CHAPTER 48. Bariatric Care
Kim A. Noble
OBJECTIVES
At the conclusion of this chapter, the reader will be able to:
1. Describe the normal anatomy and physiology of the gastrointestinal (GI) tract.
2. Describe the incidence and physiological impact of obesity.
3. Compare and contrast surgical options for weight loss with bariatric surgery.
4. Describe important considerations for patient selection for bariatric surgery.
5. List the potential complications and their physiological rationale(s) for bariatric surgery.
6. Describe the implications for the perianesthesia care of the bariatric surgical patient.
I. OVERVIEW
A. Parallel to the pandemic occurrence of obesity is the incidence of bariatric surgery (Table 48-1).
| Country | No. of Bariatric Procedures | No. of Bariatric Surgeons | Year Bariatrics Began |
|---|---|---|---|
| Argentina | 200 | 30 | 1988 |
| Australia (New Zealand) | 2750 | 68 | 1960 |
| Austria | 1396 | 38 | 1973 |
| Belgium | 6000 | 200 | 1970 |
| Brazil | 4000 | 510 | 1973 |
| Czech Republic | 400 | 6 | 1983 |
| Egypt | 2750 | 12 | 1996 |
| France | 12,000 | 200 | 1984 |
| Germany | 1100 | 54 | 1975 |
| Greece | 500 | 8 | 1978 |
| Hungary | 30 | 1 | 1999 |
| Israel | 1000 | 50 | 1978 |
| Italy | 3000 | 200 | 1973 |
| Japan | 20 | 20 | 1982 |
| Mexico | 2500 | 200 | 1971 |
| Netherlands | 800 | 40 | 1973 |
| Panama | 60 | 5 | 2002 |
| Poland | 145 | 14 | 1974 |
| Russia | 350 | 35 | 1969 |
| Spain | 2000 | 160 | 1977 |
| Sweden | 600 | 20 | 1970 |
| Switzerland | 800 | 90 | 1970 |
| Turkey | 150 | 5 | 1990 |
| Ukraine | 150 | 10 | 1978 |
| United Kingdom | 600 | 13 | 1955 |
| USA/Canada | 103,000 | 850 | 1953 |
| Totals | 146,301 | 2,839 |
B. Obesity is defined as a body mass index (BMI) >30.
1. Associated with an increased comorbidity risk (Box 48-1)
BOX 48-1
COMORBIDITY ASSOCIATED WITH BMI >25
Cardiovascular Comorbidity
Hypertension
Dyslipidemia
Coronary artery disease
Atherosclerosis
Angina
Sudden cardiac death
Congestive heart failure
Endocrine Comorbidity
Type 2 diabetes
Insulin resistance
Glucose intolerance
Neurological Comorbidity
Stroke
Gastrointestinal Comorbidity
Cholecystitis
Cholelithiasis
Gastroesophageal reflux disease
Respiratory Comorbidity
Obstructive sleep apnea
Asthma
Musculoskeletal Comorbidity
Osteoarthritis
Gout
Reproductive Comorbidity
Complications of pregnancy
Poor female reproductive health
Endometrial, breast, prostate cancers
Urological Comorbidity
Stress incontinence
Bladder infection
Renal calculi
Miscellaneous Comorbidity
Colon cancer
Depression
Eating disorders
Distorted body image
BMI, Body mass index.
2. Approximately 30% of the adult population in the United States and more than 300 million people worldwide considered obese
a. Crosses all demographic classifications
C. Morbid obesity is approximately twice ideal body weight with BMI >40.
D. Bariatric surgery has been shown to be the best weight loss option for obese patients.
E. Caring for patients undergoing bariatric surgery is challenging because they frequently have derangements leading to challenges for their perianesthetic management.
1. Respiratory
2. Metabolic
3. Endocrine
F. A comprehensive understanding of bariatric surgery and the physiological challenges of caring for obese patients can lead to potential surgical complication:
1. Prevention
2. Earlier identification
3. Treatment
II. ANATOMY AND PHYSIOLOGY OF DIGESTION AND ABSORPTION
A. Stomach
1. Gastric anatomy (Figure 48-1)
a. Pouchlike reservoir for ingested food located in the upper abdomen
b. Has three anatomic areas
(1) Fundus
(a) The upper arching area immediately distal to the cardiac sphincter
(b) Location of the gastric crypts containing secretory cells
(c) Responsible for the chemical digestion of ingested food
(d) Primary area for the accommodation of the ingested meal
(2) Body
(a) Central, thick walled muscular central area of the stomach
(b) Responsible for the mechanical digestion of ingested food
(3) Antrum
(a) Funnel-like portion of the stomach between the body and pyloric sphincter
c. Contains two sphincters
(1) Cardiac sphincter at the junction of the esophagus and stomach
(2) Pyloric sphincter at the junction of the stomach and duodenum
d. Gastric wall structure
(1) Has four layers consistent with entire GI tract
(a) Gastric mucosal layer
(i) Innermost layer
[a] Made up of epithelial cells that produce the mucous barrier
[b] Rapid cellular turnover; complete replacement every 4 to 5 days
(ii) Provides protective mucous barrier
[a] A prostaglandin grid work containing mucus and bicarbonate
[b] Protects gastric cells from acid digestion and provides lubrication
(b) Gastric submucosal layer
(i) Connective tissue
(ii) Contains blood vessels, nerves, and secretory structures
(c) Gastric muscular layer
(i) Thick muscular layer arranged in longitudinal, circular, and oblique direction
(ii) Provides grinding contractions involved in the mechanical digestion
(d) Gastric serosal layer
(i) Outermost protective layer continuous with the lesser omentum
(ii) Made up of fibrous connective tissue
e. High degree of gastric accommodation (enlargement) with ingested meals
(1) Empty stomach contains approximately 50 mL of acid with a significantly low pH.
(2) Stomach can expand to almost 1000 mL without an increase in intraluminal pressure.
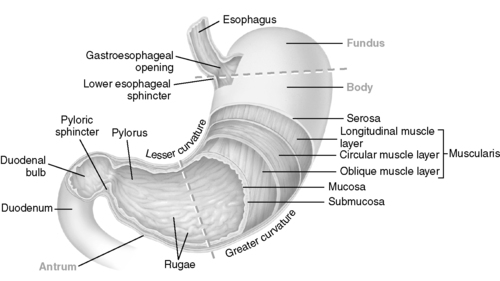 |
| FIGURE 48-1 ▪
Stomach.
(From McCance KL, Huether SE: Pathophysiology: The biologic basis for diseases in adults and children, ed 5, St Louis, 2006, Mosby.)
|
2. Gastric physiology
a. Stomach receives ingested food from the esophagus via the cardiac sphincter.
b. Chemical digestion begins in the stomach.
(1) Gastric acid secretion amounts to 2 L of fluid daily.
(a) Control of gastric acid secretion
(i) Endocrine secretion: blood-borne hormonal control of acid secretion
[a] GI tract is the largest endocrine organ in the body.
[b] Direct hydrochloric acid (HCl) release occurs when gastrin is released by:
[1] Parasympathetic nervous system
[2] Presence of alcohol
[3] Calcium-containing foods
[4] Protein in the stomach
[c] Secretin released from duodenum upon entry of chyme with pH <4.5, causes the release of large amounts of bicarbonate and water from the pancreas and liver into the common bile duct (CBD), and enters the duodenum via the sphincter of Oddi
[d] Cholecystokinin released from duodenum upon entry of protein and fat, leading to the release of pancreatic enzymes via the CBD and contraction of the gallbladder, leading to emptying of bile into the CBD and duodenum via the sphincter of Oddi.
(ii) Paracrine secretion: local control of acid secretion
[a] Histamine secretion from cells adjacent to parietal cells (local) stimulated by the endocrine release of gastrin. Histamine causes:
[1] Parietal cell stimulation
[2] Increased release of HCl
[b] Somatostatin released locally during times of fasting (decreasing pH) and leads to inhibition of gastrin and HCl release from the parietal cells
(b) Structures responsible for chemical digestion in the stomach
(i) Parietal cells
[a] Approximately 1 billion parietal cells located in the fundus
[b] Produce HCl
[c] Produce intrinsic factor necessary for vitamin B 12 absorption
(ii) Chief cells
[a] Produce pepsinogen, an inactive substance
[b] Rapidly converted to pepsin in an acidic environment
[c] Pepsin chemically digests protein.
(iii) Gastric lipase enzymatically degrades dietary fats into fatty acids.
(2) Chemical digestion is the process of chemically dividing food items into smaller parts.
(a) Starch and fibers degraded by gastric acid
(b) Protein degraded into small particle through the action of pepsin
(c) Fats delivered to the small intestine in a nondigested state
(3) Combination of the food derivative and gastric secretions called chyme
c. Mechanical digestion begins in the mouth (teeth) and continues in the stomach.
(1) Mechanical digestion (gastric motility) grinds food into chemically digestible particles.
(2) Gastric motility
(a) Peristaltic mixing and churning contractions begin in the body of the stomach and move toward the antrum, propelling the chyme toward the antrum.
(b) Large particles return to the body of the stomach for additional mechanical digestion.
(c) Opening of the pylorus and gastric empting into the duodenum is regulated by:
(i) pH of the chyme: pH is sensed by receptors on the duodenal wall, and a low pH delays gastric empting, allowing time for buffered secretions from the liver and pancreas to normalize pH before movement into the portal circulation.
(ii) Fat content of the chyme: fat delays gastric empting.
(iii) Osmolarity of the chyme: either hyperosmotic (caloric-dense foods or high protein content) or hypoosmotic chyme will delay gastric empting.
(iv) Volume of chyme in the stomach: an increase in the volume and gastric intraluminal pressure will accelerate empting.
(d) With each peristaltic contraction, a small amount of digested chyme is propelled through the pyloric sphincter.
(3) Neural control of gastric motility
(a) Enteric nervous system
(i) Local neural control in the muscular layer of the wall of GI tract
[a] Responsible for muscular contraction along the length of GI tract
(b) Autonomic nervous control of gastric motility
(i) Sympathetic nervous system stimulation
[a] Directly decreases GI motility and secretion
(ii) Parasympathetic nervous system stimulation
[a] Directly increases motility and acid secretion
(c) Endocrine control of gastric motility
(i) Gastric inhibitory peptide is released from the duodenal mucosa in response to increased concentration of glucose and/or fat in the duodenum; this causes the inhibition of
[a] Gastric acid secretion
[b] Gastric motility
[c] Gastric emptying
B. Small intestine (Figure 48-2)
1. Anatomy
a. Contains same layers as found in the stomach; anatomical variation in layers based on function
b. Muscle fibers thin compared with gastric muscle and have a longitudinal and circular arrangement allowing for coordinated peristalsis
c. Small intestine has plica, or wrinkles, that slow chyme movement to allow additional time for absorption.
(1) Plica are most numerous in:
(a) Jejunum
(b) Ileum
d. Small intestine consists of three segments:
(1) Duodenum
(a) U-shaped connection with the pylorus; entry into the small intestine
(b) Approximately 22 cm (10 inches) long
(c) Entry point for CBD via sphincter of Oddi
(i) Entry point for pancreatic enzymes and bicarbonate from the pancreas and liver after the endocrine release of secretin
(ii) Entry point for bile stored in the gallbladder after the endocrine release of cholecystokinin
(d) Large surface area for absorption related to villi and microvilli; projections of enterocyte-covered portal capillaries
(i) Villi and microvilli decrease the distance required for the diffusion of nutrients from the GI lumen into the portal blood supply, increasing absorption.
(2) Jejunum
(a) Together with the ileum approximately 7 m (23 feet) long
(b) No clear separation from duodenum or ileum
(3) Ileum
(a) Terminates into the large intestine
(b) Separated from the large intestine by the ileocecal valve
(c) Location of the appendix
2. Physiology
a. Chyme propelled through the pylorus as a liquid containing small, undigested food particles
b. Chemical digestion continues in the segments of the small intestine.
(1) Carbohydrates break down into disaccharides and monosaccharides (single sugars).
(2) Protein breaks down into amino acids and peptides.
(3) Fats emulsified into monoglycerides and fatty acids
c. Digestive role of small intestine
(1) Duodenum
(a) Digestive role for fat with entry of bile
(b) Protein digestive role with pancreatic enzymes, which activate due to acidic pH
(c) Continued digestion of carbohydrates through the secretion of digestive enzymes from the intestinal enterocytes
(d) Intestinal secretion amounts to approximately 4 L of fluid daily.
(2) Jejunum
(a) Additional intestinal length for digestion and absorption as needed
(3) Ileum
(a) Additional intestinal length for digestion and absorption as needed
d. Small intestine nutrient absorption based on anatomic location
(1) Duodenum
(a) Primary site of absorption of iron, calcium, sugars, and proteins
(b) Primary site of absorption of water and water-soluble vitamins
(c) Primary site of energy-dependent absorption of magnesium and sodium
(2) Jejunum
(a) Upper jejunum is the major site of absorption of:
(i) Bile salts
(ii) Fatty acids
(iii) Fat-soluble vitamins (A, D, E, K)
(b) Additional surface area for sugar and protein absorption
(3) Ileum
(a) Primary site for absorption of:
(i) Bile salts
(ii) Vitamin B 12 (intrinsic factor)
(iii) Chloride
e. Intestinal motility
(1) Stimulated by the arrival of chyme to mix secretions
(a) Pancreatic
(b) Gallbladder
(c) Hepatic
(2) Segmentation
(a) Produced by the contraction of circular muscle fibers
(b) More common in proximal small intestine (duodenum)
(c) Divides and mixes chyme and increases contact with absorptive surfaces
(3) Peristalsis
(a) Produced by the contraction of longitudinal muscle fibers
(b) Slow wave of contraction to propel chyme through the small intestine
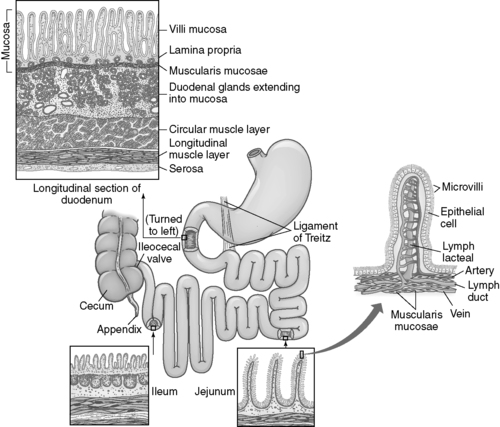 |
| FIGURE 48-2 ▪
Intestine.
(From McCance KL, Huether SE: Pathophysiology: The biologic basis for diseases in adults and children, ed 5, St Louis, 2006, Mosby.)
|
III. OBESITY
A. Overview
1. Obesity is a syndrome of increased percentage of body fat that is correlated with increased comorbidities and decreased life expectancy (see Box 48-1).
2. Definition of obesity
a. BMI in kilograms per meter squared (kg/m 2) (Table 48-2)
(1) A ratio of weight, adjusted for height, expressed as weight in kilograms (kg) divided by height in meters squared (m 2)
(2) Important to incorporate age- and gender-related differences, especially in children (Figure 48-3)
 |
| FIGURE 48-3 ▪
Sample growth chart for boys up to 36 months of age.
(From the Centers for Disease Control and Prevention.)
|
(3) Abdominal circumference should also be measured, since athletes with increased muscle mass would have high BMI without obesity.
| BMI | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height (inches) | Body Weight (lb) | ||||||||||||||||
| 58 | 91 | 96 | 100 | 105 | 110 | 115 | 119 | 124 | 129 | 134 | 138 | 143 | 148 | 153 | 158 | 162 | 167 |
| 59 | 94 | 99 | 104 | 109 | 114 | 119 | 124 | 128 | 133 | 138 | 143 | 148 | 153 | 158 | 163 | 168 | 173 |
| 60 | 97 | 102 | 107 | 112 | 118 | 123 | 128 | 133 | 138 | 143 | 148 | 153 | 158 | 163 | 168 | 174 | 179 |
| 61 | 100 | 106 | 111 | 116 | 122 | 127 | 132 | 137 | 143 | 148 | 153 | 158 | 164 | 169 | 174 | 180 | 185 |
| 62 | 104 | 109 | 115 | 120 | 126 | 131 | 136 | 142 | 147 | 153 | 158 | 164 | 169 | 175 | 180 | 186 | 191 |
| 63 | 107 | 113 | 118 | 124 | 130 | 135 | 141 | 146 | 152 | 158 | 163 | 169 | 175 | 180 | 186 | 191 | 197 |
| 64 | 110 | 116 | 122 | 128 | 134 | 140 | 145 | 151 | 157 | 163 | 169 | 174 | 180 | 186 | 192 | 197 | 204 |
| 65 | 114 | 120 | 126 | 132 | 138 | 144 | 150 | 156 | 162 | 168 | 174 | 180 | 186 | 192 | 198 | 204 | 210 |
| 66 | 118 | 124 | 130 | 136 | 142 | 148 | 155 | 161 | 167 | 173 | 179 | 186 | 192 | 198 | 204 | 210 | 216 |
| 67 | 121 | 127 | 134 | 140 | 146 | 153 | 159 | 166 | 172 | 178 | 185 | 191 | 198 | 204 | 211 | 217 | 223 |
| 68 | 125 | 131 | 138 | 144 | 151 | 158 | 164 | 171 | 177 | 184 | 190 | 197 | 203 | 210 | 216 | 223 | 230 |
| 69 | 128 | 135 | 142 | 149 | 155 | 162 | 169 | 176 | 182 | 189 | 196 | 203 | 209 | 216 | 223 | 230 | 236 |
| 70 | 132 | 139 | 146 | 153 | 160 | 167 | 174 | 181 | 188 | 195 | 202 | 209 | 216 | 222 | 229 | 236 | 243 |
| 71 | 136 | 143 | 150 | 157 | 165 | 172 | 179 | 186 | 193 | 200 | 208 | 215 | 222 | 229 | 236 | 243 | 250 |
| 72 | 140 | 147 | 154 | 162 | 169 | 177 | 184 | 191 | 199 | 206 | 213 | 221 | 228 | 235 | 242 | 250 | 258 |
| 73 | 144 | 151 | 159 | 166 | 174 | 182 | 189 | 197 | 204 | 212 | 219 | 227 | 235 | 242 | 250 | 257 | 265 |
| 74 | 148 | 155 | 163 | 171 | 179 | 186 | 194 | 202 | 210 | 218 | 225 | 233 | 241 | 249 | 256 | 264 | 272 |
| 75 | 152 | 160 | 168 | 176 | 184 | 192 | 200 | 208 | 216 | 224 | 232 | 240 | 248 | 256 | 264 | 272 | 279 |
| 76 | 156 | 164 | 172 | 180 | 189 | 197 | 205 | 213 | 221 | 230 | 238 | 246 | 254 | 263 | 271 | 279 | 287 |
B. Epidemiology
1. World Health Organization estimates 300 million people are obese worldwide.
2. Adult obesity
a. In the United States, the incidence of obesity nearly doubled over the past 25 years.
(1) 12.8% in 1962
(2) 22.5% in 1994
(3) 27% in 2000
b. Obesity at higher incidence in racial and ethnic minority populations (African Americans and Hispanic Americans) as compared with white ethnic groups
3. Childhood obesity
a. Reached when child’s weight exceeds the 95th percentile
b. Almost a 400% increase in incidence of obesity in children aged 6 to 11 years between 1963 and 2000 (from 4% to 15%)
c. For adolescents (12–19 years of age) during the same time frame (from 1963 to 2000), incidence increased 300% (from 5% to 15%)
C. Pathophysiology
1. Overview
a. Obesity is complex and multifactorial in nature.
b. Obesity follows a positive energy balance, where energy expenditure exceeds energy output.
c. Obesity carries a strong genetic predisposition with a familial pattern for excess weight.
2. Theory of ectopic fat deposition
a. When adipose tissue can no longer expand to store excess calories, fat is deposited in body tissues.
(1) Liver
(2) Skeletal muscle
(3) Pancreas
(4) Heart
b. Excess circulating fatty acids promote insulin resistance and type 2 diabetes mellitus.
c. Adipose tissue is an endocrine tissue and secretes:
(1) Hormones
(2) Inflammatory substances
3. Comorbidities of obesity affect virtually every organ system (see Box 48-1).
a. Hypertension
(1) Approximately 50% of obese individuals (BMI >30 kg/m 2) have hypertension.
(2) Hypertension is seen in overweight individuals across all demographics.
(3) Hypertension is a primary risk factor for the development of atherosclerosis.
(4) Surgical treatment of obesity improves both hypertension and cardiac function.
b. Dyslipidemia
(1) Forty percent to 50% of obese individuals have dyslipidemia with:
(a) Increased low-density lipoprotein (LDL: “bad cholesterol”)
(b) Decreased high-density lipoprotein (HDL: “good cholesterol”)
(2) Hyperlipidemia is a primary risk factor for the development of atherosclerosis.
(3) Gastric bypass has been shown to be very effective in:
(a) Lowering triglycerides and LDL
(b) Increasing HDL
c. Diabetes and impaired glucose tolerance
(1) Obesity is the primary risk factor for diabetes and 90% of type 2 diabetics are obese.
(2) Thirty-six percent of individuals with impaired glucose tolerance will progress to type 2 diabetes within 10 years.
(3) Diabetes is a risk factor for the development of:
(a) Atherosclerosis
(b) Vascular disease
(c) Obesity
(d) Combined risk factors predict lethal health consequences.
(4) Weight loss in obese type 2 diabetic patients can restore blood glucose and insulin sensitivity to near-normal levels.
d. Cardiac and peripheral vascular disease
(1) Obesity is a primary risk factor for the development of atherosclerotic cardiac and peripheral vascular disease.
(2) Obesity leads to large vessel disease.
(a) Coronary artery disease
(b) Cerebrovascular accident
(c) Carotid occlusive disease
(d) Subclavian steal syndrome
(e) Aneurysmal disease
(f) Vascular occlusive disease
(g) Vascular insufficiency
(3) Obesity and diabetes lead to small vessel disease.
(a) Retinopathy
(b) Nephropathy
e. Obstructive sleep apnea (OSA)
(1) Approximately 50% of obese individuals have OSA, with increased abdominal girth the single most important risk factor for OSA.
(2) Diagnosis of OSA is made when there are the following three findings:
(a) Individuals have breathing cessation exceeding 10 seconds during sleep.
(b) Apneic episodes occur more than five times per hour.
(c) Apneic episodes have a concurrent 4% decrease in oxygen saturation.
(3) Nocturnal OSA has been associated with cardiac dysrhythmias and sudden cardiac death.
(4) OSA may carry over into the daylight hours, leading to:
(a) Drowsiness
(b) Inattentiveness
(c) Impaired job performance
(d) Decrease in cognitive functioning
(5) OSA is categorized as:
(a) Central
(b) Oropharyngeal obstructive
(c) Combined form
(6) Marked weight loss (secondary to bariatric surgery) has been nearly 100% effective in managing OSA.
f. Asthma
(1) Asthma is a prevalent comorbidity for obesity, thought to be due to decreased lung volumes (from increased abdominal girth) sensitizing the airway and leading to reactive airways.
(2) The following contribute to asthma:
(a) OSA
(b) Respiratory stasis
(c) Gastroesophageal reflux disease (GERD)
(3) Obese children have three times greater risk for asthma (30%).
(4) Obese adults have a 25% increased risk for the development of asthma.
g. Obesity hypoventilation syndrome (OHS) or Pickwickian syndrome
(1) OHS present in 30% of patients with morbid obesity, but less common than OSA
(2) OHS caused by decreased lung volumes (increased abdominal pressure), which causes:
(a) Chronic shortness of breath
(b) Decreased expiratory reserve volume
(c) Increased oxygen consumption
(d) Increased circulating partial pressure of carbon dioxide (P co2)
(3) Long-term effects of obesity are:
(a) Pulmonary hypertension
(b) Right-sided heart failure
(c) Polycythemia
(d) Ultimately death
(4) The following is seen after bariatric surgery:
(a) Marked improvement in symptoms associated with pulmonary hypertension
(b) Improved blood oxygenation
(c) Reduced hypercarbia
h. Peripheral osteoarthritis
(1) Weight-bearing destruction (osteoarthritis) found at an accelerated rate in the obese patient’s:
(a) Knees
(b) Hips
(c) Ankles
(d) Feet
(2) Obesity increases the necessity of surgical intervention.
i. Gastroesophageal Reflux Disease (GERD)
(1) GERD is a relatively common finding in the general population.
(a) Incidence in general population: 20%
(b) Incidence in obese patients: up to 50%
(2) GERD is the retrograde movement of acidic chyme into the esophagus, leading to a chronic inflammation and the potential for precancerous lesions (Barrett esophagus).
(3) Correlation of GERD and obesity most probably related to increased abdominal pressure
j. Back and disk disease
(1) Chronic lower back pain is the most common orthopedic complaint of obese persons.
(2) With increasing age, the incidence of lower back pain in obese individuals is 100%.
(3) Decreased mobility and the use of assistive devices are common findings with obesity.
k. Nonalcoholic steatohepatitis (NASH)
(1) Fatty infiltration of the liver, or NASH, present in 100% of the morbidly obese population
(2) Severity of NASH increases linearly with increasing BMI.
(3) Over time, fatty infiltration of the liver leads to fibrosis, leading to cirrhosis and possible hepatocellular carcinoma.
l. Female endocrine and reproductive disorders
(1) Estrogen released from adipose tissue, and obese females have increased levels of estrogen
(2) Increased estrogen can cause menstrual abnormalities, dysfunctional bleeding, early menopause, and infertility.
(3) Polycystic ovarian syndrome three times more common in obese patients
(4) Obesity during pregnancy increases the risk for:
(a) Preeclampsia
(b) Urinary tract infections
(c) Gestational hypertension and/or diabetes
(d) Overdue birth
(e) Prolonged labor
(f) Increased blood loss during labor and cesarean delivery
(5) Chronically increased estrogen levels increase the risk for endometrial (3-4 times higher), ovarian (3-4 times higher), and breast (2 times higher) cancers.
m. Depression
(1) Depression related to the social and economic consequences of obesity
(2) Estimated that 50% of obese females are taking antidepressant agents
(3) Adolescent and young females at high risk for the development of depression
4. Mortality and obesity
a. BMI >35 kg/m 2 approximately doubles all causes of mortality.
b. Coronary artery disease is the major killer in both overweight and obese subjects.
c. Mortality secondary to diabetes and cancer much more common in the obese patient
IV. BARIATRIC SURGERY
A. Overview
1. Rationales for the use of bariatric surgery (Table 48-3)
a. Although traditional medical treatment for obesity has been unsuccessful, bariatric surgery has been found to lead to a significant, sustained loss of weight.
b. Obese individuals who lose significant weight can reverse:
(1) Glucose intolerance
(2) Diabetes mellitus
(3) OSA
(4) OHS
(5) Hypertension
(6) Serum lipid abnormalities
c. Patients undergoing bariatric surgery rarely achieve their ideal body weight.
| BIP/DS, Biliopancreatic diversion/duodenal switch; CV, cardiovascular; MSK, musculoskeletal; OSA, obstructive sleep apnea; PEEP, positive end-expiratory pressure; Po2, partial pressure of oxygen. | ||||
| Author | Year | Question | Sample | Findings |
|---|---|---|---|---|
| Buchwald et al. Systematic review; meta-analysis (Level I) |
2004 | Evaluate the effect of bariatric surgery on weight loss, mortality, diabetes, hyperlipemia, hypertension, and OSA |
136 studies
N = 22,094
|
1. Substantial weight losses: 47.5% for gastric banding; 61.6% for gastric bypass; 68.2% for gastroplasty; 70.1% for BIP/DS
2. Mortality at 30 days: 0.1% banding + gastroplasty; 0.5% gastric bypass; 1.1% BIP/DS
3. Improvement in type 2 diabetes seen with all surgery types
4. Significant improvement in hyperlipemia seen with all surgery types
5. Significant improvement in hypertension seen with all surgery types
6. Significant improvement in OSA seen with all surgery types
|
| Chalhoub et al. Experimental design (Level II) |
2006 | Study the effects of increased tidal volume and PEEP on oxygenation | N = 52 |
1. PEEP alone moderately and slowly increased P o2 and saturation
2. PEEP + vital capacity maneuver significantly magnified the positive effects of PEEP
|
| Madan et al.Retrospective case review (Level V) | 2007 | Looked at outcomes of morbidly obese teenagers treated in an adult program | N = 5 |
1. Five morbidly obese adolescents having laparoscopic Roux-en-Y procedures; no complications; good weight loss 2 years out
2. Difficulty maintaining follow-up noted
|
| McCullough et al. Retrospective case review (Level V) |
2006 | Evaluate the relationship between CV fitness and complications after laparoscopic Roux-en-Y | N = 109 |
1. A critical inverse relationship exists between CV fitness and complications after bariatric surgery
|
| Livingston et al. Retrospective case review (Level V) |
2006 | Evaluate the rate of surgical outcomes in patients undergoing all types of bariatric surgery in the Veterans Administration system | N = 575 |
1. 30-day mortality rate: 1.4%; 3% for males and 0.8% for females
2. 2-year mortality rate: 3.1%; 70% to 80% lacked complete follow-up
3. Postoperative complication rate: 19.7%; cardiac arrest (#1); renal failure (#2)
|
| Hooper et al. Longitudinal observation (Level V) |
2007 | Determination of prevalence of MSK diseases in patients before and after bariatric surgery | N = 48 |
1. Higher incidence of MSK disease in obese population; with upper extremity disease
2. Significant improvement in MSK in 6–12 months after bariatric surgery
|
| Haines et al. Longitudinal observation (Level V) |
2007 | Determination of prevalence of OSA disease in patients before and after bariatric surgery | N = 348 |
1. OSA found in 45% of patients having bariatric surgery
2. Weight loss in this patient population (high rate of dropout) significantly improved OSA and quality of sleep
|
| Livingston et al. Case report (Level V) |
2006 (Reprint) | Compared the rate of adverse effects after bariatric surgery as a function of age (patients <65 years of age and >65) | Record review DRG #288; pt. >65 years of age |
1. Adverse events after bariatric surgery increase with age
2. Adverse event rate non-Medicare patients <65 years: 8%; 21.6% for Medicare patients <65; 32.3% >65
|
2. Indications for bariatric surgery
a. Multidisciplinary evaluation and treatment guides patient selection and surgical care.
b. 1991 National Institutes of Health (NIH) Consensus Conference established bariatric surgical indications. Indications reviewed and endorsed by 2004 American Society of Metabolic and Bariatric Surgery (ASBS) Consensus Conference
(1) Patient must be:
(a) Adult (specifically not an adolescent)
(b) Motivated
(c) Well informed of acceptable operative risks with effective informed consent
(2) Willing to undergo lifelong medical surveillance
(3) BMI >40 kg/m 2
(4) Some cases with BMI >35 kg/m 2 acceptable if high-risk comorbid conditions present
(a) Cardiopulmonary comorbidity
(b) Severe OSA
(c) Pickwickian syndrome (OHS)
(d) Obesity-related cardiomyopathy
(e) Severe diabetes mellitus
(f) Joint disease
(g) Social effects on employment, family function, or ambulation
3. Contraindications to bariatric surgery
a. Consider risk-to-benefit ratio
(1) Active malignancy
(2) Human immunodeficiency virus infection
(3) High risk
b. High risk not prohibitive to anesthesia
(1) Cardiac ischemia
(2) Esophageal varices
(3) Active peptic ulcer
c. Absolute contraindications to surgery
(1) Active substance abuse or alcoholism diagnosed on psychological assessment
(2) Active anorexia or bulimia
d. Mild eating disorders: closely consider ability to comply with postoperative dietary requirements.
4. Other criteria for bariatric surgery according to 1991 NIH Consensus Conference
a. Age criteria: for patients younger than 18 years of age or older than 55, consider overall health status.
b. Weight criteria
(1) BMI used; excess body weight (45 kg or 100 lb) over ideal weight as a secondary indication for surgical appropriateness
(2) A maximum weight for surgical selection not identified
c. Psychological or psychiatric criteria
(1) Well-controlled major depression, bipolar disorder, and schizophrenia do not preclude surgery and may continue to improve with surgical weight loss.
(2) Prior abuse, especially sexual abuse, may lead to obesity and should be carefully evaluated on an individual basis.
d. Behavioral criteria
(1) Intelligence
(a) No intelligence limit
(b) Patient needs to be able to communicate with a multidisciplinary team.
(c) Informed consent imperative
(2) Social support
(a) Individually determined
(b) Better success adapting to the postsurgical lifestyle with adequate support system
(3) Motivation
(a) Motivation highly desirable with better surgical outcome
(b) Subjective characteristics considered individually
(4) Socioeconomic status
(a) In United States, 20 million bariatric candidates
(b) Patients with the highest BMIs have lowest socioeconomic status.
(c) Patients with lowest socioeconomic status at highest risk of disease because of:
(i) Poor medical resources
(ii) Physical environment
(iii) Social support systems
(d) All patients provided equal access regardless of socioeconomic status
(5) Pregnancy
(a) Maximal weight loss 18 to 24 months after bariatric surgery may lead to:
(i) Electrolyte imbalance
(ii) Metabolic derangement
(b) Pregnancy during the 2 years after surgery discouraged
(c) After the risk period, obesity-related derangements with pregnancy resolve.
e. Nutritional criteria
(1) Dietitian evaluation necessary and completed preoperatively
(2) Early provision of educational materials provided for adequate postoperative nutrition
(3) Eating habits may affect surgical procedure selection.
(a) Grazer eating occurs when patients eat small amounts continuously; restrictive procedures less effective.
(b) Sweeter eating occurs with the ingestion of calories mostly from sweet foods; may have side effects with malabsorptive procedures.
(c) Bloater eating occurs with the ingestion of huge meals at one sitting; successfully treated with restrictive procedures.
(d) Rarely do individuals have single eating habits; most often a combination.
B. Bariatric surgical procedures
1. Overview
a. Traditional weight loss methods ineffective; bariatric surgery is treatment of choice for long-term, significant weight loss.
b. Bariatric surgical classifications
(1) Malabsorptive procedures
(a) Lead to incomplete digestion and absorption of nutrients
(b) Degree of malabsorption controlled by length of small intestine segment
(c) May be combined with gastric resection to prolong weight loss
(d) Generally result in 10% to 20% greater loss of weight than restrictive procedures
(2) Restrictive procedures
(a) Reduce the size of the stomach to:
(i) Limit the intake of food.
(ii) Create a rapid feeling of fullness.
(b) Variety of surgical approaches and surgical procedures.
(c) Reversible only with adjustable gastric banding systems.
(3) Combined procedures (malabsorptive and restrictive)
(a) Decrease adverse effects on the GI tract
(b) Consistent long-term weight loss
2. Bariatric surgical procedures
a. Laparoscopic adjustable gastric banding (Figure 48-4)
(1) Restrictive procedure
(2) Laparoscopic technique
(a) Less invasive
(b) Small incisions
(c) Reduced pain
(d) Reduced length of stay.
(3) Completely adjustable and reversible with the addition or removal of saline in the subcutaneous reservoir
(4) Good weight loss but takes longer than with other procedures
(5) Critical need for follow-up with frequent band size adjustments (slippage rate 23%) to prevent potential esophageal complications
(6) Significantly lower rate of complications as compared with other bariatric surgical procedures
(7) Vomiting should be avoided because it may cause band slippage.
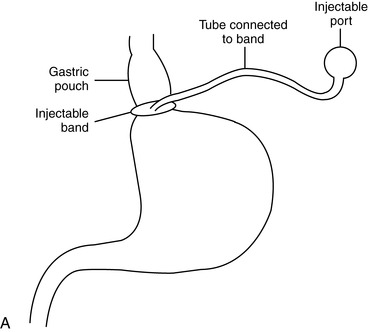 |
 |
| FIGURE 48-4 ▪
A, Laparoscopic adjustable gastric banding. B, Adjustable gastric band.
( A from Ellison SR, Ellison SD: Bariatric surgery: A review of the available surgical procedures and complications for the emergency physician. J Emerg Med 34[1]:21–32, 2008; B from Buchwald H, Cowan GS, Pories WJ: Surgical management of obesity, Philadelphia, 2007, Saunders.)
|
b. Vertical banded gastroplasty (Figure 48-5)
(1) Restrictive procedure
(2) Can be performed either laparoscopically or as open procedure
(3) Early band position was horizontal; currently using vertical banding.
(4) Gastric pouch 20 mL and reinforced to prevent dilation over time
(5) Noncompliance with dietary restrictions leads to decreased weight gain over time.
(6) Surgical complications
(a) Bleeding
(b) Leakage from stomach
(c) Deep vein thrombosis/pulmonary embolism
(d) Gastroplasty failure necessitating revisional surgery
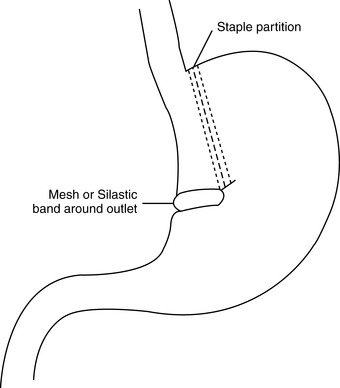 |
| FIGURE 48-5 ▪
Vertical banded gastroplasty.
(From Ellison SR, Ellison SD: Bariatric surgery: A review of the available surgical procedures and complications for the emergency physician. J Emerg Med 34[1]:21–32, 2008.)
|
c. Roux-en-Y gastric bypass (RYGBP) (Figure 48-6)
(1) Combined procedure
(2) Most frequently performed bariatric surgery in North America
(3) Surgical procedure
(a) Stomach horizontally transected leaving a 30-mL pouch
(b) Distal jejunal “Roux” limb between 50 and 150 cm in length (to ileocecal valve) brought up and attached to the gastric pouch
(c) Jejunojejunostomy created attaching the stomach stump to the Roux limb
(d) Gastric and intestinal digestive secretions (bile, pancreatic, and hepatic contribution) from stomach stump move to the jejunojejunostomy and then the distal small intestine for absorption.
(4) Can be performed either laparoscopically or as open procedure
(5) Long-limb derivation increases weight loss without altering complication rate.
(6) Optimal, long-term weight loss
(7) Nutritional deficiency risk from loss of duodenum (calcium, iron, vitamins A, D, E, and K)
(8) Surgical complications
(a) Anastomosis leak and hemorrhage
(b) Bowel obstruction
(c) Marginal ulceration
(d) Deep vein thrombosis/pulmonary embolism
(e) Hernia
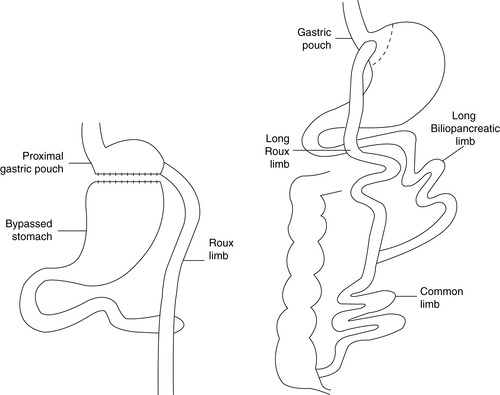 |
| FIGURE 48-6 ▪
Roux-en-Y gastric bypass.
(From Ellison SR, Ellison SD: Bariatric surgery: A review of the available surgical procedures and complications for the emergency physician. J Emerg Med 34[1]:21–32, 2008.)
|
d. Banded gastric bypass (banded RYGBP)
(1) Combined procedure
(2) Surgical procedure similar to RYGBP except:
(a) Gastric resection is vertical.
(b) Gastric pouch reinforced with a Silastic ring or polypropylene mesh bands
(c) Both segments of small intestine are relatively short (60 cm).
(3) Can be performed either laparoscopically or as open procedure
(4) Surgical complications similar to RYGBP
e. Biliopancreatic diversion
(1) Combined procedure
(2) Surgical procedure
(a) Distal vertical gastrectomy with 200- to 500-mL gastric pouch remaining
(b) Long Roux-en-Y reconstruction with jejunojejunostomy 50 cm from ileocecal valve
(3) Can be performed either laparoscopically or as open procedure; longer surgical time
(4) Lifetime malabsorption of:
(a) Fat
(b) Starch
(c) Protein
(d) Monosaccharides and disaccharides
(e) Alcohol
(f) Sweets
(g) Soft drinks
(h) Milk
(i) Creates negative reinforcement for eating restricted foods and liquids
(5) Lifetime soft stools high in fat; two to four stools and flatulence daily
(6) Larger stomach pouch allows return of “normal” eating habits as weight loss stabilizes.
(7) Extraordinarily good weight loss maintenance
(8) Surgical complications
(a) Anemia
(b) Stomal ulcer
(c) Bone demineralization
(d) Protein malnutrition
(9) Duodenal switch may be added (leaving a short portion of the duodenum attached to the pylorus); reduces the incidence of stomal ulcers.
f. Laparoscopic duodenal switch and sleeve gastrectomy procedure
(1) Combined procedure
(2) Surgical procedure
(a) Same as biliopancreatic duodenal switch
(b) Gastric resection is long and horizontal.
(3) Can be performed either laparoscopically or as open procedure
(4) Used in the super obese because of sustained large weight losses (>200 lb first year)
(5) Technically demanding especially when performed laparoscopically
(6) Surgical complications similar to those for biliopancreatic duodenal switch
g. Implantable gastric stimulator
(1) An exciting new approach to the treatment of morbid obesity
(2) Surgery less invasive than bariatric surgery
(3) Used since the 1990s and has the lowest rate of complications
(4) Pacing used to disrupt normal gastric contractions and alter digestion
(5) Patients experience meaningful weight loss.
(6) Pacemaker apparatus similar to cardiac pacemaker with a bipolar lead
(7) No reported incidence of major complications
(8) Preoperative screening tool improves weight loss by improving accurate patient selection. Only approximately 25% of all morbidly obese patients are appropriate for gastric pacing.
C. Preoperative patient preparation
1. Overview
a. Bariatric surgery unique as a surgical subspecialty
(1) Bariatric surgery is a behavior modification tool that can lead to a complete change of life for the involved patient.
(2) Bariatric surgery success is strongly related to:
(a) Skill of the surgeon
(b) Preoperative risk assessment
(c) Patient education
2. Preoperative educational priorities
a. Full informed consent
(1) Description of significant health risks and poor quality of life with morbid obesity
(2) Details of surgical GI alterations
(a) Description of surgical procedure
(b) Risks and benefits of laparoscopic versus open procedures
(c) Description of anesthesia and patient implications
(d) Description of hospital length of stay and inpatient expectations
(e) Description of early and late complications
(i) Prophylaxis for complication prevention
(ii) Anticipated implication for complication prevention while inpatient
[a] Patients with OSA using continuous positive airway pressure (CPAP)
[b] Instruct to bring their equipment to the hospital with them.
(iii) Educational points for complication prevention after hospital discharge
[a] Include description of continued prophylaxis for deep vein thrombosis.
(iv) Importance of long-term follow-up care
[a] Anticipated schedule of postoperative physician and specialist office visits for the first year
(f) Estimation of surgical outcomes and anticipated physiological implications of large weight loss
(i) Possible need for body contour procedures after stabilization of weight loss
(3) Importance of multidisciplinary evaluation and follow-up throughout the surgical experience
b. Nutritional education
(1) Implication of gastric resection and malabsorption on weight loss
(2) Details of concepts of energy balance and its application to health and weight loss
(3) Clear description of postoperative eating patterns and anticipated lifestyle changes
(4) Importance of increasing activity to facilitate weight loss
(5) Importance of careful follow-up and routine serum analysis for the prevention of nutritional deficiencies
3. Perioperative risk assessment
a. Medical history
(1) Current medication schedule
(a) Prescription medications and schedule
(b) Over-the-counter medication schedule
(c) Schedule of herbal supplements and non-Western weight loss treatments
b. Meticulous physical examination
(1) Pulmonary examination and screening
(a) Pulmonary abnormalities associated with obesity
(i) Reduction in lung and chest wall compliance
(ii) Increase in respiratory system resistance
(iii) Reduction in lung volumes
(iv) Increased effort required for the work of breathing
(b) Pulmonary function testing should be obtained for:
(i) Morbidly obese patients
(ii) Patients with self-reported respiratory illness or shortness of breath
(iii) All patients with a history of OSA
(c) Baseline arterial blood gas analysis while breathing room air
(i) Screen for perioperative hypercarbia.
(d) Planned cessation of smoking 8 weeks before surgery
(e) Detailed assessment on patients reporting OSA
(i) Careful screening and sleep studies for patients reporting:
[a] Heavy snoring
[b] Apneic episodes witnessed by bed partner
[c] Daytime somnolence
[d] Lack of restful sleep
(ii) Patients with suspected OSA need careful preoperative identification and stabilization.
(f) Thorough assessment of patient’s airway to rule in or out a difficult intubation
(i) Airway assessment using the Mallampati classification
(ii) Assessment completed by the anesthesia care provider
(iii) Assessment of mandibular opening and relative size of tongue and oral cavity opening
(g) Concerns/abnormalities discovered in the preoperative pulmonary exam must be communicated to the anesthesia team.
(2) Cardiovascular examination and screening
(a) Cardiovascular abnormalities associated with obesity
(i) Cardiac hypertrophy
[a] Left-sided secondary to hypertension
[b] Right-sided secondary to pulmonary hypertension
(ii) Increased preload
(iii) Diastolic dysfunction
(iv) Rarely systolic dysfunction associated with cardiomyopathy
(v) Cardiac dysrhythmias
(vi) Ischemic heart disease
(b) Meticulous screening of cardiovascular status including exercise tolerance
(c) Baseline electrocardiogram
(i) Identified abnormalities referred for cardiologist surgical clearance
(ii) Interventional cardiac procedures as indicated
(d) Skin and peripheral vascular assessment
(3) Endocrine examination and screening
(a) Type 2 diabetes
(i) At risk for infection and poor wound healing
(ii) Blood glucose increases substantially with physiological stress response.
(iii) Current medication schedule
(iv) Baseline blood glucose
(b) Rule out thyroid disease as detailed by history.
(i) Thyroid function testing baseline as indicated by history
(c) Rule out adrenal disease as detailed by history.
(i) Symptoms that may indicate Cushing’s syndrome
[a] Hypertension
[b] Diabetes
[c] Central obesity
[d] Weakness
[e] Muscle atrophy
[f] Hirsutism
[g] Striae
[h] Osteoporosis
[i] Acne
D. Bariatric surgical complications (Table 48-4)
1. Overview
a. Obesity increases risk of complications since there is a decreased physiological reserve.
2. Obesity-related complications
a. Pulmonary derangements
(1) Physiological overview
(a) Respiratory complications the most frequent postoperative complication, occurring in 5% of all bariatric procedures
(b) Obesity
(i) Increases the work of breathing related to an increase in the elastic work and a decrease in the efficiency of the respiratory muscles
(ii) Obese patients:
[a] Have higher metabolical demands
[b] Produce more carbon dioxide
[c] Require a higher amount of oxygen
(iii) Obesity decreases the functional reserve capacity, and when obese patients are placed supine, this is greatly increased.
(iv) Chest wall of obese patients less compliant because of fat deposition in the chest wall
(2) Potential postoperative pulmonary complications
(a) OSA
(b) OHS
(c) Atelectasis
(d) Pneumonia
(i) Patients who weighed >250 lb were found in one study to be at an almost 40% greater risk for developing pneumonia.
b. Thromboembolic derangements
c. Fluid and electrolyte derangements
3. Surgical complications
a. Early surgical complications
(1) Anastomotic leaking
(a) Caused by:
(i) Failure of anastomotic staple or suture line
(ii) Leakage of digestive juices
(b) Screened during procedure with injection of diluted methylene blue and observation
(c) Postoperative symptoms
(i) Unexplained tachycardia (>120 beats/min)
(ii) Abdominal pain not responsive to analgesia
(iii) Fever as a late sign
(d) Dependent on severity of the leak, operative exploration and correction of defect
(2) GERD
(a) Conflicting reports with gastric banding; may indicate need of band evaluation for malplacement and readjustment
(b) No effect in GERD seen with vertical gastric banding
(c) Reduction in GERD seen with Roux-en-Y surgery
b. Late surgical complications
(1) Anastomotic stricture/stenosis
(a) Relatively common occurrence with:
(i) RYGBP
(ii) Vertical gastric banding (VGB)
(b) Typically seen in first 6 months after surgery
(c) Symptoms include:
(i) Postprandial epigastric pain
(ii) Vomiting
(iii) Dysphagia
(d) Diagnosis with upper endoscopy
(e) Treatment with endoscopic dilation; rare need of surgical revision
(2) Anastomotic ulceration
(a) Most often develops at gastrojejunal anastomosis site
(b) Present in up to 16% of RYGBP procedures
(c) Contributing factors
(i) Gastric acidity
(ii) Nonsteroidal anti-inflammatory use
(iii) Helicobacter pylori infection
(iv) Local ischemia or tension at anastomosis site
(d) Symptoms consistent with peptic ulcer
(e) Diagnosis with upper endoscopy
(f) Treatment is empirical based on cause of ulcer.
(3) Anastomotic rupture or dehiscence
(a) Potential complication of RYGBP and VGB procedures
(b) May be asymptomatic or present similar to ulceration
(c) Diagnosis with upper endoscopy
(d) Treatment most often with surgical revision; however, may be successfully treated with endoscopic manipulation
(4) Band erosion
(a) Present in 1% to 2% of patients having VGB procedures
(b) Symptoms include pain or weight gain from reduction of gastric restriction.
(c) Diagnosis with upper endoscopy
(d) Treatment includes removal of band and bariatric operation.
(5) Bowel or Roux limb obstruction
(a) Small bowel obstruction
(i) Incidence of 3% with a laparoscopic RYGBP
(ii) Incidence of 2% in open procedures
(iii) May follow the development of an internal hernia
(b) Symptoms may include:
(i) Abdominal pain
(ii) Nausea/vomiting
(iii) Fever
(c) Surgical evaluation necessary if correction is warranted
(6) Hernia
(a) Several gaps created by RYGBP (incidence, 18%-20%) and VGB procedures, necessitating gap closure for both open and laparoscopic procedures
(b) Incidence of hernia also increased with rapid weight loss
(c) Symptoms may include a palpable mass or abdominal pain but would increase in severity with incarceration.
(d) Surgical evaluation necessary if correction is warranted
(7) Cholelithiasis
(a) Related to rapid weight loss
(b) Seen in up to 32% of patients after Roux-en-Y procedures; 40% of those patients symptomatic
(c) May be prevented by incidental cholecystectomy at time of bariatric surgery
(d) Symptoms consistent with nonbariatric cholelithiasis
(e) Diagnosis with abdominal ultrasonography
(f) Treatment
(i) Elective cholecystectomy easier after weight loss
(8) Dumping syndrome
(a) Caused by the rapid transit of high-caloric, high-osmolar (concentrated) foods into the small intestine
(b) Portal fluid into the lumen of the GI tract, decreasing preload
(c) Symptoms
(i) Nausea/vomiting
(ii) Diaphoresis
(iii) Palpitations/tachycardia
(iv) Abdominal cramping
(v) Dizziness
(vi) Syncope
(d) Negatively reinforces the restriction of highly concentrated sweets or alcoholic beverages and milk from the diet
4. Nutritional deficiency
a. Iron deficiency
(1) Common after RYGBP or biliopancreatic diversion (20%-49% of patients)
(2) Premenopausal patients at higher risk because of menstrual losses
(3) Mechanism for deficiency:
(a) Decreased iron intake due to intolerance of red meat
(b) Primary site of iron absorption is the duodenum, which is bypassed in RYGBP.
(4) Patients need to receive iron replacement.
(5) Concurrent supplementation with vitamin C improves iron absorption.
b. Vitamin B 12 deficiency
(1) Common in RYGBP (25%-75% of patients)
(2) Mechanism for deficiency
(a) Decreased B 12 intake due to intolerance of meat and milk
(b) Loss of intrinsic factor secretion by the parietal cells (fundus of the stomach)
(3) Leads to the development of pernicious anemia
(4) Replacement necessary with intramuscular B 12 injections or oral crystalline B 12
c. Folate deficiency
(1) Common in RYGBP
(2) Mechanism for deficiency
(a) Decreased folate intake
(b) B 12 action as a coenzyme for folate metabolism
(3) Replacement necessary with daily folate
d. Thiamine deficiency
(1) Mechanism for deficiency
(a) Decreased thiamine intake or protracted vomiting
(b) Malabsorption from surgical bypass of the duodenum
(2) Prevention, early recognition and immediate treatment necessary to prevent Wernicke’s encephalopathy
(3) Replacement necessary with daily thiamine
e. Vitamin D and calcium deficiency
(1) Fat-soluble vitamin D deficiency common in malabsorptive or combined procedures
(2) Calcium deficiency common from malabsorption secondary to bypass of duodenum
(3) Vitamin D and calcium necessary for prevention of metabolic bone disease
(4) Replacement necessary with daily 1200 to 1500 mg calcium citrate with vitamin D
f. Protein deficiency
(1) Protein deficiency common in bariatric procedures where the duodenum (site of primary absorption of protein) is bypassed
(2) Although poorly understood, protein deficiency also thought to be related to the physiological response to the starvation associated with bariatric surgery
(3) Average time for the appearance of protein deficiency is 18 months after bariatric surgery; however, may be present 3 months after surgery
(4) Protein malnutrition should be associated with any patient with pitting edema or a low serum albumin level.
(5) Severe protein wasting will affect coagulation (plasma protein based) and immune function.
(6) Nitrogen replacement is paramount with:
(a) Oral supplementation if tolerated
(b) Use of enteral feedings
5. Body contouring after massive weight loss
a. Skin of obese individuals not able to retract after large weight losses
b. Body contouring
(1) May be medically necessary
(a) Abdominal and thigh skin folds are subject to:
(i) Rashes
(ii) Fungal infections
(iii) Irritation
(iv) Ulceration
(v) Resistance to topical medical therapy
(2) Excess skin surgically removed by several staged, plastic procedures
| Decade | Procedure | Classification | Comments |
|---|---|---|---|
| 1950s | Jejunoileal (JI) bypass | Combined | Significant weight loss associated with electrolyte imbalance, diarrhea, liver failure |
| 1960s | JI bypass; less radical | Combined | Series of procedures; continued side effects |
| 1960s | Gastric bypass | Restrictive | Stomach divided horizontally; pouch 150 mL |
| 1960s | Biliopancreatic diversion | Malabsorptive | Distal horizontal gastrectomy; Roux-en-Y limb of small intestine |
| 1970s | Roux-en-Y gastric bypass (RYGB) | Combined | Stomach divided vertically; good results; short and long versions of small intestine |
| 1970s | Gastroplasty | Restrictive | Partial gastric transection (vertical) |
| 1980s | Vertical band gastroplasty | Restrictive | Partial gastric transection (horizontal); Silastic ring used to close lower end |
| 1980s | Gastric banding | Restrictive | Small pouch created by band around upper stomach; no staples so reversible; in 1986 a port added to allow manipulation of ring |
| 1990s | Laparoscopic procedures: RYGB and banding | Combined | Shorter surgery; smaller incisional lines; reduced rates of complications; surgeon experience very important |
| 1990s | Implantable gastric stimulator | Nonbariatric surgery | Safe; less invasive; improving efficacy |
V. NURSING PROCESS
A. Receive report from anesthesia care provider
1. Preoperative data
a. Past medical history including medication history
b. Allergies
c. Preoperative diagnostic data
2. Intraoperative data
a. Surgical procedure performed; intraoperative surgical complications
b. Anesthetic
(1) Type(s) of anesthetic used for surgery
(2) Agents and dosages administered; patient response
(3) Vital signs throughout procedure
(4) Anesthetic complications or difficulties
c. Airway status
(1) Intubation history
(a) Paralytic agent used
(b) Presence of difficult airway
(c) Number of intubation attempts
(d) Assistive equipment if used
(2) Extubation history
(a) Reversal agents timing
(b) Neuromuscular response at time of reversal
(i) Peripheral nerve stimulator
(ii) Train of four: number of twitches present at reversal
(c) Patient response before extubation
(i) Presence of adventitious sounds, if any
(ii) Strength and ability to follow commands
(iii) Additional medications given (i.e., bronchodilators, narcotics)
(3) Presence of any artificial airway devices
d. Fluid balance
(1) Fluid intake
(a) Crystalloids: type and amount
(b) Colloids: type and amount
(c) Irrigations if used: type and amount
(2) Fluid output
(a) Estimated blood loss
(b) Urine output
(c) Additional losses
e. Blood glucose response
(1) Perioperative blood glucose
(2) Intraoperative blood glucose
(3) Any blood glucose regulation during anesthetic
f. Additional medications administered
(1) Antibiotics
(2) Narcotics
(3) Antiemetics
(4) Local anesthetic infiltration
B. Admission assessment
1. Complete a head-to-toe admission assessment.
a. Neurological assessment
(1) Assess level of consciousness and orientation status.
(2) Assess extremity movement and strength in response to verbal command.
(3) Assess patient’s pain level using the pain scale included in patient’s preoperative educational plan.
b. Pulmonary assessment
(1) Apply supplemental oxygen as ordered or per protocol.
(2) Evaluate the effectiveness of gas exchange.
(a) Observe ventilatory rate, depth, and pattern.
(3) Elevate head of bed as soon as stable blood pressure obtained.
(a) Uses gravity to remove redundant abdominal fat from the chest
(b) Eases pressure on diaphragm to decrease the work of breathing
(c) Increases tidal volume and reduces tendency toward atelectasis and intrapulmonary shunting
(4) Apply CPAP as ordered in patients with preoperative history of OSA.
(5) Monitor continuous pulse oximetry.
(6) Encourage deep breathing and coughing exercises included in patient’s preoperative educational plan.
c. Cardiovascular assessment
(1) Initiate frequent vital sign and continuous cardiac monitoring.
(2) Obtain 12-lead electrocardiogram as ordered. Report results to anesthesia care provider.
(3) Assess skin and nail bed color and timing of capillary refill.
(4) Assess extremity circulation.
(a) Presence of peripheral pulses
(b) Presence and location of edema
(c) Application of compression boots/compressive stockings as ordered
d. Gastrointestinal assessment
(1) Assess surgical dressings.
(a) Location and number of dressings
(b) Presence of drainage
(i) Note location, character, and color.
(ii) Reinforce dressing as ordered.
(iii) Report excessive drainage to surgical team and anesthesia care provider as indicated.
(2) Assess drainage tubes.
(a) Location, type, and number of drainage tubes
(b) Presence of drainage
(i) Note location, character, and color.
(ii) Report excessive drainage to surgical team and anesthesia care provider as indicated.
(3) Assess for presence of postoperative nausea/vomiting.
(a) Report occurrence of nausea/vomiting to anesthesia care provider.
(b) Obtain orders for pharmacological management of nausea/vomiting.
(c) Administer ordered pharmacological interventions.
(d) Assess and record patient response to pharmacological agent.
(e) Continue communication with anesthesia care provider as needed.
e. Pain assessment
(1) Assess patient’s pain level at admission using the pain scale discussed in patient’s preoperative educational classes.
(a) Note characteristics of the pain.
(i) Location, character, quality, aggravating and alleviating factors
(ii) Visually inspect pain loci for swelling, drainage, discoloration, or redness as a cause of the pain.
(b) Report occurrence of pain to anesthesia care provider.
(c) Obtain orders for pharmacological management of pain.
(d) Administer ordered pharmacological interventions.
(e) Assess and record patient’s response to pharmacological agent.
(f) Continue communication with anesthesia care provider as needed.
(g) Begin patient-controlled analgesia (PCA) as soon as patient’s condition warrants its use.
(i) Describe the use/purpose of PCA to patient.
(ii) Monitor patient’s use of PCA and success of pain management.
(iii) Document and communicate the effectiveness of PCA use to surgeon.
f. Fluid balance assessment
(1) Reassess perioperative fluid management.
(2) Measure the volume of urinary output on admission and as per protocol.
(a) Report any abnormal findings to the anesthesia care provider.
(b) Initiate physician orders as received.
(3) Obtain ordered postoperative lab work.
(a) Communicate results to ordering physician and anesthesia care provider.
(b) Initiate physician orders as received.
g. Skin assessment
(1) Assess skin integrity, especially at pressure points.
(a) Ensure arms not resting on side rails because that may place pressure on the median nerve and lead to potential peripheral nerve injury
(i) Place patient in size-appropriate bed as available.
(ii) Pad side rails and reposition arms frequently to prevent the development of pressure.
(2) Assess bilateral lower extremities for circulatory compromise.
(a) Pad and reposition as indicated.
h. Musculoskeletal assessment
(1) Assess for musculoskeletal pain.
(a) Position of comfort (as long as adequate gas exchange is maintained) for lower back pain
(b) Patients in supine position; may place pillow under knees to remove lower back pressure
(2) Assess for extremity strength.
(a) Encourage foot and leg movement as per preoperative teaching.
i. Thermal balance assessment
(1) Admission temperature measurement as per protocol
(a) Apply warming blankets as indicated.
(b) Report occurrence of postanesthetic shivering to anesthesia care provider.
(c) Obtain orders for pharmacologic management of postanesthetic shivering.
(d) Administer ordered pharmacologic interventions.
(e) Assess and record patient response to pharmacologic agent.
(f) Continue communication with anesthesia care provider as needed.
j. Psychosocial assessment
(1) Complete an assessment of patient’s anxiety and emotional well-being.
(2) Reassure patient as appropriate.
(a) Place patient in calm, quiet environment.
(b) Provide patient reassurance of nurse’s presence and touch.
(c) Question source of anxiety and use factual statements to relieve anxiety.
(3) Reorient patient as to completion of procedure and current location.
(4) Initiate visitation of family or significant other as per protocol.
k. Additional data collection
(1) Obtain postoperative blood glucose reading.
(2) Report results to anesthesia care provider.
(3) Obtain orders for pharmacologic management of blood glucose as indicated.
(4) Administer ordered pharmacologic interventions.
(5) Assess and record patient response to pharmacologic agent.
(6) Continue communication with anesthesia care provider as needed.
2. Admission auscultation
a. Pulmonary auscultation
(1) Auscultate patient’s regular breathing for adventitious sounds.
(a) Wheezing indicative of:
(i) Increased airway resistance
(ii) Bronchospasm
(b) Snoring may indicate a partial obstruction of the upper airway from redundant tissue of the neck or mouth.
(i) Remove pillow and reposition head using chin lift maneuver to clear snoring.
(ii) If snoring is from retained secretions, ask patient to cough and clear airway.
(iii) Suction secretions as needed for patients with ineffective airway clearance.
(iv) Insert artificial airway as indicated.
(v) Report any abnormality to anesthesia care provider.
(c) Stridor or crowing may indicate a partial laryngospasm from mechanical manipulation of the larynx with intubation.
(i) Notify anesthesia care provider immediately.
(ii) Stridor can be broken by using positive pressure ventilation with 100% oxygen and an Ambu bag.
(iii) Nebulized racemic epinephrine or the administration of corticosteroids may also be used to reduce swelling of the vocal cords.
(iv) Have intubation equipment available.
(2) Auscultate all lung fields bilaterally, asking patient to take deep, slow breaths.
(a) As above, listen for adventitious breath sounds.
(b) Ask patient to take additional deep breaths and cough to clear abnormal sounds.
(c) Record and report any abnormality to anesthesia care provider.
b. Auscultate gastrointestinal function.
(1) Auscultate gently, all four abdominal quadrants.
(a) Absence of bowel sounds a normal finding, especially in open surgical procedures
(b) Presence of hypoactive bowel sounds also a normal finding in laparoscopic procedures
(c) Presence of subcutaneous emphysema common after laparoscopic surgery and placement of pneumoperitoneum
3. Develop perianesthesia plan of care.
a. Interact with anesthesia care provider.
(1) Report elicited physiological abnormalities.
(2) Receive medical orders for interventions as indicated by patient status.
b. Perform ongoing assessment for current status and response to interventions.
c. Evaluate and revise plan of care based on patient response as needed.
d. Upon anesthesia care provider order, follow established nursing protocol and medical orders for patient transfer of care to admitting unit.
(1) Patient care hand-off as per hospital policy via written or oral report
(2) Assemble needed equipment and personnel for safe transfer of care.
4. Evaluation of outcomes
a. Ongoing evaluation of patient response to treatment plan conducted throughout length of stay
(1) Physiological indicators
(a) Adequate gas exchange
(b) Stable vital signs (including oxygen saturation)
(c) Absence of cardiac dysrhythmias
(d) Adequate fluid administration to maintain perfusion and sufficient urinary output
(e) Blood glucose within normal range
(f) Abdominal dressings clean, dry, and intact; minimal drainage from tubes
(g) Absence of nausea/vomiting
(h) Control of surgical pain
(i) Intact distal nervous function
(j) Prophylactic measures implemented for prevention of thromboembolism
(2) Cognitive indicators
(a) Follows instructions correctly
(b) Institutes postoperative behaviors per preoperative teaching plan
(i) PCA
(ii) Deep breathe and cough
(iii) Foot movement
(iv) Foley
(c) Appropriate use of pain scale for adequate pain relief
(3) Affective indicators
(a) Verbalizes individual needs
(b) Verbalizes and demonstrates compliance with treatment plan
(4) Supportive resources
(a) Family members involved in patient plan of care
(b) Identification of appropriate support groups and community resources
(5) Patient satisfaction
BIBLIOGRAPHY
1. Ali, M.R.; Maguire, M.B.; Wolfe, B.M., Assessment of obesity-related comorbidities: A novel scheme for evaluating bariatric surgical patients, J Am Coll Surg 202 (1) ( 2006) 70–77.
2. AORN bariatric surgery guideline, AORN J 79 (5) ( 2004) 1026–1052.
3. Apovian, C.M.; Lenders, C.M., A clinical guide for management of overweight and obese children and adults. ( 2007)CRC Press, Boca Raton, FL.
4. Bagchi, D.; Preuss, H.G., Obesity: Epidemiology, pathophysiology and prevention. ( 2007)CRC Press, Boca Raton, FL.
5. Barrow, C., Roux-en-Y gastric bypass for morbid obesity, AORN J 76 (4) ( 2002) 590–604.
6. Blouw, E.L.; Rudolph, A.D.; Narr, B.J.; et al., The frequency of respiratory failure in patients with morbid obesity undergoing gastric bypass, AANA J 71 (1) ( 2003) 45–50.
7. Buchwald, H., Consensus conference statement for bariatric surgery for morbid obesity: Health implications for patients, health professionals, and third-party payers, J Am Coll Surg 200 (2005) 593–604.
8. Buchwald, H.; Avidor, Y.; Braunwald, E.; et al., Bariatric surgery: A systematic review and meta-analysis, JAMA 292 (14) ( 2004) 1724–1737.
9. Buchwald, H.; Cowan, G.S.; Pories, W.J., Surgical management of obesity. ( 2007)Saunders, Philadelphia.
10. Buchwald, H.; Williams, S.E., Bariatric surgery worldwide, Obes Surg 14 (2004) 1157–1164.
11. Chaloub, V.; Yazigi, A.; Sleilaty, G.; et al., Effect of vital capacity manoeuvres on arterial oxygenation in morbidly obese patients undergoing open bariatric surgery, Eur J Anaesthesiol 24 (2006) 283–288.
12. Daniels, J., Obesity: America’s epidemic: What goes up does not always come down. Is there a solution?Am J Nurs 106 (1) ( 2006) 40–49.
13. Decker, G.A.; Swain, J.M.; Crowell, M.D.; et al., Gastrointestinal and nutritional complications after bariatric surgery, Am J Gastroenterol 102 (2007) 2571–2580.
14. DeMaria, E.J.; Latifi, R.; Sugerman, H.J., Laparoscopic bariatric surgery: Techniques and outcomes. ( 2002)Landes Bioscience Vademecum, Georgetown, TX.
15. Ellison, S.R.; Ellison, S.D., Bariatric surgery: A review of the available surgical procedures and complications for the emergency physician, J Emerg Med 34 (1) ( 2008) 21–32.
16. Ezri, T.; Muzikant, G.; Medalion, B.; et al., Anesthesia for restrictive bariatric surgery (gastric bypass not included): Laparoscopic vs open procedures, Int J Obes 28 (2004) 1157–1162.
17. Fatima, J.; Houghton, S.G.; Iqbal, C.W.; et al., Bariatric surgery at the extremes of age, J Gastrointest Surg 10 (10) ( 2006) 1392–1396.
18. Goldberg, S.; Rivers, P.; Smith, K.; Homan, W., Vertical banded gastroplasty: A treatment for morbid obesity, AORN J 72 (6) ( 2000) 987–1010.
19. Gould, J.C.; Garren, M.J.; Gutowski, K.A., Bariatric surgery, Clin Obstet Gynecol 49 (2) ( 2006) 375–388.
20. Haines, K.L.; Nelson, L.G.; Gonzalez, R.; et al., Objective evidence that bariatric surgery improves obesity-related obstructive sleep apnea, Surgery 141 (3) ( 2007) 354–358.
21. Han, S.H.; Gracia, C.; Mehran, A.; et al., Improved outcomes using a systematic and evidence-based approach to laparoscopic Roux-en-Y gastric bypass in a single academic institution, Am Surg 73 (10) ( 2007) 955–958.
22. Hooper, M.M.; Hallowell, P.T.; Seitz, B.A.; et al., Musculoskeletal findings in obese subjects before and after weight loss following bariatric surgery, Int J Obes 31 (2007) 114–120.
23. Hydock, C.M., A brief overview of bariatric surgical procedures currently being used to treat the obese patient, Crit Care Nurs Q 28 (3) ( 2005) 217–226.
24. Jazet, I.M.; Groot, G.H.; Tuijnebreyer, W.E.; et al., Cardiovascular risk factors after bariatric surgery: Do patients gain more than expected from their substantial weight loss?Eur J Intern Med 18 (2007) 39–43.
25. Livingston, E.H., Obesity and its surgical management, Am J Surg 184 (2002) 103–113.
26. Livingston, E.H.; Arterburn, D.; Schifftner, T.L.; et al., National Surgical Quality Improvement Program analysis of bariatric operations: Modifiable risk factors contribute to bariatric surgical adverse outcomes, J Am Coll Surg 203 (5) ( 2006) 625–633.
27. Livingston, E.H.; Langert, J., The impact of age and Medicare status on bariatric surgery outcomes, Arch Surg 141 (2006) 1115–1120.
28. Madan, A.K.; Dickson, P.V.; Ternovitis, C.A.; et al., Results of teenaged bariatric patients performed in an adult program, J Laparoendosc Adv Surg Tech 17 (4) ( 2007) 473–477.
29. Marley, R.A.; Hoyle, B.; Ries, C., Perianesthesia respiratory care of the bariatric patient, J Perianesth Nurs 20 (6) ( 2005) 404–431.
30. McCance, K.L.; Huether, S.E., Pathophysiology: The biologic basis for disease in adults and children. ed 6 ( 2010)Mosby, St Louis.
31. McCullough, P.A.; Gallagher, M.J.; deJong, A.T.; et al., Cardiovascular fitness and short-term complications after bariatric surgery, Chest 130 (2) ( 2006) 517–525.
32. McGlinch, B.P.; Que, F.G.; Nelson, J.L.; et al., Perioperative care of patients undergoing bariatric surgery, Mayo Clin Proc 81 (10) ( 2006) S25–S33.
33. McNatt, S.S.; Longhi, J.J.; Goldman, C.D.; et al., Surgery for obesity: A review of the current state of the art and future directions, J Gastrointest Surg 11 (2007) 382–402.
34. Moos, D.D.; Cuddelford, J.D., Implications of obstructive sleep apnea syndrome for the perianesthesia nurse, J Perianesth Nurs 21 (2) ( 2006) 103–118.
35. Murray, D., Morbid obesity-psychosocial aspects and surgical interventions, AORN J 78 (6) ( 2003) 990–995.
36. Nguyen, N.T.; Ho, H.S.; Palmer, L.S.; et al., A comparison study of laparoscopic versus open gastric bypass for morbid obesity, J Am Coll Surg 191 (2) ( 2000) 149–155.
37. Pinkney, J.; Kerrigan, D., Current status of bariatric surgery in the treatment of type 2 diabetes, Obes Rev 5 (2004) 69–78.
38. Porth, C.M., Pathophysiology: Concepts of altered health states. ed 8 ( 2009)Lippincott Williams & Wilkins, Philadelphia.
39. Rea, J.D.; Yarbrough, D.E.; Leeth, R.R.; et al., Influence of complications and extent of weight loss on quality of life after laparoscopic Roux-en-Y gastric bypass, Surg Endosc 21 (2007) 1095–1100.
40. Steinbrook, R., Surgery for severe obesity, N Engl J Med 350 (11) ( 2004) 1075–1079.
41. Venable, H.D.; Schlink, C.L., Anesthesia for bariatric surgery, Plast Surg Nurs 24 (3) ( 2004) 99–101.
42. Voelker, M., Assessing quality of life in gastric bypass clients, J Perianesth Nurs 19 (2) ( 2004) 89–104.
43. Voelker, M.; Foster, T.G., Nursing challenges in the administration of oral antidepressant medications in gastric bypass patients, J Perianesth Nurs 22 (2) ( 2007) 108–124.
44. Woodard, C.B., Pregnancy following bariatric surgery, J Perinat Neonatal Nurs 18 (4) ( 2004) 329–340.



