History
Comments
Current Medications
Current Symptoms
Physical Examination
Comments
Laboratory Data
Comments
Electrocardiogram
Findings
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
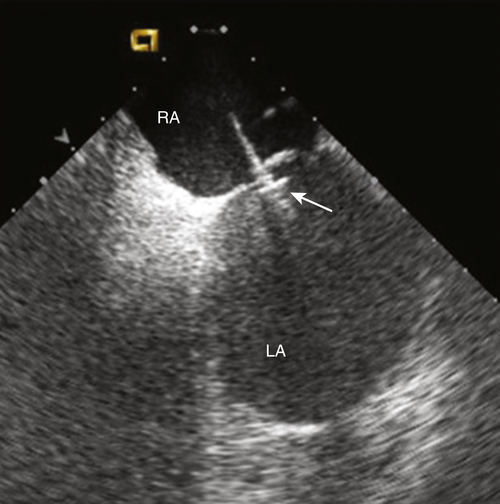
FIGURE 47-1 Intracardiac echocardiogram showing the left atrial pressure sensor in situ, anchored across the intraatrial septum from the right atrium. CAUTION: This is an investigational device, restricted by U.S. law to investigational use. LA, Left atrium; RA, right atrium.
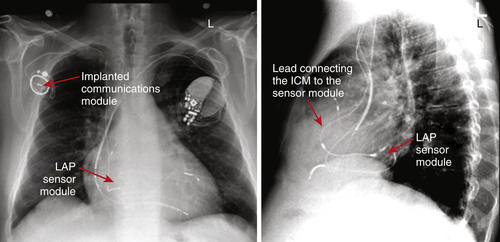
FIGURE 47-2 Pulmonary artery and lateral chest radiograph demonstrating proper placement of the left atrial pressure (LAP) monitoring system. The implanted communications module is used to transmit the pressure readings from the sensor module. The patient also has a cardiac resynchronization therapy device with an implantable cardioverter-defibrillator. CAUTION: This is an investigational device, restricted by U.S. law to investigational use. ICM, Implanted Communications Module.
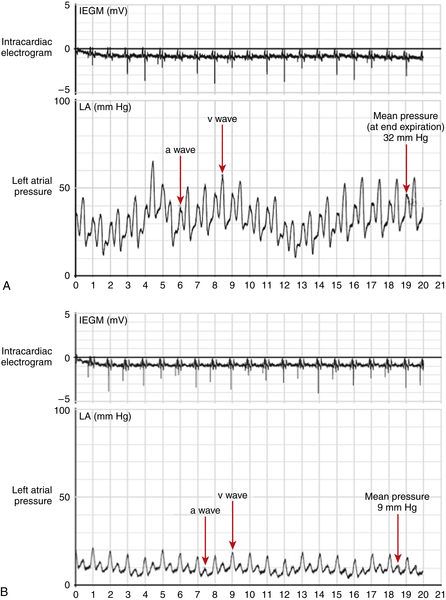
FIGURE 47-3 A, Left atrial pressure tracing obtained 3 months after implantation of the left atrial pressure (LAP) sensor, before initiation of a tailored regimen based on pressure readings. The waveform can be correlated with the atrial electrocardiogram and demonstrates significantly elevated pressure. B, LAP tracing obtained 12 months after implantation of the LAP sensor, after treatment was tailored using pressure readings. The waveform demonstrates a significant reduction in LAP in contrast to 9 months previously CAUTION: This is an investigational device, restricted by U.S. law to investigational use. IEGM, Intracardiac electrogram.
Selected References
1. Abraham W., Adamson P., Bourge R. et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666.
2. Adamson P.B. Continuous heart rate variability from an implanted device: a practical guide for clinical use. Congest Heart Fail. 2005;11:327–330.
3. Adamson P.B., Gold M.R., Bennett T. et al. Continuous hemodynamic monitoring in patients with mild to moderate heart failure: results of The Reducing Decompensation Events Utilizing Intracardiac Pressures in Patients. Congest Heart Fail. 2011;17:248–254.
4. Adamson P.B., Magalski A., Braunschweig F. et al. Ongoing right ventricular hemodynamics in heart failure: clinical value of measurements derived from an implantable monitoring system. J Am Coll Cardiol. 2003;41:565–571.
5. Bourge R.C., Abraham W.T., Adamson P.B. et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073–1079.
6. Hunt S.A., Abraham W.T., Chin M.H. et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119 e391.
7. Ritzema J., Troughton R., Melton I. et al. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121:1086–1095.
8. Yu C.M., Wang L., Chau E. et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848.





