History
Comments
Current Medications
Comments
Current Symptoms
Comments
Physical Examination
Comments
Laboratory Data
Comments
Focused Clinical Questions and Discussion Points
Discussion
Question
Discussion
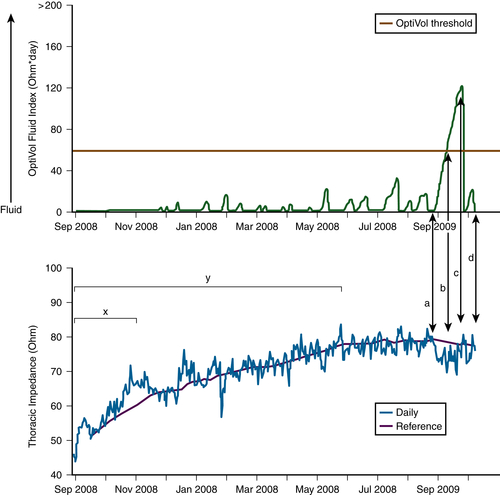
FIGURE 45-1
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Selected References
1. Braunschweig F., Ford I., Conraads V. et al. Can monitoring of intrathoracic impedance reduce morbidity and mortality in patients with chronic heart failure? Rationale and design of the Diagnostic Outcome Trial in Heart Failure (DOT-HF). Eur J Heart Fail. 2008;10:907–916.
2. Conraads V.M., Tavazzi L., Santini M., Oliva F. et al. Sensitivity and positive predictive value of implantable intrathoracic impedance monitoring as a predictor of heart failure hospitalizations: the SENSE-HF trial. Eur Heart J. 2011;18:2266–2273.
3. Daubert J.C., Saxon L., Adamson P.B. et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–1286.
4. Dickstein K., Bogale N., Priori S. et al. The European cardiac resynchronization therapy survey. Eur Heart J. 2009;30:2450–2460.
5. Fonarow G.C. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003;4(Suppl 7):S21–S30.
6. Gudmundsson K., Lynga P., Karlsson H. et al. Midsummer Eve in Sweden: a natural fluid challenge in patients with heart failure. Eur J Heart Fail. 2011;13:1172–1177.
7. Tang W.H., Warman E.N., Johnson J.W. et al. Threshold crossing of device-based intrathoracic impedance trends identifies relatively increased mortality risk. Eur Heart J. 2012:2189–2196.
8. van Veldhuisen D.J., Braunschweig F., Conraads V. et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726.
9. Whellan D.J., Ousdigian K.T., Al-Khatib S.M. et al. Combined heart failure device diagnostics identify patients at higher risk of subsequent heart failure hospitalizations: results from PARTNERS HF study. J Am Coll Cardiol. 2010;55:1803–1810.
10. Yu C.M., Wang L., Chau E. et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841–848.





