History
TABLE 5-1
Hemodynamic Values at Baseline and after Administration of Inhaled Nitric Oxide
| Hemodynamics | Baseline | NO to 80 ppm |
| BP (mm Hg) | 93/61 | 71/46 |
| RA (mm Hg) | 21 | 18 |
| PA (mm Hg) | 71/26/45 | 63/21/42 |
| PAWP (mm Hg) | 12 | 16 |
| TPG (mm Hg) | 33 | 26 |
| CO (L/min), Fick | 5.2 | 6.1 |
| CI (L/min/m2), Fick | 2.5 | 2.9 |
| PVR (Wood units) | 6.4 | 4.3 |
| PVRI (Wood units/m2) | 13.2 | 9.0 |
BP, Arterial blood pressure; CI, Cardiac index; CO, cardiac output; NO, nitric oxide; PA, pulmonary arterial pressure; PPM, parts per million; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index; RA, right atrial pressure; TPG, transpulmonary gradient.
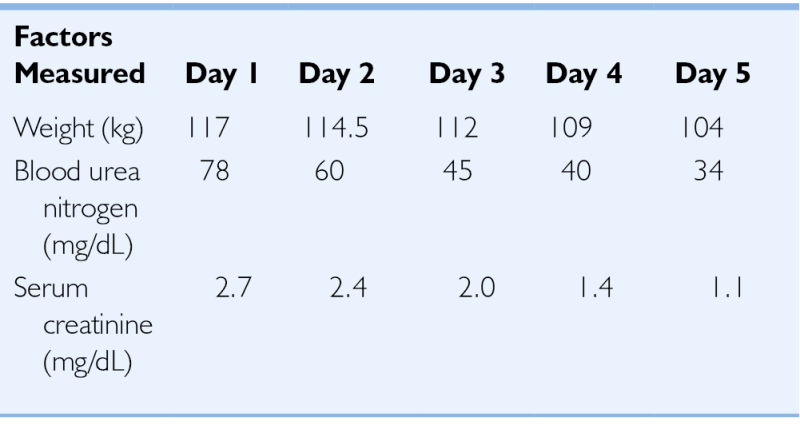
TABLE 5-2
Weight and Renal Function Changes Observed with Extracorporeal Fluid Removal
| Factors Measured | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 |
| Weight (kg) | 117 | 114.5 | 112 | 109 | 104 |
| Blood urea nitrogen (mg/dL) | 78 | 60 | 45 | 40 | 34 |
| Serum creatinine (mg/dL) | 2.7 | 2.4 | 2.0 | 1.4 | 1.1 |
Comments
Current Medications
Comments
Current Symptoms
Comments
Physical Examination
Comments
Laboratory Data
Comments
Electrocardiogram
Findings
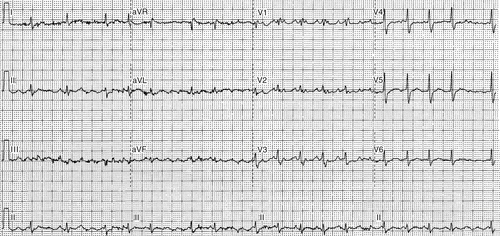
FIGURE 5-1
Comments
Echocardiogram
Findings
Comments
Computed Tomography
Findings
Comments
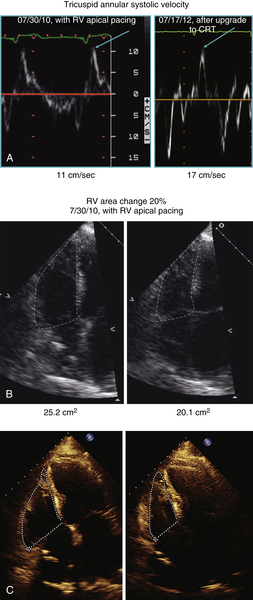
FIGURE 5-2 A to C.
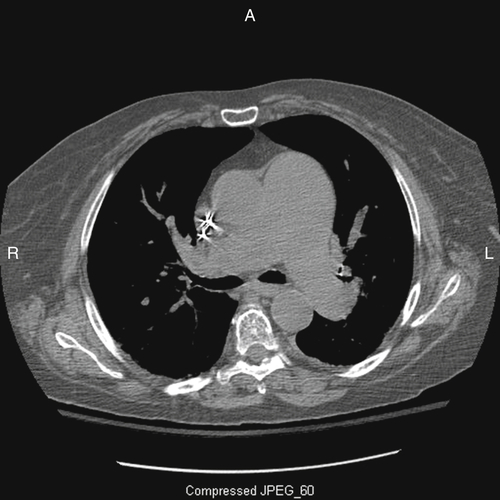
FIGURE 5-3
Hemodynamics
Findings
Comments
Focused Clinical Questions and Discussion Points
Question
Discussion
TABLE 5-3
Comparison of Hemodynamic Monitoring Results before Atrial Fibrillation and before Upgrade to Cardiac Resynchronization Therapy
| Factors Measured | Before Onset of AF | Before Upgrade to CRT |
| BP (mm Hg) | 110/70 | 92/60 |
| RA (mm Hg) | 2 | 13 |
| PA (mm Hg) | 32/13/22 | 59/36/43 |
| PAWP (mm Hg) | 5 | 27 |
| TPG (mm Hg) | 17 | 16 |
| CO (L/min) | 3.8 | 4.1 |
| CI (L/min/m2) | 2.0 | 2.0 |
| PVR (Wood units) | 4.5 | 3.9 |
| PVRI (Wood units/m2) | 8.4 | 7.9 |
AF, Atrial fibrillation; BP, arterial blood pressure; CI, cardiac index; CO, cardiac output; CRT, cardiac resynchronization therapy, PA, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure: PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index; RA, right atrial pressure; TPG, transpulmonary gradient.
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Findings
Comments
TABLE 5-4
Hemodynamic Values with Right Ventricular Pacing and After Upgrade to Cardiac Resynchronization Therapy
| Factors Measured | RV Pacing | CRT |
| RA (mm Hg) | 13 | 9 |
| PA (mm Hg) | 59/36/43 | 39/14/22 |
| PAWP (mm Hg) | 27 | 7 |
| TPG (mm Hg) | 16 | 15 |
| CO (L/min) | 4.1 | 4.7 |
| CI (L/min/m2) | 2.0 | 2.3 |
| PVR (Wood units) | 3.9 | 3.2 |
| PVRI (Wood units/m2) | 7.9 | 6.5 |
CI, Cardiac index; CO, cardiac output; CRT, cardiac resynchronization therapy; PA, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index; RA, right atrial pressure; RV, right ventricular; TPG, transpulmonary gradient.
Selected References
1. Costanzo M.R., Jessup M. The cardiorenal syndrome: do we need a change of strategy or a change of tactics? J Am Coll Cardiol. 2009;53:597–599.
2. Costanzo M.R., Ronco C. Isolated ultrafiltration in heart failure patients. Curr Cardiol Rep. 2012;14:254–364.
3. de Vos C.B., Pisters R., Nieuwlaat R. et al. Progression from paroxysmal to persistent atrial fibrillation. J Am Coll Cardiol. 2010;55:725–731.
4. Dell’Italia L.J. Anatomy and physiology of the right ventricle. Cardiol Clin. 2012;30:167–187.
5. Doshi R.N., Daoud E.G., Fellows C. et al. Left ventricular-based pacing cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol. 2005;16:1160–1165.
6. Hardziyenka M., Campian M.E., Verkerk A.O. et al. Electrophysiologic remodeling of the left ventricle in pressure overload-induced right ventricular failure. J Am Coll Cardiol. 2012;59:2193–2202.
7. Jessup M., Abraham W.T., Casey D.E. et al. 2009 Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;119:1977–2016.
8. McLaughlin V.V., Davis M., Cornwell W. Pulmonary arterial hypertension. Curr Probl Cardiol. 2011;36:461–517.
9. Santamore W.P., Dell’Italia L.J. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40:289–308.
10. Tops L.F., Schlij M.J., Bax J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony. J Am Coll Cardiol. 2009;54:764–776.





