769-5779.
91 Fallowfield L, Cella D, Cuzick J, et al. Quality of life of postmenopausal women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) adjuvant breast cancer trial. J Clin Oncol. 2004;22:4261-4271.
92 Howell A, Cuzick J. Vascular effects of aromatase inhibitors: data from clinical trials. J Steroid Biochem Mol Biol. 2005;95:143-149.
93 Rapkin AJ. Vasomotor symptoms in menopause: physiologic condition and central nervous system approaches to treatment. Am J Obstet Gynecol. 2007;196(2):97-106.
94 Loprinzi CL, Kugler JW, Sloan JA, et al. Venlafaxine in management of hot flashes in survivors of breast cancer: a randomized controlled trial. Lancet. 2000;356:2059-2063.
95 Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes. JAMA. 2003;280:2827-2834.
96 Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol. 2002;20:1578-1583.
97 Pandya KJ, Morrow GR, Roscoe JA, et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomized double-blind placebo-controlled trial. Lancet. 2005;355:818-824.
98 Perez DG, Loprinzi CL, Sloan J, et al. Pilot evaluation of bupropion for the treatment of hot flashes. J Palliat Med. 2006;9:631-637.
99 Roth AJ, Scher HI. Sertraline relieves hot flashes secondary to medical castration a treatment of advanced prostate cancer. Psycho-oncology. 1998;7(2):129-132.
100 Jacobsen PB, Garland LL, et al. Relationship of hemoglobin levels to fatigue and cognitive functioning among cancer patients receiving chemotherapy. J Pain Symptom Manage. 2004;28:7-18.
101 Sawyer M, Antoniou G, Toogood I, et al. Childhood cancer: a two-year prospective study of the psychological adjustment of children and parents. J Am Acad Adolesc Psychiatry. 1997;36:1736-1743.
102 Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;6:293-310.