History
Current Medications
Comments
Current Symptoms
Physical Examination
Comments
Laboratory Data
Comments
Electrocardiogram
Findings
Echocardiogram
Findings
Comments
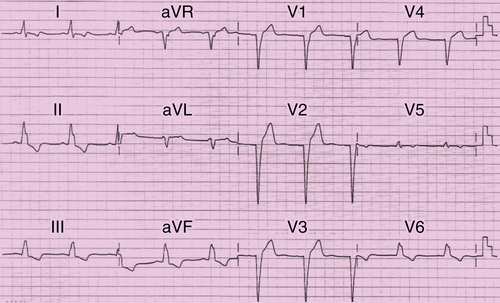
FIGURE 46-1 Resting electrocardiogram.
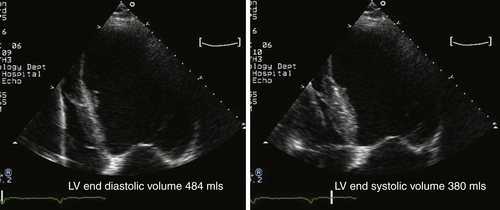
FIGURE 46-2 Apical four-chamber view of the left ventricle. LV, Left ventricle.
Findings
Comments
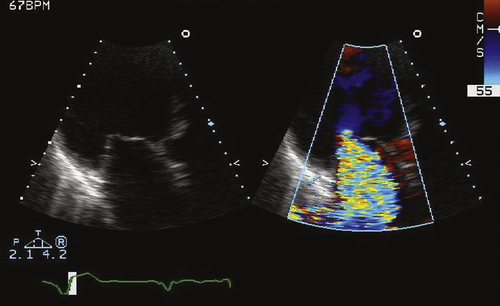
FIGURE 46-3 Apical long axis view of the mitral valve.
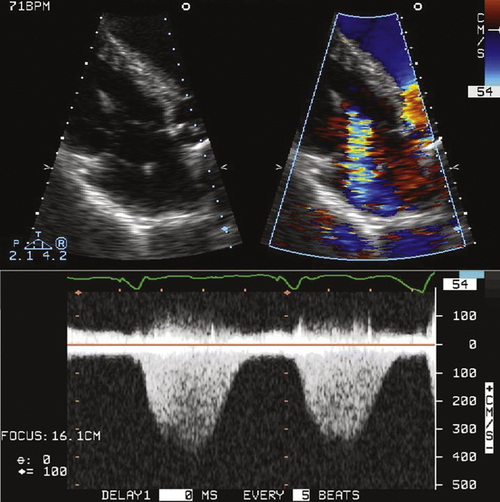
FIGURE 46-4 Apical four-chamber view of the tricuspid valve.
Findings
Physiologic Tracings
Findings
Catheterization
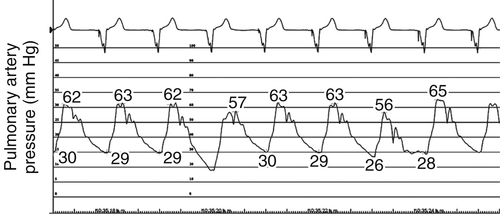
FIGURE 46-5 Pulmonary artery pressure trace at baseline before cardiac resynchronization therapy.
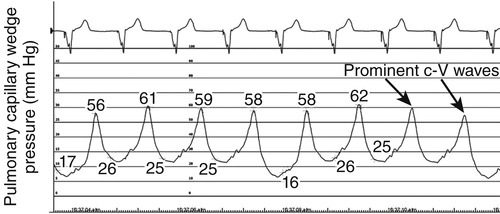
FIGURE 46-6 Pulmonary capillary wedge pressure trace at baseline before cardiac resynchronization therapy.
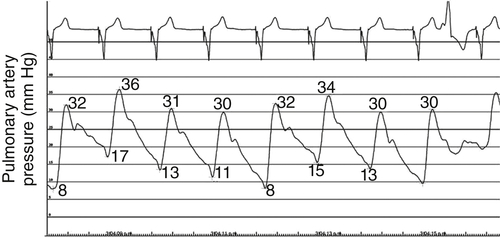
FIGURE 46-7 Pulmonary artery pressure trace after intravenous nitroglycerin 300 mcg demonstrating a significant fall in mean pulmonary artery pressure.
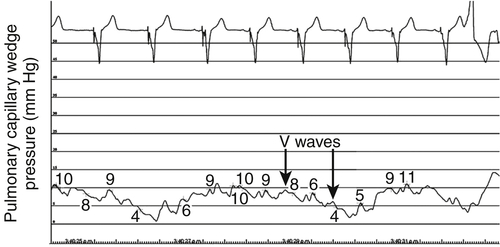
FIGURE 46-8 Pulmonary capillary wedge pressure trace after intravenous nitroglycerin 300 mcg demonstrating a significant fall in mean capillary wedge pressure.
Hemodynamics
Baseline
After Intravenous Nitroglycerin 300 mcg
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Question
Discussion
Question
Discussion
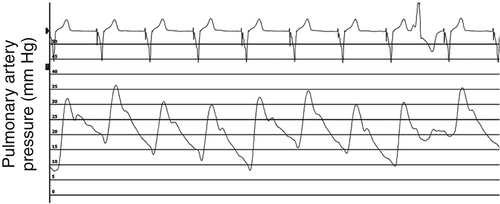
FIGURE 46-9 Pulmonary artery pressure trace 3 months after cardiac resynchronization therapy.
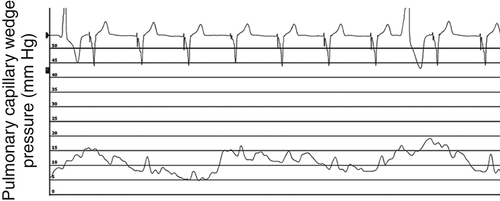
FIGURE 46-10 Pulmonary capillary wedge pressure trace 3 months after cardiac resynchronization therapy.
Final Diagnosis
Plan of Action
Outcome
Findings
Comments
Selected References
1. Aronson D., Eitan A., Dragu R. et al. Relationship between reactive pulmonary hypertension and mortality in patients with acute decompensated heart failure. Circ Heart Fail.. 2011;4:644–650.
2. Chatterjee N.A., Lewis G.D. What is the prognostic significance of pulmonary hypertension in heart failure? Circ Heart Fail.. 2011;4:541–545.
3. Grigioni F., Potena L., Galie N. et al. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant. 2006;25:1241–1246.
4. Guazzi M., Borlaug B.A. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990.
5. Liang Y.J., Zhang Q., Fung J.W. et al. Different determinants of improvement of early and late systolic mitral regurgitation contributed after cardiac resynchronization therapy. J Am Soc Echocardiogr. 2010;23:1160–1167.
6. McLaughlin V.V., Archer S.L., Badesch D.B., Barst R.J., Farber H.W., et al.Writing Committee, American College of Cardiology Foundation Task Force on Expert Consensus Documents, American Heart Association, American College of Chest Physicians, American Thoracic Society, Pulmonary Hypertension Association, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension. Circulation. 2009;119:2250–2294.
7. Shalaby A., Voigt A., El-Saed A. et al. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiolo. 2008;101:238–241.
8. Stern J., Heist E.K., Murray L. et al. Elevated estimated pulmonary artery systolic pressure is associated with an adverse clinical outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007;30:603–607.
9. Tedrow U.B., Kramer D.B., Stevenson L.W. et al. Relation of right ventricular peak systolic pressure to major adverse events in patients undergoing cardiac resynchronization therapy. Am J Cardiol. 2006;97:1737–1740.
10. van Bommel R.J., Marsan N.A., Delgado V. et al. Cardiac resynchronization therapy as a therapeutic option in patients with moderate-severe functional mitral regurgitation and high operative risk. Circulation. 2011;124:912–919.