History
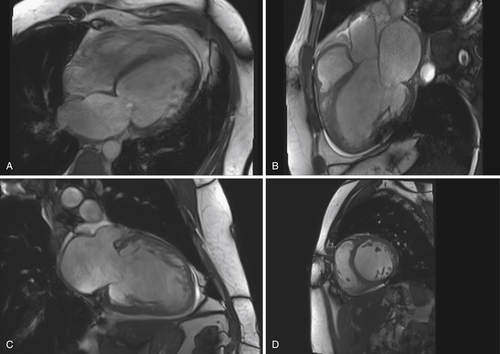
FIGURE 41-1 Examination obtained February 2011. A, Four-chamber view. B, Three-chamber view. C, Two-chamber view. D, Short-axis view.
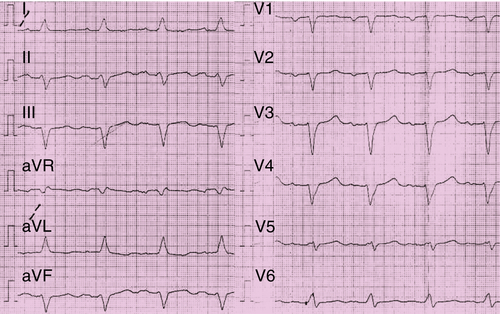
FIGURE 41-2 Electrocardiogram before implantation of the biventricular implantable cardioverter-defibrillator in February 2011.
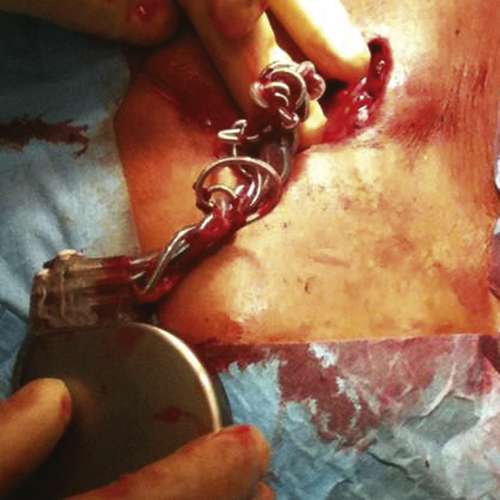
FIGURE 41-3 During the operative revision the device was observed to have been rotated around its axis 18 times.
Current Medications
Current Symptoms
Physical Examination
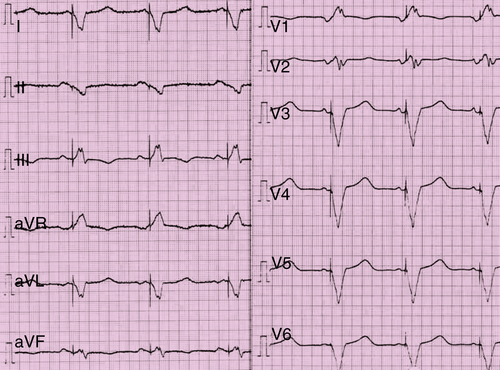
FIGURE 41-4 Electrocardiogram with active biventricular implantable cardioverter-defibrillator stimulation.
Laboratory Data
Electrocardiogram
Findings
Findings
Findings
Chest Radiograph
Findings
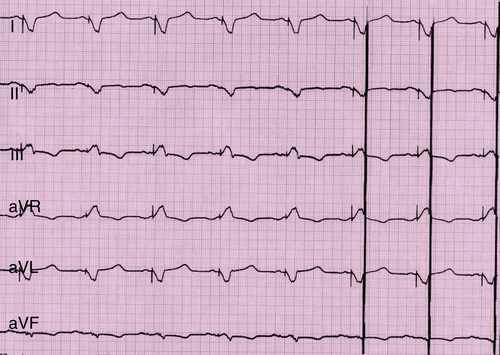
FIGURE 41-5 Electrocardiogram with active stimulation of the Optimizer III system.
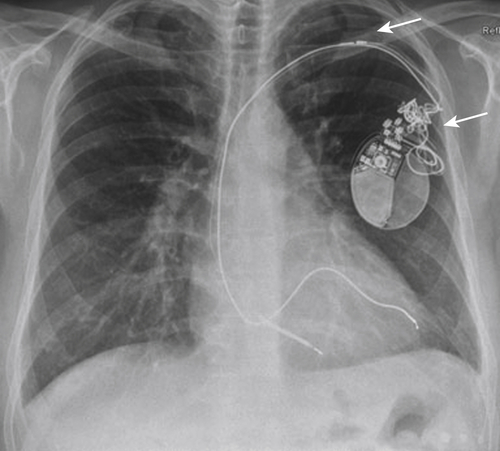
FIGURE 41-6 Chest radiograph from August 2011, 6 months after implantation of the biventricular implantable cardioverter-defibrillator.
Findings
Exercise Testing
Echocardiogram
Findings
Magnetic Resonance Imaging
Findings
Catheterization
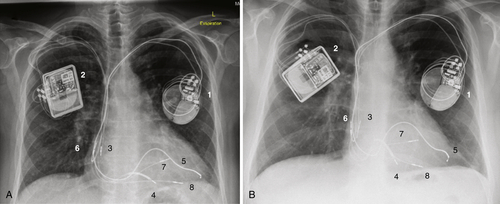
FIGURE 41-7 A, Chest radiograph obtained March 2012 after implantation of the Optimizer III device. B, Follow-up chest radiograph from July 2012, 4.5 months after implantation of the Optimizer III device. The visualized parts of the two devices are marked with numbers 1-8 to make the identification clearer.
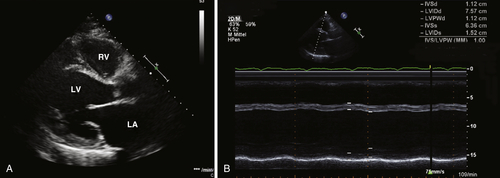
FIGURE 41-8 Echocardiogram.
Findings
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Question
Discussion
FIX-CHF-4
FIX-CHF-5
Consideration of Both Studies
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Selected References
1. Abraham W.T., Nadamanee K., Volosin K. et al. Subgroup analysis of a randomized controlled trial evaluation the safety and efficacy of cardiac contractility modulation in advanced heart failure. J Card Fail. 2011;17:710–717.
2. Borggrefe M.M., Lawo T., Butter C. et al. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J. 2008;29:1019–1028.
3. Bristow M.R., Saxon L.A., Boehmer J. et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150.
4. Butter C., Meyhofer J., Seifert M. et al. First use of cardiac contractility modulation (CCM) in a patient failing CRT therapy: clinical and technical aspects of combined therapies. Eur J Heart Fail. 2007;9:955–958.
5. Cleland J.G., Daubert J.C., Erdmann E. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549.
6. Kadish A., Nademanee K., Volosin K. et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J. 2011;161:329–337.
7. Moss A.J., Hall W.J., Cannom D.S. et al. Cardiac resynchronization therapy for the prevention of heart failure events. N Engl J Med. 2009;14:1329–1338.
8. Nägele H., Behrens S., Eisermann C. et al. Cardiac contractility modulation in non-responders to cardiac resynchronization therapy. Europace. 2008;10:1375–1380.