CHAPTER 44. Peripheral Vascular Care
Maureen E. Lisberger
OBJECTIVES
At the conclusion of this chapter, the reader will be able to:
1. Explain three factors that affect peripheral circulation.
2. Describe three causes of arteriosclerosis.
3. Compare the signs and symptoms of arterial and venous vascular disease.
4. Identify the risk factors that contribute to the development of peripheral vascular disease.
5. List three postarteriography assessment criteria.
6. Identify the most common sites of occurrence of peripheral vascular disease.
7. Describe operative and interventional radiology procedures performed on patients with peripheral vascular disease.
8. Describe the immediate postoperative nursing considerations for each operative procedure.
9. List postoperative complications of vascular surgery.
10. Describe complications of endovascular repair.
11. Describe the preoperative assessment, intraoperative, and postoperative care of the vascular patient.
I. ANATOMY AND PHYSIOLOGY
A. Peripheral vascular anatomy
1. Includes:
a. Peripheral arterial
b. Venous systems
2. Excludes:
a. Cardiac
b. Pulmonary
c. Cerebral systems
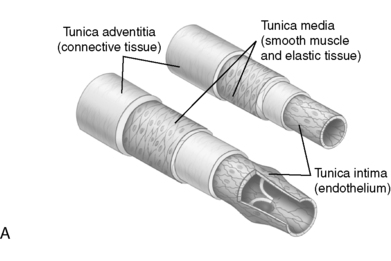
B. Arterial and venous wall structure contains three layers (Figure 44-1).
1. Adventitia—thin outer layer containing:
a. Collagen
b. Lymphatics
2. Media: thick middle layer containing smooth muscle cells arranged into strong, intertwining sheets of elastin that constrict or dilate. Medial layer is thinner in veins.
3. Intima: thin, inner, single endothelial layer; easily traumatized
 |
 |
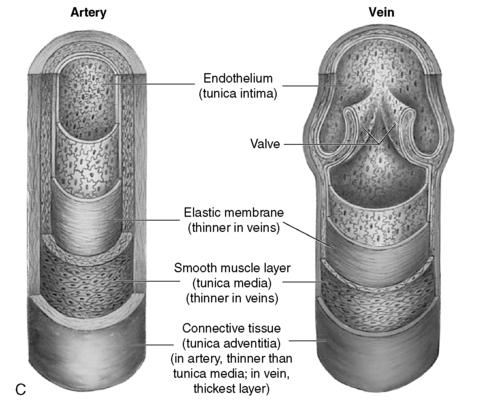 |
| FIGURE 44-1 ▪
A, Layers of artery and vein. B, Microcirculation. C, Cross section of an artery and vein showing the three layers: tunica intima, tunica media, and tunica adventia. Note the difference in wall thickness between the artery and the vein and the lack of valves within the artery.
( A and B from Thibodeau GA, Patton KT: Anatomy and physiology, ed 5, St Louis, 2003, Mosby; C from Thompson JM, McFarland GK, Hirsh JE, et al: Mosby’s clinical nursing, ed 5, St Louis, 2002, Mosby.)
|
C. Circulatory path (Figure 44-2)
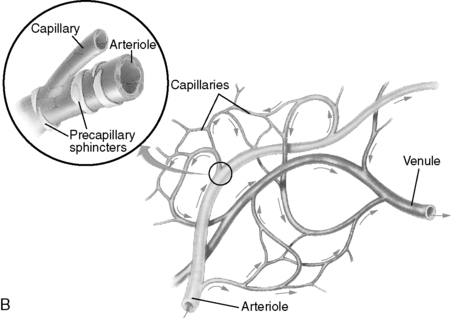
1. Artery → Arteriole → Precapillary sphincter → Capillary (Figure 44-3)
a. Artery: high pressure, low volume
b. Arteriole (diameter <0.5 mm)
(1) Offers resistance to blood flow
(2) Regulates blood flow into capillary bed
c. Precapillary sphincters
(1) Rings of smooth muscle located at proximal end of a true capillary
(2) Regulates flow of blood and oxygen (see Figure 44-1)
d. Capillary: site of gas and nutrient exchange
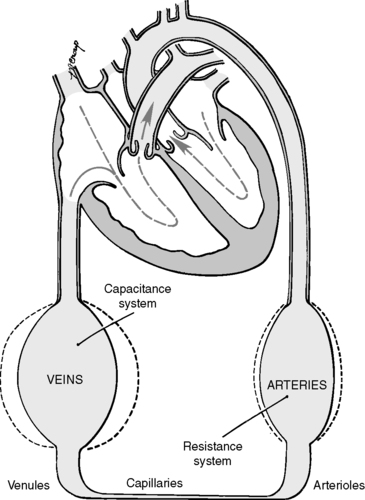 |
| FIGURE 44-2 ▪
Systemic circulation.
(From Price SA, Wilson LM: Pathophysiology: Clinical concepts of disease processes, ed 6, St Louis, 2003, Mosby.)
|
2. Capillary → Venule → Vein (see Figure 44-3)
a. Venule: as venules merge, rate of blood flow increases.
b. Vein
(1) Low pressure
(2) High volume
(3) Veins are capacitance vessels because they accommodate large volumes of blood.
(4) Unidirectional valves direct venous flow from feet toward heart and prevent reflux.
(5) Approximately 70% of blood volume contained in venous circulation (Figure 44-4)
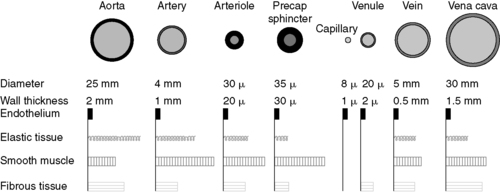 |
| FIGURE 44-3 ▪
Internal diameter, wall thickness, and relative amounts of the principal components of the vessel circulatory system. Cross sections of the vessels are not drawn to scale because of the huge range from aorta to vena cava to capillaries.
(From Berne RM, Levy MN: Cardiovascular physiology, ed 8, St Louis, 2001, Mosby.)
|
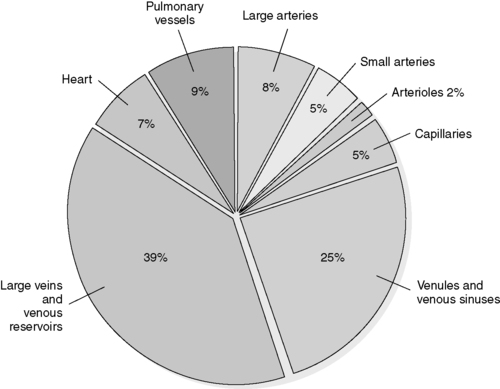 |
| FIGURE 44-4 ▪
Percentage of the total blood volume in each portion of the circulation system.
(From Urden LD, Stacy KM, Lough ME: Thelan’s critical care nursing diagnosis and management, ed 5, Philadelphia, 2006, Mosby.)
|
D. Arterial circulation (Figure 44-5)
1. Aorta: largest peripheral vessel, which includes four sections (Figure 44-6)
a. Ascending aorta: from aortic valve to arch
b. Arch: where brachiocephalic and carotid vessels originate
c. Descending thoracic aorta: from aortic arch to level of diaphragm
d. Abdominal aorta: from thoracic to aortic bifurcation
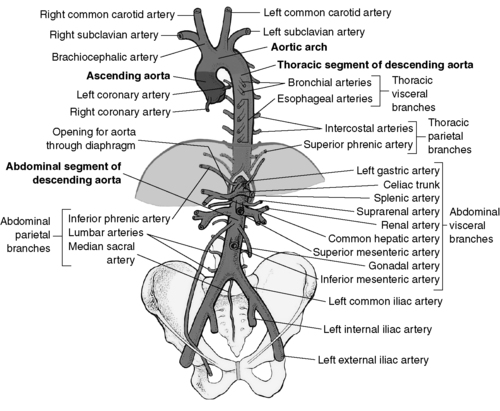 |
| FIGURE 44-6 ▪
The aorta.
(From Rothrock J: Alexander’s care of the patient in surgery, ed 12, St Louis, 2003, Mosby.)
|
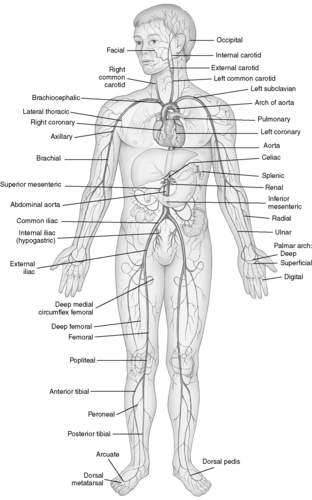 |
| FIGURE 44-5 ▪
Principal arteries of the body.
(From Thibodeau GA, Patton KT: Anatomy and physiology, ed 6, St Louis, 2007, Mosby.)
|
2. Aortic bifurcation: where aorta divides into common right and left iliac arteries
a. Common iliac divides into:
(1) Internal iliac (hypogastric)
(2) External iliac: continuation of common iliac artery that becomes common femoral artery in thigh
b. Common femoral (thigh) (Figure 44-7)
(1) Lateral and medial femoral circumflex
(2) Profunda (deep) femoral
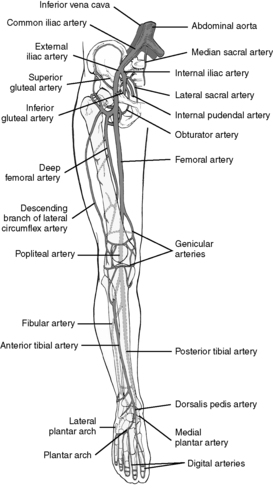 |
| FIGURE 44-7 ▪
Major arteries of the lower extremity.
(From Rothrock J: Alexander’s care of the patient in surgery, ed 12, St Louis, 2003, Mosby.)
|
c. Popliteal: continuation of common femoral located posterior to knee surface divides into:
(1) Anterior tibial
(a) Dorsalis pedis
(b) Posterior tibial
(i) Medial and lateral plantar
(ii) Peroneal
E. Venous circulation (Figure 44-8)
1. Superficial system: in subcutaneous tissue
a. Greater saphenous: longest vein in body extending from malleolus of ankle to femoral vein (saphenous junction)
b. Lesser saphenous: extends from ankle to popliteal vein in knee (saphenopopliteal junction)
2. Deep veins: in muscular layers
a. Anterior and posterior tibial
b. Peroneal
c. Popliteal
d. Femoral, profunda femoris
e. Iliac
3. Perforating (communicating): vascular channels (Figure 44-9)
a. Communicate between deep and superficial veins
b. Flow shunted from superficial to deep system with help of unidirectional valves and finally to inferior vena cava
c. Muscle contraction promotes forward flow; valves prevent backflow during muscular relaxation.
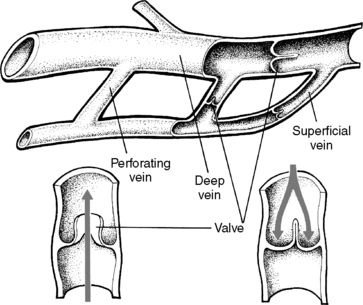 |
| FIGURE 44-9 ▪
Anatomy of the venous system of the leg.
(From Price SA, Wilson LM: Pathophysiology: Clinical concepts of disease processes, ed 6, St Louis, 2003, Mosby.)
|
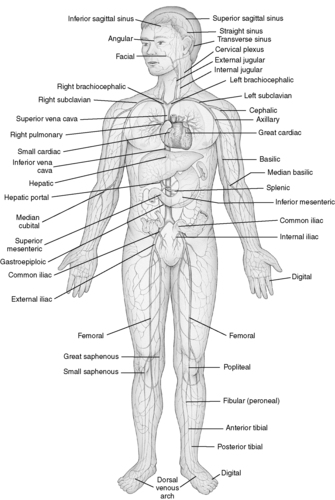 |
| FIGURE 44-8 ▪
Principal veins of the body.
(From Thibodeau GA, Patton KT: Anatomy and physiology, ed 6, St Louis, 2007, Mosby.)
|
F. Factors affecting circulation
1. Cardiac output (Cardiac output = Stroke volume × Heart rate): venous capacity will determine venous return that will affect stroke volume of heart.
2. Arteriolar resistance: systemic vascular resistance (SVR) depends on:
a. Degree of arteriolar constriction
b. Resistance
(1) Increases as vessels constrict
(2) Decreases as vessels dilate
c. High SVR will:
(1) Decrease blood flow
(2) Increase myocardial workload
3. Vessel wall elasticity
a. With low compliance, pressure is greater.
b. Increased pressure will increase myocardial oxygen consumption.
4. Fluid volume status: low fluid volume will reduce peripheral resistance.
5. Diameter of vessel (arteriole diameter <0.5 mm)
a. Vasoconstriction: exposure to cold, vasoconstrictive agents
b. Vasodilation: exposure to heat, vasodilator agents
6. Sympathetic nervous system: regulates amount of vasoconstriction
G. Common sites of vascular disease (Figure 44-10)
1. Internal carotid arteries
2. Aorta above inguinal ligament inflow disease
3. Aortoiliac: bifurcation of aorta and iliac arteries inflow disease
4. Superficial femoral: middle to distal thigh below inguinal ligament outflow disease
5. Popliteal artery outflow disease
6. Tibial arteries: common in patients with diabetes outflow disease
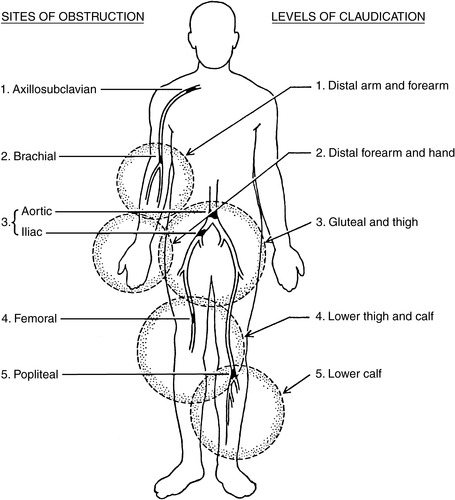 |
| FIGURE 44-10 ▪
Sites of arterial obstruction and corresponding levels of claudication.
(From Fahey VA: Vascular nursing, ed 4, St Louis, 2004, Saunders.)
|
H. Incidence and risk factors associated with peripheral vascular disease
1. Highest incidence among elderly, men, persons with diabetes, smokers
2. Gender
a. More common in men
b. Earlier onset in men
c. Postmenopausal women susceptible
3. Age
a. Occurs beyond 30 years of age
b. Symptoms worsen after 65 years of age.
I. Risk factors of atherosclerosis
1. Lifestyle habits
a. Psychophysiologic stress triggers vasoconstriction.
b. Sedentary; lack of exercise
c. Smoking (major risk factor) for smokers and passive smoking (environmental tobacco smoke exposure)
(1) Vasoconstrictive effect of nicotine
(2) Inhalation of carbon monoxide in cigarette smoke
(a) Increases carboxyhemoglobin levels (carbon monoxide binds with hemoglobin)
(b) Impaired oxygen transport
(c) Hypoxic injury to intimal lining of artery
(d) Increased platelet aggregation caused by enhanced platelet adhesiveness
d. Diet
(1) Hyperlipidemia (hyperlipoproteinemia): accumulation of lipids in arterial wall
(a) Elevated cholesterol: total serum levels
(b) Elevated triglycerides
(i) Low-density lipoproteins (LDL): high serum levels related to premature development of atherosclerotic process
(ii) High-density lipoproteins (HDL): high serum levels demonstrate protective effect against atherosclerosis.
(iii) Homocysteine: high serum levels block production of nitric oxide on vascular endothelium, making cell walls less elastic and permitting plaque to build up.
(2) Obesity
2. Positive family history
3. Disease processes
a. Diabetes mellitus
b. Hypertension (major risk factor)
J. Indications for surgical intervention
1. Ischemic pain at rest
2. Significant limb ischemia
3. Limiting claudication
II. PATHOPHYSIOLOGY
A. Arterial occlusive disease
1. Classified as inflow or outflow above or below inguinal ligament (Figure 44-11)
a. Inflow obstruction involves distal end of aorta.
(1) Common iliac arteries
(2) Internal iliac arteries
(3) External iliac arteries
b. Outflow obstruction involves infrainguinal arteries below superficial femoral artery (SFA).
(1) Femoral arteries
(2) Popliteal arteries
(3) Tibial arteries
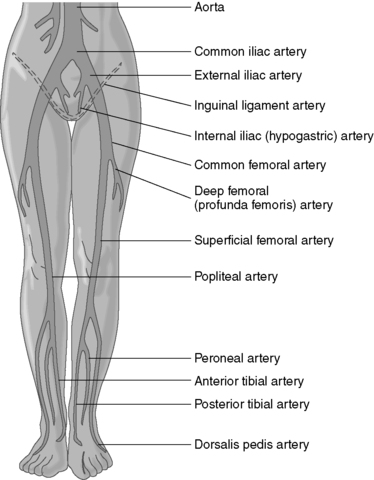 |
| FIGURE 44-11 ▪
Common locations of inflow and outflow lesions.
(From Ignatavicius DD, Workman L: Medical-surgical nursing: Critical thinking for collaborative care, Philadelphia, 2006, Saunders.)
|
2. Obstruction or stenosis of vessel
a. Decreased peripheral vessel blood flow
b. Decreased vessel diameter
c. Increased peripheral vascular resistance
d. Decreased blood flow velocity
3. Degenerative changes
a. Reduced tissue oxygen and nutrient supply
(1) Inadequate tissue integrity
(2) Ischemic tissue
(3) Destruction of muscle and elastic fibers
b. Formation of calcium and/or cholesterol deposits
(1) Thickening of arterioles
(2) Loss of elasticity
B. Venous disease
1. Deep vein thrombosis (DVT): disease of deep veins of lower extremity, often accompanied by intraluminal clot
2. Superficial thrombophlebitis: inflammation and clot in superficial veins
3. Virchow’s triad: three factors that increase incidence of venous thrombosis
a. Hypercoagulability caused by alteration of platelet and clotting factors
b. Venous stasis caused by incompetent venous valves
c. Intimal damage caused by trauma, intravenous infusions, ischemia
4. Pulmonary embolism: dislodged DVT with migration to pulmonary vasculature
5. Varicose veins
a. Structural weakness
b. Vessel tortuosity
c. Dilation
(1) Incompetent venous valves
(2) Reflux of blood results in venous pooling.
6. Venous hypertension: hereditary
a. Incompetent valves result in reduced blood return to heart.
b. Venous stasis and pooling of blood results in venous hypertension.
C. Arterial insufficiency: arterial occlusive disease
1. Arteriosclerosis obliterans
a. Atherosclerosis: most common form of arteriosclerosis obliterans
(1) Accumulation of lipids and connective tissue
(2) Intraluminal plaque formation
(3) Platelet aggregation
(4) Thrombus formation
(5) Loss of elasticity
b. Mönckeberg’s arteriosclerosis: arteriosclerosis of peripheral arteries
(1) Characterized by calcium deposits within medial layer
c. Arteriolosclerosis: sclerosis of arterioles
2. Aneurysm: abnormal dilation of vessel wall with high incidence of rupture and mortality when greater than 6 cm in diameter (Figures 44-12, 44-13 and 44-14)
a. Fusiform: diffuse circumferential dilation of artery
b. Saccular: area of pouching; affects localized part of arterial wall
c. Dissecting: intimal layer torn; blood accumulates between layers.
d. False aneurysm—when palpable hematoma often present, a complete tear of all three layers of arterial wall occurs caused by:
(1) Trauma
(2) Needle puncture
(3) Suture failure at anastomosis site of prosthetic graft
e. Pseudoaneurysm: dilated or tortuous segment of arterial wall without interruption of layers
f. Theories of aneurysm pathogenesis (Table 44-1)
| Etiology | Clinical Evidence | Theory |
|---|---|---|
| Genetic |
Genetically linked enzyme deficiencies are associated with aneurysms.
Familial clustering is observed.
Male siblings have up to 25% lifetime risk of aneurysm.
|
X chromosome linked and autosomal dominant inheritance pattern
Specific deficits in collagen
|
| Atherosclerotic |
Risk factors are similar to occlusive disease including smoking, hypertension, and aging.
Aneurysm wall contains calcium and atherosclerotic lesions.
|
Compensatory dilation of the artery becomes uncontrolled. |
| Immunologic | A variant called inflammatory aneurysm is characterized by gross inflammation and microscopic leukocyte infiltrates. | Antigen, possibly through molecular mimicry, precipitates autoimmune response. |
| Degenerative |
Disruption of normal aortic wall architecture
Decreased amounts of elastin and collagen are found in aneurysms.
Hernias are common in patients with aneurysms.
|
Elastin and collagen are aberrantly formed or digested. |
| Hemodynamic | Aneurysms typically occur proximal to bifurcations or distal to stenoses. | Wall tension, turbulence, vibration, and shear stress are increased dramatically in these areas. |
| Iatrogenic | Occur at graft anastomosis, after endarterectomy, angioplasty, or full-thickness traumatic disruption | Structural injury, end-to-side anastomosis |
| Infectious | Salmonella, Chlamydia pneumoniae, Streptococcus species, Staphylococcus species, Treponema pallidum are associated with aneurysms. | Microorganisms by direct extension, emboli, or infection from unknown primary may stimulate inflammation or degradation. |
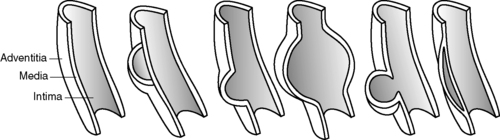 |
| FIGURE 44-12 ▪
Aneurysm types.
(From Smeltzer SC, Bare BG: Brunner and Suddarth’s textbook of medical-surgical nursing, ed 10, Philadelphia, 2004, Lippincott Williams & Wilkins.)
|
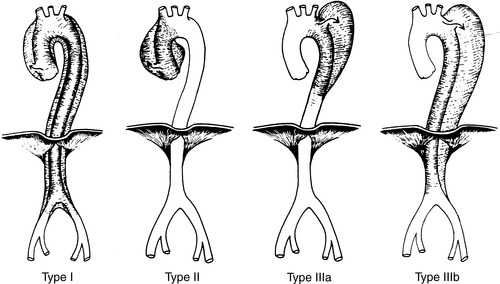 |
| FIGURE 44-13 ▪
Two popular classification schemes for aortic dissections. Diagnosis of involvement of ascending aorta has important prognostic and therapeutic implications. DeBakey types I and II are called type A in the Stanford classifications; DeBakey type III is equal to Stanford type B.
(From Hamilton IN, Hollier LH: Thoracoabdominal aortic aneurysms. In Moore W, ed: Vascular surgery: A comprehensive review, ed 5, Philadelphia, 1998, W.B. Saunders.)
|
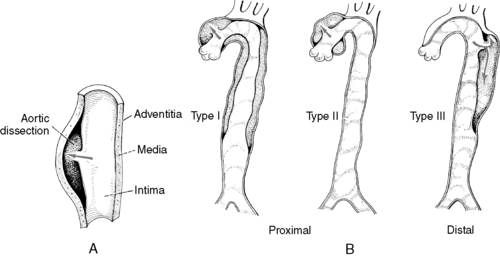 |
| FIGURE 44-14 ▪
Aortic dissection. A, Separation of vascular layers. B, Classification of aortic dissection.
(From Price SA, Wilson LM: Pathophysiology: Clinical concepts of disease processes, ed 6, St Louis, 2003, Mosby.)
|
D. Vascular diseases and conditions
1. Acute
a. Arterial embolism: sudden onset of symptoms of acute arterial insufficiency
(1) Originates in myocardium or arterial aneurysm
(2) May be secondary to external or iatrogenic trauma (catheter placement)
b. Trauma: arterial wall tear or dissection
2. Chronic
a. Diabetes mellitus: medial layer calcification; arteries become noncompressible.
b. Hypertension: increases permeability of intimal endothelium
c. Polycythemia: increased blood viscosity caused by increase in red blood cell count
d. Inflammatory processes: may cause occlusive lesions
(1) Arteritis: inflammation of arterial wall
(a) Polyarteritis nodosa (PAN): systemic disease causing arterial inflammation and aneurysm rupture in adults
(b) Kawasaki: similar to PAN; occurs in children
(c) Cogan’s: (rare condition) similar to PAN; inflammatory infiltration of large veins and muscular arteries
(d) Behcet’s: similar to PAN; affects both arteries and veins
(e) Drug abusers: similar to PAN; necrotizing arteritis (intra-arterial injection of drugs)
(2) Fibromuscular dysplasia: multiple areas of arterial stenosis and dilation
(3) Buerger’s disease: thromboangiitis obliterans, autoimmune disease
(a) Inflammation of arterial walls
(b) Thrombus formation caused by intimal thickening
(c) Affects plantar and digital vessels
(d) Pain at rest, extremity cold, cyanotic
(4) Granulomatous/giant cell arteritis
(a) Takayasu’s arteritis: transmural inflammatory process
(i) Type I aortic arch and vessels originating from arch
(ii) Type II abdominal aorta and visceral vessels
(iii) Type III both the arch and abdominal aorta
(iv) Type IV pulmonary arteries
(b) Temporal arteritis: thickening of intima, necrosis of media
(5) Hypersensitivity angiitis: arterial damage from antigen-antibody complexes
e. Raynaud’s phenomenon and Raynaud’s disease: vasospastic diseases that are related
(1) Intense vasospasm of arteries and arterioles of extremities
(2) Precipitated by exposure to cold
(3) Ischemic changes: cyanosis, numbness, tingling
(4) Occurs in 40% of patients with systemic lupus erythematosus, and 90% of patients with scleroderma
(5) Raynaud’s phenomenon occurs unilaterally in both men and women older than 30 years.
(6) Raynaud’s disease occurs bilaterally, mostly in females, between 17 and 50 years of age.
f. Compartment syndrome: swelling within osteofascial compartments of leg or arm
(1) Intracompartmental pressure increases from bleeding within the compartment, or external compression from dressings, cast, traumatic crush injury.
(2) Vascular perfusion decreases, compromising tissue.
(3) Ischemia affects nerves and muscles.
(a) Pain
(b) Tenseness of compartment
(c) Paresthesia
(d) Pulselessness
(e) Paralysis
(f) Pallor
III. CLINICAL SIGNS AND SYMPTOMS
A. Arterial insufficiency
1. Decreased blood flow may cause inadequate tissue oxygenation distal to lesion.
2. A 70% to 90% occlusion of a large artery usually must occur before a decrease in blood flow or pressure causes symptoms at rest.
3. A 60% obstruction may be sufficient to precipitate signs and symptoms during exercise.
4. Acute
a. Peripheral pulses diminished, weak, or absent
b. Cold and pale extremity (sudden onset)
c. Sudden, severe pain may occur during exercise or at rest: moderate to severe inflow disease.
(1) Lower back and buttock pain: common iliac or abdominal aorta inflow disease (Figure 44-11)
(2) Thigh pain at or above profunda femoris artery
d. Limited sensory and motor function
(1) Possible paresthesia
(2) Atrophied skeletal muscle: restricted limb movement
e. Minimal edema: usually unilateral
f. Bruit present with partial occlusion; no bruit with total occlusion
5. Chronic
a. Diminished or weak distal pulses outflow disease (see Figure 44-11)
(1) Below SFA
(2) Popliteal artery
b. Pain at rest related to severe ischemia: burning or cramping in calves, ankles, feet, toes
c. Tissue necrosis: gangrene
d. Intermittent claudication
e. Skin
(1) Skin ulceration
(2) Delayed healing of skin lesions
(3) Skin texture: thin, shiny, dry
(4) Cool skin: poikilothermic
f. Color: pale extremity
(1) Increased pallor when elevated
(2) Rubor or cyanosis or both when dependent
g. Possible paresthesia of limb
h. Edema: none or mild
(1) Hair loss distal to occlusion
i. Nails: thick, brittle
j. Impotence: associated with aortoiliac disease
B. Venous insufficiency
1. Acute
a. Moderate pain localized to area of inflammation
b. Pulses present or diminished (absent in presence of concomitant disease)
c. Skin warm, cyanotic, mottled, or pale
d. Engorged veins when legs slightly dependent
e. Moderate to severe peripheral edema
2. Chronic
a. Minimal to moderate pain
b. Moderate to severe edema, unilateral or bilateral
c. Sensation of heaviness at site of occlusion
d. Muscle cramps, aching
e. Ulceration of ankle area
f. Superficial veins may be prominent.
g. Skin
(1) Warm
(2) Brawny (reddish brown) color
(3) Pronounced lower leg pigmentation
(4) Texture: thickening, scaling, and/or scarring
IV. DIAGNOSTIC ASSESSMENT
A. Arterial tests
1. Noninvasive laboratory studies
a. Segmental pressure measurement: measurement of systolic blood pressure along selected segments of each extremity
(1) Gradient >20 mm Hg: evidence of arterial stenosis in lower extremity
(2) Gradient >10 mm Hg: evidence of arterial stenosis in upper extremity
b. Ankle/brachial index (ABI): ratio of ankle to brachial pressure (normal ABI, ≥1.0)
(1) One limitation is calcified vessels as in renal failure or diabetes.
c. Toe pressure measurements: assess distal arterial flow.
(1) Useful in diabetics with calcification of larger vessels
d. Pulse volume recording: quantifies arterial flow to determine location of lesion and severity.
e. Doppler ultrasound: determines blood flow and velocity
f. B-mode ultrasonography: projects two-dimensional image in real time
g. Duplex ultrasound imaging: assesses both anatomic characteristics and stenosis of peripheral arteries; combination of Doppler and B-mode ultrasonogram
h. Air plethysmography (APG) and photoplethysmography: record volume changes in limb
i. Treadmill exercise testing: objective evidence of walking capacity and evaluation of peripheral stenosis
j. Computed tomography (CT): a tomograph is an image of a cross-sectional slice of a body part.
(1) CT image is three dimensional: a camera rotates around selected body part taking two-dimensional images at multiple angles, which are converted to a composite three-dimensional image by a computer.
(2) Contrast material (usually iodine) injected to heighten contrast between vessel wall and blood
(3) Used for diagnosis of aortic aneurysms and aortic dissection
(4) Able to detect hematomas or thrombi better with CT than with arteriography
k. Magnetic resonance imaging: detailed and three-dimensional imaging of vessel lumen where contrast not needed; contraindicated in patients with pacemakers and cerebral aneurysm clips
l. Magnetic resonance angiography: has replaced angiography for severe carotid stenosis and the lower extremities; uses intravenous gadolinium; no arterial puncture required
2. Invasive laboratory studies
a. Arteriography: invasive radiographic procedure in which radiopaque contrast injected into artery
b. Transcatheter therapy: percutaneous transluminal angioplasty, stenting, lyse clot, therapeutic embolization
(1) Purposes
(a) Depict location of stenosis, occlusion, or view aneurysm
(b) Visualize collateral, proximal, and distal arterial circulation to determine surgical treatment options
(2) Complications
(a) Intimal disruption
(i) Hematoma formation at puncture site
(ii) Plaque dislodgement
(iii) Arterial occlusion: thrombosis
(iv) Distal embolization
(v) Arteriovenous (AV) fistula
(vi) Arterial dissection
(vii) Renal failure
(b) Transient ischemic attack (TIA) or cerebrovascular accident (CVA)
(c) Toxic reaction to contrast media: renal or cardiac
(d) Allergic reaction
(i) Rash
(ii) Bronchospasm
(iii) Altered consciousness
(iv) Convulsions
(v) Anaphylaxis
(vi) Cardiac arrest
(3) Postarteriography assessment and intervention
(a) Assessment
(i) Vital signs
(ii) Hematoma and/or bleeding at puncture site
(iii) Signs and symptoms of acute arterial insufficiency
[a] Skin: color, temperature
[b] Pulses distal to puncture site
[c] Pain
[d] Urinary output
[e] Neurologic status
[f] Signs of heart failure or respiratory distress
(b) Intervention
(i) Observe for rash.
(ii) Maintain adequate hydration to flush contrast.
(iii) Head of bed at 30° or less
(iv) Keep affected extremity straight for 4 to 6 hours after procedure.
(v) Wait a minimum of 4 hours to resume heparin if previously receiving heparin.
B. Venous tests
1. Noninvasive laboratory studies
a. Venous Doppler ultrasonography examinations: used to determine blood flow patterns and velocity
(1) During inspiration, intrathoracic pressure decreases and venous return to heart increases.
(2) During expiration, venous flow to lower extremities will increase.
b. APG
(1) Used to evaluate venous obstruction, reflux, and calf muscle pump function
(2) Able to differentiate deep and superficial venous insufficiency
c. Duplex imaging of valvular closing times indicates severity of venous reflux.
d. Arm/foot pressure gradient measures outflow obstruction: normal difference between arm and foot is <4 mm Hg.
2. Invasive testing
a. Ascending phlebography: used to assess venous patency
b. Descending phlebography: used to assess valvular function
V. GENERAL SYSTEMS ASSESSMENT OF VASCULAR PATIENTS
A. Cardiovascular (see Chapter 32)
1. Myocardial infarction remains leading cause of death after peripheral vascular procedures because of coexisting cardiovascular disease.
B. Evaluation of cardiac status
1. Hemodynamic profile: cardiac output, SVR
2. Electrocardiogram: dysrhythmias
a. Increased myocardial oxygen demands
b. Increased cardiac ischemia: angina
3. Signs and symptoms of myocardial infarction
a. Chest pain
b. Dysrhythmias
c. Diaphoresis
d. Nausea and vomiting
e. Dyspnea
f. Hypotension
4. Chest x-ray film to assess heart size and fluid status
5. Cardiac enzymes
a. Creatine Kinase (CK)
b. CK-MB isoenzyme
c. Troponin T (cTnT) and troponin I (cTnI)
d. Myoglobin never used alone but in conjunction with cardiac-specific markers
6. Serum electrolytes
a. Hyperkalemia
(1) Oliguric renal failure
(2) Volume depletion
(3) Decreased effect of aldosterone: Addison’s disease, chronic heparin administration
b. Hypokalemia
(1) Diuretic or digitalis therapy
(2) Stress
(3) Gastrointestinal disorders
(a) Long-term steroid therapy: arthritis, chronic obstructive pulmonary disease (COPD)
(b) Hypoaldosteronism
c. Hypernatremia
(1) Mechanical ventilation without humidification
(2) Fever
d. Hyponatremia
(1) Diuretics
(2) Gastrointestinal disorders
(3) Hypotonic irrigating solutions
(4) Hyperlipidemia
(5) Hyperglycemia
e. Hypermagnesemia
(1) Renal failure
(2) Adrenal insufficiency
(3) Shock
(4) Hypothermia
f. Hypomagnesemia
(1) Excessive loss of body fluids
(2) Diuretics
(3) Cardiac glycosides, aminoglycosides
(4) Decreased intestinal absorption
(5) Primary hyperaldosteronism
(6) Hypercalcemia associated with hyperparathyroidism and hyperthyroidism
(7) ECG reflects prolonged QT interval, decreased T-wave amplitude, shortened ST segment.
C. Accompanying cardiovascular disorders
1. Hypertension
a. High incidence (40%-60%) associated with peripheral vascular disease (PVD)
b. Adds stress to anastomotic sites
c. Precipitates postoperative incisional bleeding
2. Hypotension
a. Decreases cerebral, coronary, and renal artery perfusion
b. Decreases stroke volume: decreased cardiac output
3. Valvular disease: associated with decreased cardiac output and left ventricular failure
a. Mitral valve stenosis: associated with pulmonary fibrosis and hypertension
b. Aortic insufficiency: associated with circulatory collapse with sudden hypotension
D. Pulmonary status (see Chapter 41)
1. Baseline parameters
a. Respirations
(1) Rate, quality
(2) Pattern, excursion
b. Auscultation of lungs
(1) Wheezes
(2) Rales
(3) Rhonchi
(4) Crackles
c. Arterial blood gases (ABGs), mixed venous oxygen saturation (Sv o2) monitoring
d. Pulmonary function studies
(1) Forced vital capacity (FVC)
(a) Measurement of volume of air expelled by fully inflated lung
(b) Compromised FVC indicative of lung parenchymal restriction
(2) Forced expiratory volume (FEV)
(a) Decreased FEV indicative of impaired elastic recoil (emphysema)
(b) Increased FEV indicative of airway resistance (chronic bronchitis or asthma)
(3) Ventilation-to-perfusion ratio
e. Chest x-ray film
(1) Pulmonary infiltrate or lesion
(2) Heart size
(3) Congestive heart failure (CHF)
(4) Fibrosis or effusion
2. Pulmonary history
a. Obstructive disorders (COPD, emphysema, asthma)
b. Infections (bronchitis, tuberculosis)
c. Presence of cough (lesions, smoking, bronchitis)
d. Complaints of shortness of breath
e. Orthopnea
E. Neurologic status (see Chapter 33)
1. Postoperative assessment: compare with preoperative baseline.
a. Level of consciousness
b. Pupillary reactions
c. Sensory and motor ability
d. Evaluation of following cranial nerves
(1) Facial (VIII): controls facial muscles; affects ability to smile, show teeth, wrinkle forehead, raise eyebrows
(2) Hypoglossal (XII): most frequently traumatized; controls tongue; affects side-to-side motion of tongue
(3) Glossopharyngeal (IX): controls posterior third of tongue, uvula; affects gag reflex
(4) Vagus (X): controls pharynx, larynx, soft palate; affects gag reflex
(5) Spinal accessory (XI): controls trapezius and sternocleidomastoid muscles; affects strength and tone of shoulder muscles
(6) Phrenic nerve: controls diaphragm; affects diaphragmatic function in respiratory excursion
2. Neurological history
a. Vertigo
b. Syncope
c. TIA
d. CVA
e. Spinal cord ischemia with descending thoracic aorta repair
F. Renal status (see Chapter 37)
1. Preoperative baseline studies
a. Blood urea nitrogen
b. Creatinine
c. Electrolytes
d. Calcium
e. Phosphorus
f. Urinalysis
2. Specific renal function studies, if indicated
a. Osmolar, free water, and sodium clearances
b. Creatinine or insulin clearance to evaluate glomerular filtration
c. Para-aminohippurate clearance to evaluate renal blood flow
d. Postangiographic renal function to evaluate possibility of renal failure caused by radioactive dye
3. Compromised renal system during vascular surgery caused by:
a. Hemorrhage
b. Trauma
c. Renal vessel damage, tubular damage
d. Anoxia
e. Prolonged hypotension
G. Diabetes (see Chapter 34)
1. Relationship to PVD
a. High incidence of occurrence in patients with PVD
b. Higher incidence of postoperative complications
c. Altered fluid requirements
2. Management of diabetes in a surgical patient (insulin regimen dependent on institutional policy)
a. Preoperative dose: usually less than routine daily dose
b. Stress of surgery with release of epinephrine and glucocorticoids increases need for insulin.
c. Diet-controlled diabetics may require insulin in immediate postoperative period.
3. Laboratory assessment
a. Fasting glucose levels
b. Electrolyte series
c. Serum ketones and acetones
d. Renal function studies
e. ABGs
H. Hematologic evaluation (see Chapter 29)
1. Laboratory studies
a. Prothrombin time, partial thromboplastin time
b. D-dimer assay
c. Platelet count
d. Bleeding time, clotting time
e. Type and crossmatch
f. Complete blood count
2. Anticoagulant and antiplatelet aggregation medications
a. Heparin (sodium warfarin, Coumadin)
b. Aspirin
c. Dipyridamole (Persantine)
d. Ticlopidine (Ticlid)
e. Clopidogrel (Plavix)
f. Pentoxifylline (Trental) decreases blood viscosity and increases erythrocyte flexibility.
g. Cilostazol (Pletal) inhibits platelet aggregation and increases vasodilation.
3. Previous postoperative bleeding and clotting problems
a. Disseminated intravascular coagulopathy
b. Blood transfusion reactions
c. Thrombophlebitis
d. Pulmonary emboli
e. History of any postoperative bleeding
VI. OPERATIVE PROCEDURES
A. Endarterectomy (Figure 44-15)
1. Opening of occluded portion of artery
2. Removal of atheromatous material or plaque
3. Excision of artery’s intimal lining
4. Performed on carotid, subclavian, iliac, or femoral artery
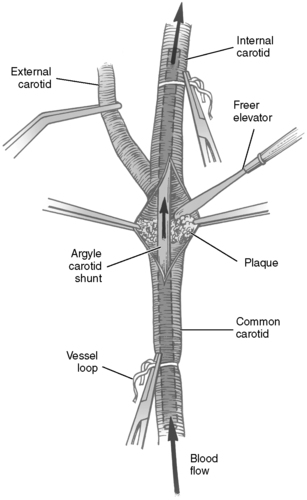 |
| FIGURE 44-15 ▪
Left carotid endarterectomy. Argyle carotid shunt in place to allow flow to the brain. Stenotic plaque being removed with Freer elevator.
(From Rothrock JC, McEwen DR: Alexander’s care of the patient in surgery, ed 13, St Louis, 2007, Mosby.)
|
B. Carotid-subclavian bypass
1. Anastomosis of carotid and subclavian arteries to improve circulation
2. Common carotid used as donor for subclavian lesions
3. Subclavian used to restore circulation for carotid lesions
C. Aortocarotid-subclavian bypass
1. Insertion of bypass graft from ascending aorta into carotid or subclavian artery
2. For occlusive lesions of both common carotid or innominate and subclavian arteries
D. Carotid artery ligation
1. Surgical occlusion of carotid artery
2. Temporary control of hemorrhaging during intracranial vessel surgery
3. Permanent control of intracranial or nasal hemorrhaging
4. Treatment of carotid-cavernous fistula
E. Aorto-innominate-subclavian bypass: thoracic aortic graft into innominate, subclavian arteries
F. Aneurysmectomy
1. Excision of weakened dilated area of artery
2. Insertion of synthetic prosthesis to reestablish circulatory continuity
3. Usually occurring in abdominal aorta, thoracic aorta, or carotid, popliteal, or femoral artery
G. Thoracoabdominal aortic aneurysm repair
1. Clots removed before anastomosis of Dacron graft
2. Spinal catheter placed at L1 to L2 to allow for cerebrospinal fluid (CSF) drainage
3. Spinal cord ischemia evaluated by monitoring CSF pressure
H. Bypass approaches for aortoiliac occlusions (Figure 44-16)
1. Aortoiliac bypass: insertion of vascular graft from distal aorta into iliac artery or arteries
2. Aortofemoral bypass (Figure 44-17)
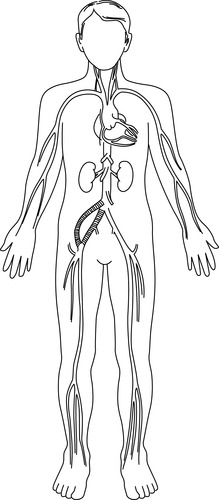 |
| FIGURE 44-17 ▪
Right iliac artery occlusion and graft: aortofemoral bypass.
(From Kinney MR, Dunbar SB, Brooks-Brunn JA, et al: AACN’s clinical reference for critical care nursing, ed 4, St Louis, 1998, Mosby.)
|
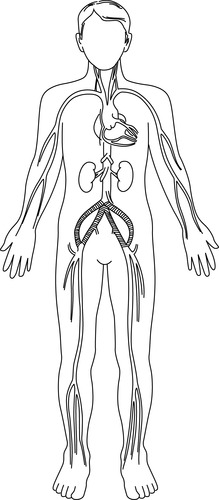 |
| FIGURE 44-16 ▪
Aortoiliac occlusion and graft: aortobifemoral bypass.
(From Kinney MR, Dunbar SB, Brooks-Brunn JA, et al: AACN’s clinical reference for critical care nursing, ed 4, St Louis, 1998, Mosby.)
|
3. Anastomosis of distal aorta to femoral arteries
4. Lesion bypassed with vascular graft
I. Axillofemoral bypass (Figure 44-18)
1. Superficial flank placement
2. Anastomosis of prosthetic graft from one axillary artery to one or both femoral arteries
3. Restores blood flow beyond occlusive lesion
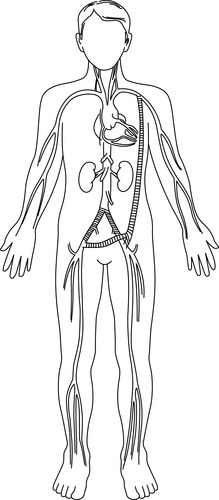 |
| FIGURE 44-18 ▪
Left iliac artery occlusion and graft: right-to-left femorofemoral prosthetic bypass graft.
(From Kinney MR, Dunbar SB, Brooks-Brunn JA, et al: AACN’s clinical reference for critical care nursing, ed 4, St Louis, 1998, Mosby.)
|
J. Femorofemoral bypass: femoral crossover graft (Figure 44-19)
1. Extra-anatomic bypass procedure with subcutaneous placement across suprapubic area
2. End-to-side anastomosis from patent femoral to stenotic femoral artery
3. Diverts blood flow from one donor femoral artery to recipient stenotic artery
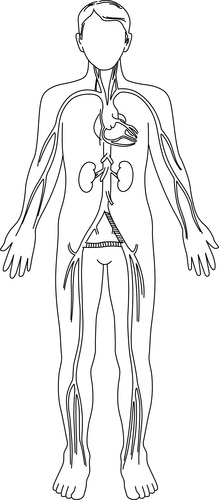 |
| FIGURE 44-19 ▪
Aortoiliac occlusion with extra-anatomic graft: left axillofemorofemoral bypass.
(From Kinney MR, Dunbar SB, Brooks-Brunn JA, et al: AACN’s clinical reference for critical care nursing, ed 4, St Louis, 1998, Mosby.)
|
K. Aortorenal bypass: anastomosis of abdominal aorta to renal artery with vascular graft
L. Femoropopliteal bypass
1. Establishes adequate circulation to leg and foot through popliteal artery and branches
2. Graft used for superficial femoral artery occlusion
M. Femorotibial bypass
1. Autogenous saphenous vein graft from common femoral artery to proximal anterior tibial artery
2. Procedure indicated for superficial femoral and popliteal artery occlusion
N. Angioplasty: percutaneous insertion of balloon-tipped catheter to dilate areas of localized vessel stenosis has major limitations—recurrence restenosis within 1 year.
O. Vena cava ligation
1. Partial or total surgical occlusion of vena cava to prevent emboli from entering pulmonary vasculature (Figure 44-20)
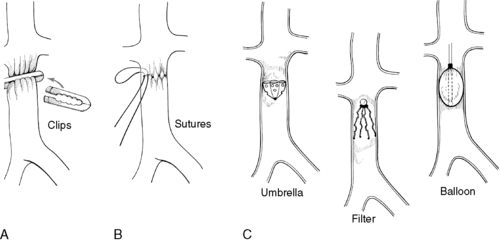 |
| FIGURE 44-20 ▪
Vena caval interruption techniques. A, Clipping. B, Suturing. C, Transvenous devices.
(Modified from Moore WS: Vascular surgery: A comprehensive review, ed 3, Philadelphia, 1991, Saunders. In Price SA, Wilson LM: Pathophysiology: Clinical concepts of disease processes, ed 6, St Louis, 2003, Mosby.)
|
2. Common ligation sites
a. Superficial femoral
b. Inferior vena cava below renal veins
P. Vena caval umbrella filter
1. Insertion of intravascular device through jugular or femoral vein to occlude inferior vena cava (Figure 44-21)
 |
 |
| FIGURE 44-21 ▪
Vena cava filters. A, Actual filters. B, Radiographic images. Left to right, Kimray-Greenfield, titanium Greenfield, Simon nitinol, Gianturco bird’s nest, and Vena Tech.
(From Ballinger PW: Merrill’s atlas of radiographic positions and radiologic procedures, vol 2, ed 8, St Louis, 1995, Mosby.)
|
2. Prevents emboli from entering pulmonary vessels
3. Risk of developing deep vein thrombosis
Q. Vein ligation and stripping: surgical ligation and removal of varicose vein(s) of leg(s)
R. Sympathectomy
1. Interruption of some portion of sympathetic nervous system pathway
2. Causes vasodilation, improvement in circulation to extremity
3. Treatment of partial arterial obstruction with resultant distal trophic changes
S. Interventional radiology
1. Catheter-directed thrombolysis
2. Urokinase enzyme produced from human neonatal kidney tissue cells, lysis faster than streptokinase
3. Streptokinase has a high rate of allergic reactions.
4. Recombinant tissue plasminogen activator (rtPA)
5. Alteplase weak plasminogen
6. Reteplase plasminogen activator penetrates and destroys fiber in matrix.
7. Platelet inhibitors: abciximab (ReoPro), tirofiban (Aggrastat), eptifibatide (Integrilin)
T. Percutaneous mechanical thrombectomy used in combination with thrombolytics
U. AV fistula
1. Long-term vascular access for hemodialysis
2. Primary AV fistula directly connects an artery and a vein via anastomosis (Figure 44-22).
a. Endogenous connection of an artery and a vein via anastomosis (Figure 44-23)
(1) Radial artery to cephalic vein (Figure 44-24)
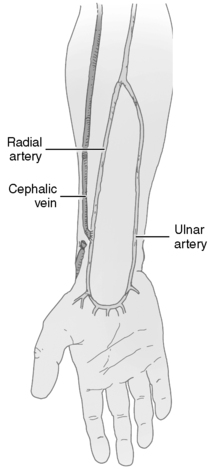 |
| FIGURE 44-24 ▪
End of the cephalic vein anastomosed to the side of the radial artery at a site superior to the usual location of the radiocephalic fistula. This technique can be useful if the distal radial artery is small or the cephalic vein at the wrist is thrombosed.
(From Wilson SE: Vascular access: Principles and practice, ed 3, St Louis, 1996, Mosby.)
|
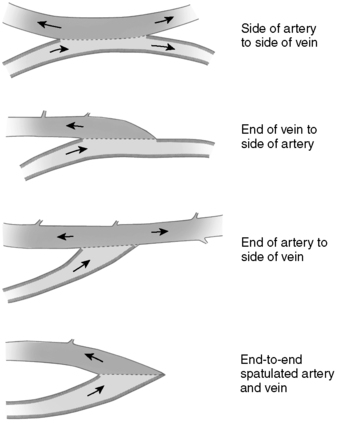 |
| FIGURE 44-22 ▪
Four types of anastomoses between radial artery and cephalic vein.
(From Wilson SE: Vascular access: Principles and practice, ed 3, St Louis, 1996, Mosby.)
|
(2) Ulnar artery to basilic vein
(3) Brachial artery to basilic or cephalic vein
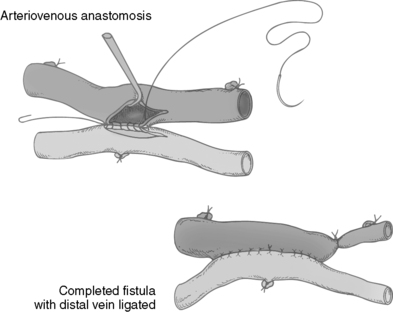 |
| FIGURE 44-23 ▪
Arteriovenous anastomosis. The artery is anastomosed to the vein.
(From Calne R, Pollard SG: Operative surgery, London, 1992, Gower.)
|
b. Graft fistula anastomosis of a conduit between an artery and a vein (Figure 44-25)
 |
| FIGURE 44-25 ▪
An example of a loop fistula. A synthetic graft has been used to create a loop brachiocephalic fistula.
(From Wilson SE: Vascular access: Principles and practice, ed 3, St Louis, 1996, Mosby.)
|
V. Endovascular surgery: minimally invasive treatment for vascular diseases
1. Requires appropriate screening, of lesion type and location
2. Percutaneous transluminal angioplasty: dilation of vessel with a balloon
3. Transcatheter therapy endovascular graft, stent placement (Figures 44-26 and 44-27)
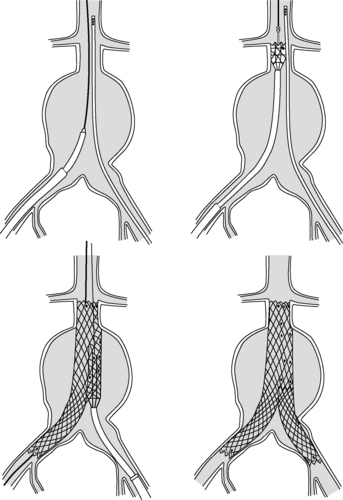 |
| FIGURE 44-26 ▪
Endovascular repair of infrarenal abdominal aortic aneurysms.
(From Souba WW, Fink MP, Jurkovich GJ, et al (eds): ACS surgery principles & practice, New York, 2006, Web MD.)
|
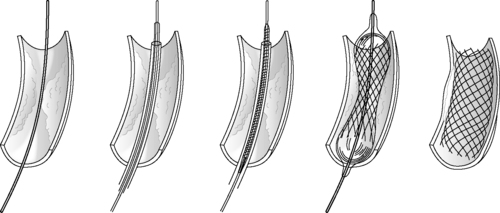 |
| FIGURE 44-27 ▪
Deployment of a balloon-expandable stent.
(From Souba WW, Fink MP, Jurkovich GJ, et al (eds): ACS surgery principles & practice, New York, 2006, Web MD.)
|
4. Coronary, drug-eluting stents versus bare metal stents in lower extremity lesions are promising, but there needs to be more research and elimination of restenosis.
5. Two other promising technologies
a. Tiny razor (Silver Hawk) threaded through a catheter shaves off plaque.
b. Cryoplasty is angioplasty with freezing nitrous oxide.
6. Angiogenesis growth factors: intra-arterial infusions of vascular endothelial growth factor and fibroblast growth factor; much further study remains
W. Intrathoracic vascular procedures: thoracoabdominal aneurysm (Figure 44-28)
1. Lung deflation during procedure
a. To protect lung from injury
b. For adequate exposure to operative site
2. Use of extracorporeal circulation, depending on location of lesion
3. Use of hypothermia and/or temporary shunts to minimize organ ischemia
4. Intraoperative complications
a. CVA
b. Pneumothorax, hemothorax
c. Myocardial injury
d. Severe hypotension
e. Renal failure
f. Spinal cord ischemia
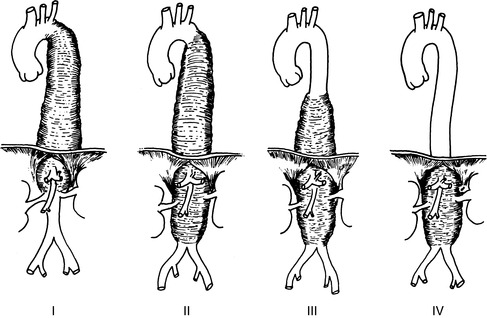 |
| FIGURE 44-28 ▪
Crawford classification of extent thoracoabdominal aneurysms. Extent I and extent II aneurysms are associated with higher risks for paraplegia.
(From Hamilton IN, Hollier LH: Thoracoabdominal aortic aneurysms. In Moore W, ed: Vascular surgery: A comprehensive review, ed 5, Philadelphia, 1998, W.B. Saunders.)
|
X. Abdominal vessel procedures: abdominal aortic aneurysm repair
1. Bowel preparation
a. Decreases incidence of ischemic bowel injury
b. Minimizes postoperative ileus
2. Anesthesia choices
a. Spinal and epidural for elective lower abdominal procedures
(1) Advantages
(a) Elimination of vasospasm
(b) Reduction of respiratory complications
(2) Disadvantages
(a) Positional discomfort if procedure prolonged
(b) Anxiety increases tachycardia, dysrhythmias.
(c) Prolonged decreased sensory and motor function
b. General anesthesia (as previously outlined)
3. Aortic cross-clamping
a. Extreme hypertension can occur as aorta is clamped.
b. Hypotension occurs after clamping released because of:
(1) Vasodilation of lower extremities
(2) Third-space fluid shifting
(3) Metabolic acidosis: products of catabolism and ischemia released systemically
4. Renal status changes
a. Acute renal failure develops in approximately 20% of postoperative abdominal vessel patients.
b. Transient oliguria if hypovolemia occurs
(1) Fluid challenge
(2) Mannitol: osmotic diuretic
(3) Furosemide (Lasix): loop diuretic
c. Hematuria
(1) Possible reaction to transfusion
(2) Ureteral damage
(3) Dislodged microemboli in renal arteries (renal failure)
5. Decreased core temperature related to:
a. Massive fluid replacement
b. Length of procedure
c. Extensive viscera exposure
d. Cold irrigation fluid
e. Rapid heat loss in elderly patients
f. General anesthesia
6. Intraoperative complications
a. Hemorrhage: abdominal aorta, iliac vessels, inferior vena cava
b. Injury to ureters
c. Injury to duodenum, renal arteries and veins, kidney, or spleen
d. Hemiplegia
e. Ischemic bowel
7. Anticoagulation and reversal
a. Heparin administered during vessel clamping and anastomosis
b. Protamine sulfate administered to reverse effects of heparinization before completion of procedure
Y. Sympathectomy: palliative surgical option for patients with PVD
1. Peripheral blood vessels: under continuous control of sympathetic nervous system
a. With normal vasculature, sympathetic system regulates amount of vasoconstriction.
(1) To keep extremities warm, dry, and comfortable
(2) To supply adequate amount of blood to periphery
b. With compromised peripheral circulation
(1) Surgical division of sympathetic chain (variable response in patients with PVD)
(a) Permits permanent, maximal vasodilation
(b) Allows for maximal blood supply to affected extremity
(c) Not primary treatment for vascular obstructive disease
(2) Benefits of sympathectomy
(a) Increases warmth and comfort of extremity
(b) Infection subsides; ulcers heal.
(c) Small areas of gangrene or fibrosis improve.
(d) Ischemic pain less severe
2. Surgical approaches
a. Lumbar sympathectomy: resection of ganglions L2, L3, L4
(1) Indications for surgery
(a) Vasospastic disease
(b) Ischemic ulcers with pain at rest
(c) Certain forms of causalgia (severe sensation of burning skin)
(2) Specific surgical risks
(a) Hemorrhage caused by lumbar arterial or venous damage
(b) Impotence related to genitofemoral nerve damage
(c) Ureteral damage: inadvertent ligation or clipping during excision of lumbar sympathetic chain
(3) Nursing considerations
(a) Supine, lateral recumbent position
(b) Increased sensitivity to position change; turning, elevating of head must be performed slowly.
(c) Flank dressing should remain dry.
(d) Presence of urine on dressing: ureteral damage
(e) Presence of blood on dressing: lumbar vessel damage
(f) Nasogastric decompression to prevent paralytic ileus
(g) Pain: usually moderate, relieved by analgesics; severe flank pain indicative of ureteral ligation, hydronephrosis; requires surgical reexploration
(h) Urine output: bladder distention and acute retention associated with operative discomfort
(4) Neurovascular assessment: both lower extremities
(a) Increase in warmth and vasodilation: desired result
(b) Neuralgia may occur from damaged nerve.
b. Cervical sympathectomy: resection of thoracic ganglia T2 to T6 and half of stellate ganglia C8 to T1
(1) Effectively denervates upper extremity of all extrinsic vasoconstrictor influences arising in sympathetic nervous system, permitting return of normal vasodilation
(2) Surgical approach: usually supraclavicular; may use thoracic, transaxillary, or transpleural approach
(3) Specific surgical risks
(a) Hemothorax or pneumothorax
(b) Phrenic nerve dysfunction: ipsilateral paralysis of diaphragm
(c) Chylous leak caused by ligation of divided thoracic duct
(4) Nursing considerations: cervical sympathectomy
(a) Elevation of head enhances respiratory exchange.
(b) Position on side opposite chest tube; permits optimal lung inflation.
(c) Chest tube drainage should be less than 200 mL in first 8 hours.
(d) Vital signs: changes may indicate intrathoracic or intercostals bleeding.
(5) Cardiopulmonary assessment: includes care of mechanically ventilated patients and monitoring of cardiac parameters
(6) Neurovascular assessment
(a) Palpable radial pulse: confirm with Doppler apparatus if necessary.
(b) Circulation to affected extremity: warm, dry, pink
(c) Observe for Horner’s syndrome: common after cervical sympathectomy
(i) Ptosis of upper eyelid
(ii) Slight elevation of lower lid
(iii) Constriction of affected pupil
(iv) Increased salivation and drooping of mouth on affected side
(7) Pain management: per nursing diagnosis and intervention appropriate to unit policy
(8) Complications
(a) Persistent pneumothorax: damage to underlying lung during thoracotomy
(b) Intrathoracic bleeding: undetected intercostal vessel interruption
(c) Radial nerve and artery damage
(d) Pleural effusion
(9) Postanesthesia concerns—examples of related nursing diagnostic categories include:
(a) Ineffective airway clearance
(b) Pain
(c) Ineffective breathing pattern
(d) Altered peripheral tissue perfusion
(e) Decreased cardiac output
(f) Hypothermia
(g) Paralysis
VII INTRAOPERATIVE CONCERNS
A. Carotid and other neck vessel procedures
1. Anesthesia choices
a. Local anesthesia
(1) Advantages
(a) Quick evaluation of level of consciousness and neurologic changes
(b) Minimizes risk of cerebral ischemia
(2) Disadvantages
(a) Difficult to manage systemic complications (convulsions, dysrhythmias, hypotension, hypertension)
(b) Positional discomfort
b. General anesthesia
(1) Advantages
(a) Facilitates control of:
(i) Hypertension
(ii) Hypoxia
(iii) Dysrhythmias
(iv) Blood loss
(b) Temperature control
(2) Disadvantages
(a) Inability to assess immediate neurologic status
(b) May require postoperative ventilatory support
(c) Anesthetic side effects
2. Extubation as soon as possible allows for accurate neurologic evaluation.
3. Maintenance of adequate cerebral blood flow: avoidance of hypotension
4. Intraoperative complications of carotid surgery
a. Hemorrhage
b. Acute CVA: higher incidence when stenosis of opposite carotid and vertebral artery prevents adequate cerebral perfusion
c. Facial and hypoglossal nerve damage (see Neurologic Status, section V.E.1)
VIII. GENERAL POST ANESTHESIA CARE UNIT (PACU) CARE OF VASCULAR PATIENT
A. Postoperative report includes:
1. Preoperative preparation
a. Sedation: control of anxiety
b. Anticholinergics for reduction of secretions
c. Antibiotics
d. Insulin: adjusted dose (according to regimen of institution)
e. Heparin infusion rate
2. Preoperative medications: often continued until time of surgery
a. Nitroglycerin
(1) Increases coronary perfusion
(2) Decreases peripheral resistance
b. Antihypertensives
(1) Beta-blocker
(2) Calcium channel blocker
(3) Angiotensin-converting enzyme inhibitors
c. Antidysrhythmics
3. Background information
a. Patient identification
b. Baseline vital signs
c. Procedure performed
d. Anesthesia administered
e. Drugs received
f. Length of procedure
g. Estimated blood loss
4. Intraoperative vital signs and monitoring data
5. Intraoperative problems encountered
6. Anticipated problems
B. Postoperative monitoring data: observe for compensatory mechanisms.
1. ECG: rhythm, ST-segment changes
2. Arterial line and/or noninvasive blood pressure
3. Central venous pressure (CVP)
4. Pulmonary artery pressures (Swan-Ganz catheter)
5. Core temperature
6. Ventilation and oxygen support
a. Mechanical ventilation; parameters
(1) Fraction of inspired oxygen (Fio2)
(2) Mode—continuous mechanical ventilation (CMV)
(3) Synchronous intermittent mechanical ventilation (SIMV)
(4) Continuous positive airway pressure (CPAP)
(5) Tidal volume (TV)
(6) Rate
(7) Positive end-expiratory pressure (PEEP)
(8) Pressure support
b. Spontaneous ventilation: face mask
c. Oxygen saturation (pulse oximetry)
d. End-tidal carbon dioxide
e. Sv o2
C. Vascular assessment
1. Skin
a. Temperature: warm, cool, or cold
b. Skin color: pink, ruddy, dusky, pale, or mottled
2. Capillary refill
a. Normal color return after nail bed blanching
b. Color return should occur within 2 seconds.
3. Peripheral pulses
a. Head and neck arteries
b. Carotid
c. Temporal
d. Upper extremity arterial pulses
(1) Radial
(2) Brachial
(3) Axillary
e. Lower extremity arterial pulses
(1) Femoral artery
(2) Popliteal artery
(3) Posterior tibial artery
(4) Dorsalis pedis artery
4. Quality of pulse
a. Reflection of cardiac output and peripheral vascular patency
b. Use of objective pulse quality scale for charting purposes
(1) 0: Absent pulse
(2) 1+: Weak, thready pulse
(3) 2+: Normal quality
(4) 3+: Increased volume, strong and bounding
5. Doppler ultrasound confirmation: device amplifies sound waves produced by pulsating blood flow in vessel and allows for detection of pulsatile flow in absence of palpable pulse.
6. Marking of pulses: facilitates comparison of pulses and promotes continuity of care
7. Extra-anatomic graft pulses: placed subcutaneously to improve recipient vessel circulation
D. Neurologic assessment (see Chapter 33)
1. Level of consciousness: orientation to person, place, time
2. Motor and sensory function
a. Motion and sensation of all extremities
b. Bilateral and equal hand grasp
3. Pupillary function: equal reaction and accommodation to light
4. Abnormal findings
a. Tics
b. Tremors
c. Gazing
d. Seizures
E. Neurovascular assessment: evaluate the six p’s.
1. Pulses and pulselessness
2. Pain
3. Paresthesia
4. Paralysis
5. Pallor
6. Poikilothermia (coldness)
F. Fluid volume status
1. CVP, pulmonary artery pressures
2. Assessment of vital signs
3. Laboratory data
a. Hemoglobin, hematocrit
b. Serum sodium, potassium
c. Coagulation studies
4. Replacement of blood and (third space) fluid loss
a. Colloid
b. Crystalloid
c. Plasma expanders
G. Operative site observation
1. Dressing site and condition
2. Drains and drainage
3. Presence of abnormalities
a. Hematoma formation
b. Discolorations
4. Changes in abdominal girth, diameter
H. Limb protection
1. Bed cradle
2. Heel and elbow padding
3. Lanolin for dry skin
4. Lamb’s wool between toes
5. No pressure under knee
6. Avoidance of joint (graft) flexion at hip or knee
IX. PACU CARE FOR SPECIFIC VASCULAR PROCEDURES
A. Carotid vessel procedures
1. Neurological assessment
a. Presence of swallow and gag reflexes
b. Cranial nerve function: affected by intraoperative retraction and stretching of nerves
2. Respiratory concerns
a. Instruct patient to inhale deeply and minimize deep cough response to avoid elevation of venous pressure.
b. Incentive spirometry encourages deep inhalation.
c. Assess for possible respiratory obstruction.
(1) Vocal cord edema and injury, surgical trauma
(2) Tracheal deviation: hematoma development at operative site; may present with stridor
3. Blood pressure concerns: maintain adequate blood pressure to maximize cerebral perfusion and minimize possible sequelae of hypertension or hypotension.
a. Hypertension
(1) Sequelae
(a) Suture line disruption: tension at site of anastomosis may cause bleeding.
(b) Hematoma formation: tracheal compression
(c) Cerebral hemorrhage, edema
(2) Nursing interventions
(a) Elevate head of bed to decrease venous pressure.
(b) Comfort measures to minimize pain and maintain desired blood pressure parameters
(c) Ensure adequate ventilation.
b. Hypotension
(1) Sequelae resulting from hypersensitive carotid sinus
(a) Sluggish blood flow through operative artery and graft
(b) Difficult pulse assessment
(c) Decreased cerebral or coronary artery perfusion
(2) Nursing interventions
(a) Increase fluids if indicated.
(b) Reduce high Fowler’s to more moderate position.
(c) Titrate vasopressor.
c. Pharmacologic intervention
(1) Sodium nitroprusside (Nipride): vasodilator
(a) Direct effect on arterial and venous smooth muscle
(b) Used to treat severe acute hypertension: rapid onset
(c) Reduces peripheral resistance and increases cardiac output
(2) Nitroglycerin: vasodilator
(a) Relaxes smooth muscle in small blood vessels
(b) Causes venous and arterial dilation; increases coronary artery perfusion
(c) Used for treatment of myocardial ischemia and hypertension
(3) Trimethaphan (Arfonad): antihypertensive
(a) Ganglionic blocking agent
(b) Causes peripheral vasodilation; used to treat hypertension
(4) Dopamine (Intropin): vasopressor
(a) Directly stimulates beta-receptors and dopamine receptors
(b) Low dose causes renal and mesenteric vasodilation and subsequently increases urine output.
(c) Midrange dose produces a positive inotropic effect on myocardium.
(d) High dose stimulates alpha-adrenergic receptors and causes renal vasoconstriction, increased peripheral resistance, and increased blood pressure.
(5) Milrinone (Primacor)
(a) Positive inotropic agent with vasodilator properties
(b) Causes thrombocytopenia and may be contraindicated for some patients
(6) Phenylephrine (Neo-Synephrine): vasopressor
(a) Acts on alpha-adrenergic receptors
(b) Produces vasoconstriction and increased peripheral resistance
(c) Increases systolic and diastolic blood pressure
(d) Reflex bradycardia occurs because of increased vagal activity.
(7) Labetalol hydrochloride (Normodyne, Trandate): alpha-receptor and nonspecific beta-receptor blocking agent
(a) Used for treatment of hypertension
(b) Administer supine to avoid orthostatic hypotensive effect.
(8) Esmolol (Brevibloc): beta-blocking agent used to treat supraventricular tachyarrhythmias
(a) Rapid onset of action, short half-life
(b) Hypotension most common side effect
(9) Nifedipine (Procardia): calcium channel blocker used for treatment of chronic hypertension, acute hypertensive emergencies, and angina
(a) Decreases systemic vascular resistance
(b) Augments cardiac output
4. Bradycardia
a. Causes
(1) Altered baroreceptor responses
(2) Vagal manipulation
(3) Vagal pressure from hematoma formation
(4) Myocardial infarction
b. Interventions
(1) Pharmacologic
(a) Atropine (anticholinergic, parasympatholytic): inhibits action of acetylcholine; stimulates or depresses central nervous system depending on dose; used to treat bradycardia
(b) Glycopyrrolate (Robinul; anticholinergic): inhibits action of acetylcholine; used to treat bradycardia
(2) Surgical
(a) Excision of hematoma
(b) Reexploration of wound
5. Positioning: elevation of head
a. Decreases venous pressure
b. Facilitates respiratory excursion
6. Dressings and drains
a. Dressings: light, nonconstricting
b. Drains: Penrose, Jackson-Pratt, Hemovac
B. Intrathoracic vessel procedures
1. Respiratory support
a. Principles of care of intubated and mechanically ventilated patient
b. Head elevation permits respiratory excursion and allows proper chest tube function.
c. Turn, cough, deep breathe every 2 hours and as needed.
2. Assess for complications.
a. Atelectasis
b. Pneumothorax, hemothorax
c. Adult respiratory distress syndrome
d. CHF and pulmonary edema
3. Ensure proper chest tube functioning.
a. Make sure connections are secure.
b. Observe for air leaks.
c. Measure drainage.
d. Keep bottles below chest level.
e. Auscultate lung sounds.
f. Palpate for subcutaneous emphysema (crepitus).
4. Neurovascular assessment (as previously outlined)
a. Pulse assessment: upper and lower extremities
b. Motor and sensory function
(1) Spinal cord ischemia
(a) Paraplegia can occur with prolonged thoracic and aortic occlusion.
(b) Decreased perfusion pressure to spinal cord
(2) Embolization to distal arteries, originating from aortic clot
5. Monitor for cardiac, pulmonary, renal function (as previously outlined).
6. Pain management (according to unit policy)
a. Prevent splinting and permit lung expansion.
b. Allay apprehension and fear.
c. Decrease tachycardia and hypertension.
d. Enhance mechanical ventilation compliance.
C. Abdominal vessel procedures
1. Continuous cardiopulmonary assessment (as previously outlined)
2. Observe for signs and symptoms of hypovolemic shock caused by hemorrhage.
3. Gastrointestinal assessment
a. Nasogastric tube: decompresses stomach, prevents paralytic ileus
b. Complications
(1) Ileus
(2) Occlusion of inferior mesenteric artery, causing colon ischemia
(3) Hemorrhage: measure and monitor abdominal girth.
4. Renal assessment (as previously outlined)
a. Hematuria: aortic cross-clamping, kidney and/or bladder trauma
b. Oliguria: renal failure, tubular necrosis
5. Neurovascular status (as previously outlined)
a. Pedal pulses may be absent for 6 to 12 hours postoperatively.
(1) Vascular spasm
(2) Peripheral vasoconstriction
(3) Vessel patency, verified by surgeon
(4) Confirm absence with Doppler ultrasonography.
b. Absence of previously palpable pulse
(1) Signifies occlusion of vessel or graft
(2) Requires immediate surgical reexploration
6. Positioning
a. Abdominal procedures: head elevation
(1) Facilitates respiratory excursion
(2) Decreases suture line stress
b. Vena cava plication: supine to slight Trendelenburg
(1) Prevents further reduction of venous return
(2) Decreased venous return results in decreased cardiac output.
7. Pain or vascular spasm
a. Severe pain indicative of retroperitoneal bleeding
b. Spasms
(1) Usually after aortic surgery
(2) Aggravated by:
(a) Hypotension
(b) Hypothermia
(c) Pain
(d) Carbon dioxide retention
8. Hypothermia or shivering
a. Sequelae
(1) Increases oxygen requirement
(2) ST-segment depression can occur with increased myocardial oxygen requirement.
(3) Prolonged somnolence occurs with decreased cerebral perfusion.
(4) Increases vasoconstriction and vasospasm
(a) Increases difficulty in palpating pulses
(b) Aggravates hypertension
(5) Increases patient anxiety and discomfort
b. Corrective nursing interventions
(1) Heated blankets, automatic hyperthermia blanket
(2) Warming lights
(3) Heated aerosol nebulizers with oxygen delivery
9. Complications
a. Acute arterial occlusion
b. Debris embolization: pulmonary, cerebral, peripheral
c. Graft suture line hemorrhage
d. Cardiopulmonary complications
(1) Dysrhythmias
(2) Myocardial infarction
(3) CHF
e. Third-space fluid accumulation
f. Renal complications: failure, trauma
D. Extra-anatomic vessel bypasses (femoral crossover, axillofemoral bypass)
1. Positioning: turn only to unoperated side.
a. Avoid external pressure on graft.
b. Avoid flexion of graft; careful pillow positioning.
2. Pulse checks with femoral crossover
a. Across symphysis pubis (femoral to femoral)
b. Both lower extremities
3. Pulse checks with axillofemoral bypass: monitor donor arm and revascularized limb.
a. Avoid damage to donor artery; obtain blood pressure, draw blood from opposite arm.
b. Specific complications of axillofemoral bypass
(1) Brachial plexus injury
(2) Subclavian or axillary artery injury
(3) Upper extremity embolization
E. Extremity vessel procedures (arterial bypass grafts, embolectomies, vein stripping and ligation)
1. Nursing concerns: arterial procedures
a. Positioning: avoid severe joint flexion, crossing of legs, pillows under popliteal area.
b. Nonrestrictive dressings
c. Neurovascular assessment
(1) Comparison of both extremities
(2) Doppler confirmation
(3) If no pulses expected by surgeon, successful revascularization assessed by dry, pink, warm legs and feet
d. Limb protection (as previously outlined)
e. Laboratory data
(1) Monitor glucose level in diabetic patient: control of blood sugar can prevent infection.
(2) Monitor potassium level: extracellular potassium increases with limb ischemia and infection.
(3) Monitor for metabolic acidosis: causes increased serum potassium level.
f. Administer low–molecular weight dextran (500 mL over 10-24 hours).
(1) Anticoagulation effect: interrupts action of fibrinogen and clotting factors
(2) Reduces platelet accumulation and adhesiveness
(3) Increases tissue perfusion
(4) Reduces blood viscosity
(5) Increases colloid osmotic pressure
g. Control of pain to prevent spasms
2. Complications of extremity vessel procedures
a. Graft occlusion
b. Vein, nerve injury
c. Pulmonary or cerebral emboli
3. Nursing concerns: vein procedures
a. Positioning
(1) Supine to slight head elevation with leg elevation
(2) Avoidance of knee bending or leg crossing
b. Dressing: multiple wounds covered by ACE bandages
c. Neurovascular assessment: bilateral comparison as previously outlined
d. Pain assessment: incisional discomfort versus deep calf pain of thrombophlebitis
4. Complications of vein ligation, stripping procedures
a. Hematoma and wound bleeding
b. Femoral vein or femoral saphenous nerve damage
c. Thrombophlebitis
d. Edema
F. Endovascular repair stent grafts of abdominal aortic aneurysm
1. Selected by location of aneurysm and risk factor of open surgery
2. Graft inserted via catheter through skin
3. Stent deployed away from renal arteries and anchored in place with hooks
a. Advantages of endovascular repair
(1) Decreases length of stay
(2) Minimally invasive
(3) Shorter recovery time
b. Disadvantages of endovascular repair
(1) Aneurysm rupture
(2) Peripheral embolization
(3) Bleeding
(4) Misdeployment of stent graft
(5) Requiring open surgical procedure
BIBLIOGRAPHY
1. American Association of Critical Care Nurses, Core curriculum for critical care nursing. ed 6 ( 2006)Saunders, Philadelphia.
2. Arko, F.R.; Zarins, C.K., Repair of infrarenal abdominal aortic aneurysms, In: (Editors: Souba, W.W.; Fink, M.P.; Jurkovich, G.J.; et al.) ACS surgery principles & practice ( 2006)Web MD, New York.
3. Bartley, M.K., Keep venous thromboembolism at bay, Nursing 36 (10) ( 2006) 36–41.
4. Beese-Bjurstrom, S., Hidden danger: Aortic aneurysms and dissections, Nursing 34 (2) ( 2004) 36–41.
5. Bickley, L.S.; Szilagyi, P.G., Bate’s guide to physical examination and history taking. ed 8 ( 2003)Lippincott Williams & Wilkins, Philadelphia.
6. Blach, D.E.; Ignatavic, D.D., Interventions for clients with vascular problems, In: (Editors: Ignatavic, D.D.; Workman, M.L.) Medical-surgical nursing: Critical thinking for collaborative care ( 2006)Saunders, Philadelphia.
7. Block, P.C., ACC/AHA guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic), ACC Cardiosource Rev J 15 (3) ( 2006) 16–19.
8. Bussard, M.E., Reteplase. Nursing implications for catheter-directed thrombolytic therapy for peripheral vascular occlusion, Crit Care Nurse 22 (3) ( 2003) 57–63.
9. Casserly, J.S.; Yadav, S.; Sachar, R., Manual of peripheral vascular intervention. ( 2005)Lippincott Williams & Wilkins, Philadelphia.
10. Croft, J.A.; Todd, B.A., Thoracoabdominal aneurysms, Advance for Nurses 7 (20) ( 2005) 16–17.
11. Crowther, M.; McCourt, K., Get the edge on deep vein thrombosis, Nurs Manage 35 (1) ( 2004) 22–29.
12. Decousis, H., Eight year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism, Circulation 12 (2005) 416–422.
13. Dilainas, I.; Nano, G.; Kashyap, A.; et al., Balloon angioplasty or nitinol balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery, N Engl J Med 355 (5) ( 2003) 521–524.
14. Drain, C.B.; Odom-Forren, J., Perianesthesia nursing: A critical care approach. ed 5 ( 2009)Saunders, Philadelphia.
15. Fahey, V.A., Vascular nursing. ed 4 ( 2004)Saunders, St Louis.
16. Hirsch, A.T.; Haskal, Z.J.; Hertzer, N.R.; et al., ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patents With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation, Circulation 113 (2006) e463–e465.
17. Horlander, K.T.; Mannino, D.M.; Leeper, K.V., Pulmonary embolism mortality in the United States, 1997–1998, Arch Intern Med 163 (14) ( 2003) 1711–1717.
18. Jarvis, C., Physical examination and health assessment. ed 4 ( 2004)Mosby, St Louis.
19. Kinney, M.R.; Packa, D.R.; Dunbar, S.B., AACN’s clinical reference for critical-care nursing. ( 1998)Mosby, St Louis.
20. Kuznar, K.A., Peripheral arterial disease, Advance for Nurses 6 (13) ( 2004) 19–24.
21. Lipsitz, E.C.; Kim, S., Antithrombotic therapy in peripheral arterial disease, Clin Geriatr Med 22 (1) ( 2006) 183–198.
22. Owings, J.T., Venous thromboembolism, In: (Editors: Souba, W.W.; Fink, M.P.; Jurkovich, G.J.; et al.) ACS surgery principles & practice ( 2006)Web MD, New York.
23. Pamoukian, V.N.; Shortell, C.K., Pulseless extremity and atheroembolism. Approach to the acutely ischemic limb, In: (Editors: Souba, W.W.; Fink, M.P.; Jurkovich, G.J.; et al.) ACS surgery principles & practice ( 2006)Web MD, New York.
24. Price, S.A.; Wilson, L.M., Pathophysiology: Clinical concepts of disease processes. ed 6 ( 2001)Mosby, St Louis.
25. Rice, K.L., How to measure ankle/brachial index, Nursing 35 (1) ( 2005) 56–57.
26. Rothrock, JC., Alexander’s care of the patient in surgery. ed 13 ( 2007)Mosby, St Louis.
27. Smeltzer, S.C.; Bare, B.G., Brunner & Suddarth’s textbook of medical-surgical nursing. ed 10 ( 2004)Lippincott Williams & Wilkins, Philadelphia.
28. Society of Interventional Radiology, Peripheral vascular disease statistics. ( September 4, 2003) ; Available at:www.sirweb.org; Accessed.
29. Sontheimer, D.L., Peripheral vascular disease: Diagnosis and treatment, Am Fam Physician 73 (11) ( 2006) 1971–1976.
30. Souba, W.W.; Fink, M.P.; Jurkovich, G.J.; et al., ACS surgery principles & practice. ( 2006)WebMD, New York.
31. Stoney, R.J.; Effeney, D.J., Wylie’s atlas of vascular surgery: Thoracoabdominal aorta and its branches. ( 1992)Lippincott, Philadelphia.
32. Patton, K.T.; Thibodeau, G.A., Anatomy & physiology. ed 7 ( 2010)Mosby, St. Louis.
33. Tzou, W.S.; Mohler, E.R.I.I.I., Peripheral arterial disease: Diagnosis and medical management, Hosp Physician 42 (7) ( 2006) 17–25; 54, 72.
34. Urden, L.D.; Stacy, K.M.; Lough, M.E., Thelan’s critical care nursing: Diagnosis and management. ed 5 ( 2006)Mosby, St Louis.
35. Zelenock, G.B., Mastery of vascular and endovascular surgery. ( 2006)Lippincott Williams & Wilkins, Philadelphia.