History
Current Medications
Comments
Current Symptoms
Physical Examination
Laboratory Data
Comments
Electrocardiogram
Findings
Echocardiogram
Findings
Comments
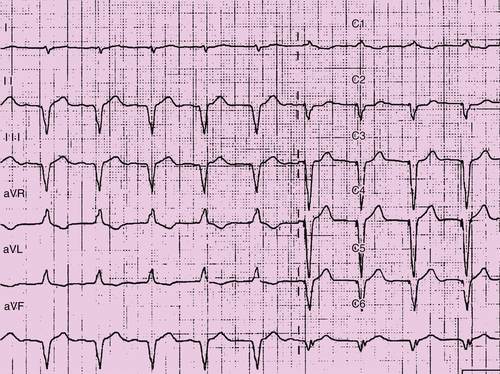
FIGURE 44-1
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
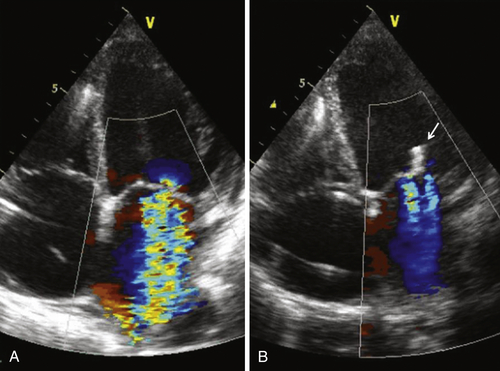
FIGURE 44-2
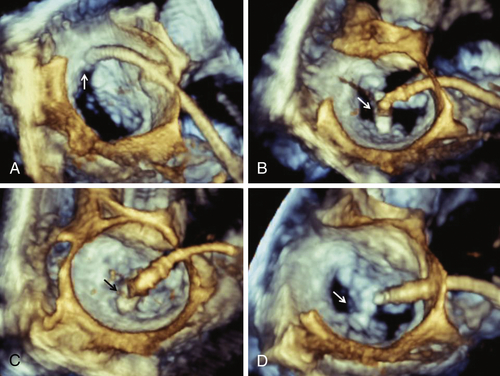
FIGURE 44-3
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
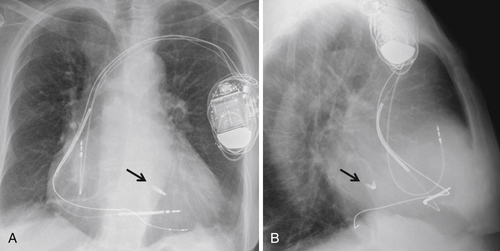
FIGURE 44-4
Findings
Selected References
1. Feldman T., Kar S., Rinaldi M. et al. EVEREST Investigators Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54:686–694.
2. Levy W.C., Mozaffarian D., Linker D.T. et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433.
3. Roques F., Michel P., Goldstone A.R. et al. The logistic EuroSCORE. Eur Heart J. 2003;24:882–883.
4. Auricchio A., Schillinger W., Meyer S. et al. PERMIT-CARE Investigators Correction of mitral regurgitation in nonresponders to cardiac resynchronization therapy by MitraClip improves symptoms and promotes reverse remodeling. J Am Coll Cardiol. 2011;58:2183–2189.
5. Vahanian A., Alfieri O., Andreotti F. et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496.
6. Boriani G., Gasparini M., Landolina M. et al. InSync/InSync ICD Italian Registry Investigators. Impact of mitral regurgitation on the outcome of patients treated with CRT-D: data from the InSync ICD Italian Registry. Pacing Clin Electrophysiol. 2012;35:146–154.
7. Di Biase L., Auricchio A., Mohanty P. et al. Impact of cardiac resynchronization therapy on the severity of mitral regurgitation. Europace. 2011;13:829–838.






