History
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
Electrocardiogram
Findings
Chest Radiograph
Findings
Focused Clinical Questions and Discussion Points
Question
Discussion
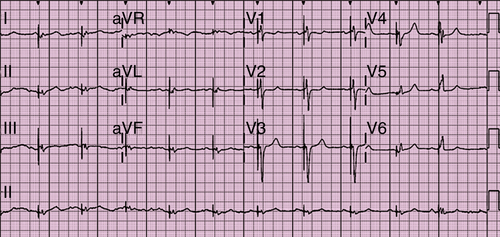
FIGURE 42-1 Electrocardiogram.
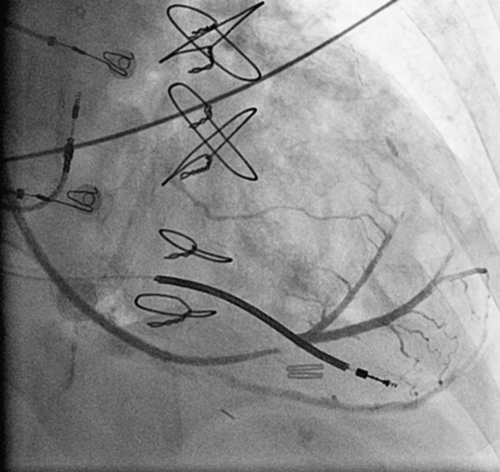
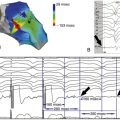
FIGURE 42-2 Coronary sinus venogram.
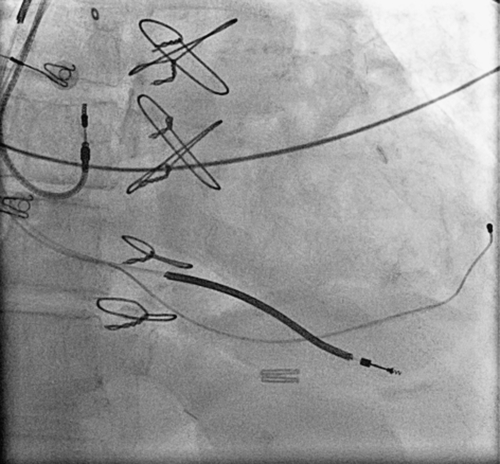
FIGURE 42-3 Chest radiograph of final coronary sinus lead position corresponding to venogram in Figure 42-2.
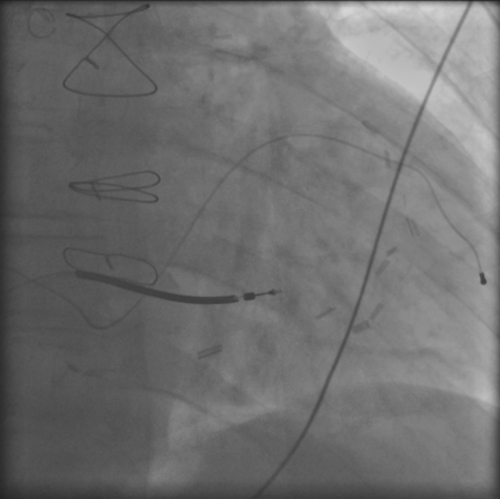
FIGURE 42-4 Chest radiograph in a different patient in whom the apical position of the coronary sinus lead is a better example of an apical coronary sinus lead position.
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Selected References
1. Chung E.S., Leon A.R., Tavzzi L. et al. Results of the predictors of response to CRT (PROSPECT) Trial. Circulation. 2008;117:2608–2616.
2. Delgado V., Van Bommel R.J., Bertini M. et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–78.
3. Fornwalt B.K., Sprague W.W., BeDell et al., et al. Agreement is poor among current criteria used to define response to cardiac resynchronization therapy. Circulation. 2010;121:1985–1991.
4. Goldenberg I., Moss A.J., Hall W.J. et al. Predictors of response to cardiac resynchronization therapy in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT). Circulation. 2011;124:1527–1536.
5. Hsing J.M., Selzman K.A., Leclercq C. et al. Paced left ventricular QRS width and ECG parameters predict outcomes after cardiac resynchronization therapy: PROSPECT-ECG substudy. Circ Arrhythm Electrophysiol. 2011;4:851–857.
6. Khan F.Z., Virdee M.S., Palmer C.R. et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518.
7. Knappe D., Pouleur A.C., Shah A.M. et al. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail. 2011;4:433–440.
8. Singh J.P., Klein H.U., Huang D.T. et al. Left ventricular lead position and clinical outcome in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Trial. Circulation. 2011;123:1159–1166.
9. Burger H., Schwarz T., Ehrlich W. et al. New generation of transvenous left ventricular leads: first experience with implantation of multipolar left ventricular leads. Exp Clin Cardiol. 2011;16:23–26.
10. Lampert R., Hayes D.L., Annas G.J. et al. HRS expert consensus statement on the management of cardiovascular implantable electronic devices (CIEDs) in patients nearing end of life or requesting withdrawal of therapy. Heart Rhythm. 2010;7:1008–1026.