CHAPTER 4
Sodium, water and potassium
CHAPTER OUTLINE
Extracellular fluid and sodium
Extracellular fluid, intracellular fluid and potassium
DISORDERS OF SODIUM METABOLISM
PHYSIOLOGY
Introduction
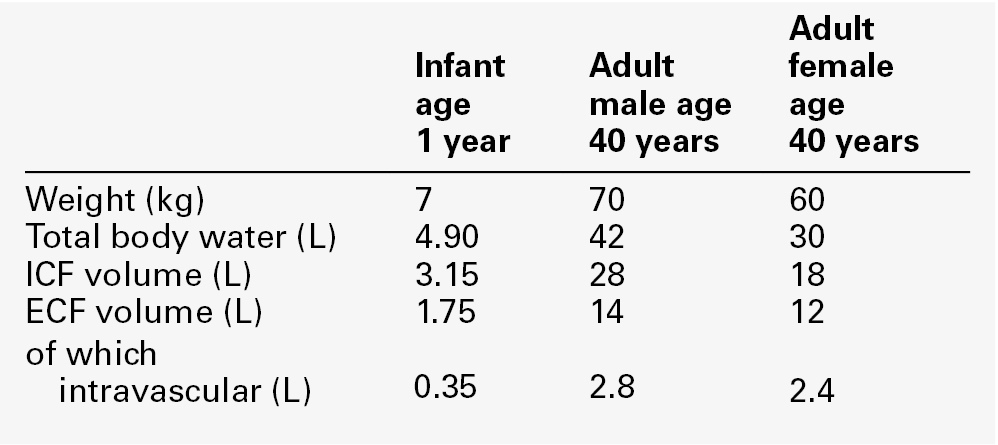
Water is the most abundant molecule in the human body and is the major solvent (the only other of consequence is fat). The physiological control of the composition and distribution of the fluid spaces is a highly sensitive and complex homoeostatic process necessary to maintain a constant milieu interieur. Two main fluid spaces exist: the intracellular fluid (ICF) and the extracellular fluid (ECF). The latter is further separated into the intravascular space (plasma), the interstitial space (which includes lymph) and transcellular fluid (pleural, pericardial, peritoneal, cerebrospinal and gastrointestinal fluids), formed by the transport activity of cells. Table 4.1 summarizes the water content of the body and the distribution of fluid between the main body spaces; the proportion of body water to total body weight is affected by age, gender and body fat content.
The electrolyte compositions of the ECF and the ICF are different – in essence, the extracellular space is a predominantly sodium-containing solution and the intracellular space a potassium-containing solution (Table 4.2). This fundamental difference in electrolyte composition is maintained by cell membrane transport pumps (energy-consuming ATPases). Electrolyte and protein concentrations of blood are now most commonly measured in serum. In this chapter, unless specified, the term plasma is used for describing in vivo concentrations and the term serum for measured in vitro concentrations.
TABLE 4.2
Representative molal concentration of electrolytes within the body fluid spaces
Other predominant intracellular counter anions are sulphates and proteinates
| Electrolyte | ECF (mmol/kg) | ICF (mmol/kg) |
| Sodium | 152 | 10 |
| Potassium | 4.3 | 160 |
| Calcium | 2.7 | 1.0 |
| Magnesium | 1.1 | 13 |
| Chloride | 109 | 10 |
| Bicarbonate | 29 | 10 |
| Phosphate | 1.5 | 50 |
Body water moves between the main body spaces through water channels (aquaporins), predominantly under the influence of the osmotic pressures resulting from dissolved particles in the ECF and ICF on either side of the cell membrane. Under steady-state physiological conditions, the osmotic pressure of the ICF equates exactly with the plasma osmotic pressure. Osmolality represents the molal concentration of solute in a litre of solvent (water) and is expressed as mmol/kg, as opposed to a molar (or calculated osmolar) solution, which is the concentration in the space of a litre of solution (which includes solute space) and is expressed as mmol/L. This apparent nicety in definition does have its uses in differentiating certain real from apparent electrolyte disorders (see p. 46).
The measured osmolality in ECF cannot, however, always be equated with transcellular osmotic pressure. The cell membrane is selectively permeable to a variety of solutes, but certain natural solutes such as urea, or exogenous solutes such as alcohol, are freely permeable. Thus, an increase in plasma osmolality due to sodium implies an increase in osmotic pressure across the cell membrane, tending to withdraw water from the cell to equalize osmolalities. However, an increase in plasma osmolality due to urea does not have this effect because of the free permeability of urea between the ICF and ECF. This leads to the concept of effective osmolality or tonicity, which, under physiological conditions, is primarily dependent on plasma sodium concentration, but under pathological or iatrogenic conditions, may be dependent on other solutes, for example, the effect of glucose in untreated diabetes mellitus or mannitol following its intravenous infusion (used therapeutically precisely for this effect).
The distribution of water between different body spaces is thus dependent on the permeability of the relevant membrane barriers to water, and the quantity of solute within each space. Because water is freely permeable across all cell membranes (except some highly specialized membranes in the nephrons and sweat glands), the water content of body spaces is dependent on the solute content of the space. The Gibbs–Donnan effect is an important force that influences the distribution of solutes. If the barrier separating two compartments is permeable to water and small ions, but impermeable to large ionized molecules, and the larger molecules are confined to one compartment, the concentration of small ions will differ between compartments at equilibrium, and the compartment containing the protein will exert an osmotic force.
Oncotic pressure (colloid osmotic pressure) is the osmotic pressure resulting from the difference within the ECF between the protein contents of plasma and interstitial fluid. The major contribution to oncotic pressure under physiological conditions is plasma albumin concentration. The hydrostatic pressure of the plasma across the afferent capillary membrane creates a counter force to the oncotic pressure: the combination of the changing hydrostatic and oncotic pressure gradients across the capillary bed is known as the Starling forces.
Because the total solute content of cells under physiological conditions is essentially fixed and water is freely permeable across most cell membranes, the volume of the ICF is determined by the body water content. Intracellular fluid tonicity will in turn determine ECF tonicity, but ECF volume is essentially dependent on its sodium content.
Extracellular fluid and sodium
The sodium content of a normal adult is 55–65 mmol/kg body weight. The concentration of sodium in plasma is ~ 140 mmol/L (~ 152 mmol/kg). Under physiological conditions, the control of the ECF volume is through the control of functioning or effective plasma volume (that part of the plasma volume actively perfusing tissues). There are a variety of afferent mechanisms to monitor effective plasma volume (and thus ECF volume), which include intrathoracic volume receptors such as atrial stretch receptors, hepatic volume receptors, arterial baroreceptors, intrarenal baroreceptors and, possibly, tissue receptors monitoring tissue perfusion. Whatever the actual or relative function of all these sensory systems, their resultant influence is fine control over the renal conservation of sodium and the appetite for oral sodium intake.
Sodium intake varies considerably between different human cultural and ethnic groups. Variations in intake between 5 and 500 mmol/24 h have been recorded and physiological mechanisms balance this with the renal excretion of sodium. Sodium delivery to the renal tubules is a function of plasma sodium concentration and the glomerular filtration rate (GFR). Every 24 h, the kidneys of an average healthy adult male will filter in excess of 24 000 mmol of sodium, most of which is reabsorbed in the tubules so that, in health, sodium balance is achieved. In a healthy individual, renal sodium conservation can be extremely efficient, with urine sodium concentration falling to < 1 mmol/L urine. Conversely, when sodium intake is excessive, the capacity to excrete sodium can result in urine sodium concentrations up to 300 mmol/L urine.
Renal control of sodium output
Intrinsic renal control of tubular reabsorption of sodium
Under normal physiological conditions, approximately 80% of the sodium in the glomerular filtrate is reabsorbed in the proximal tubules. The protein concentration of the blood within the post-glomerular peritubular capillary beds is believed to exert a strong oncotic pressure on fluid in the proximal tubules, and this in turn helps to regulate the volume of fluid reabsorbed. This process contributes to the autoregulation of filtration and reabsorption known as glomerulo–tubular balance. There has been considerable physiological interest in the control of proximal tubular sodium reabsorption and other intrinsic renal control mechanisms, such as redistribution of filtering activity from superficial nephrons (relatively salt-losing) to juxtamedullary nephrons (relatively salt-retaining). However, to date, the major humoral influences on sodium reabsorption appear to reside in the distal tubules and collecting ducts.
Renin–angiotensin–aldosterone axis
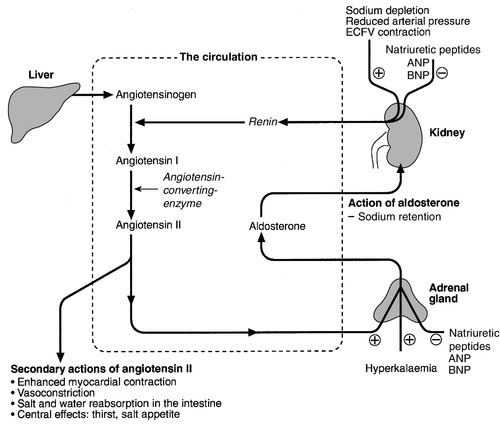
Aldosterone is a steroid hormone released from the zona glomerulosa of the adrenal cortex. The major control of aldosterone secretion is through angiotensin II, an octapeptide produced in the circulation as a final product of the action of renin. Renin is a proteolytic enzyme secreted by a group of cells (the juxtaglomerular apparatus) situated between the afferent and efferent glomerular arterioles, and specialist chemoreceptor cells found within the distal convoluted tubular epithelium of the kidneys – the macula densa. Renin release is stimulated by decreased sodium delivery to the distal tubules (sodium depletion, ECF volume contraction), renal artery hypotension (due to systemic hypotension or renal artery stenosis) and by sympathetic nerve activity via β1-adrenergic receptors. The substrate for renin is angiotensinogen, an α2-globulin produced by the liver. Renin releases an amino-terminal decapeptide from angiotensinogen known as angiotensin I, which in turn is acted upon by angiotensin-converting enzyme (ACE), predominantly within the pulmonary capillaries. The action of ACE is to cleave the carboxy-terminal dipeptide of angiotensin I to produce angiotensin II. The renin–angiotensin–aldosterone axis is summarized in Figure 4.1. The major physiological influences on aldosterone are body sodium content and renal perfusion pressure, although hyperkalaemia can stimulate aldosterone release directly.
FIGURE 4.1 The renin–angiotensin–aldosterone system. +, stimulatory signal; −, inhibitory signal; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide.
Aldosterone acts through the specific nuclear mineralocorticoid receptor, which is protected from cortisol (for which it has equal affinity) through the intracellular production of 11β-hydroxysteroid dehydrogenase (11β-HSD). This enzyme converts cortisol to cortisone, for which the receptor has weak affinity. The response within the principal cells lining the distal tubules and collecting ducts is the apical influx of sodium via epithelial Na+ channel (ENaC) stimulation and its efflux via basolateral Na+,K+-ATPase. The net effect is active sodium reabsorption in exchange for potassium. In addition, angiotensin II has direct vasoconstrictive actions, thus having an immediate influence on effective plasma volume.
Natriuretic peptides
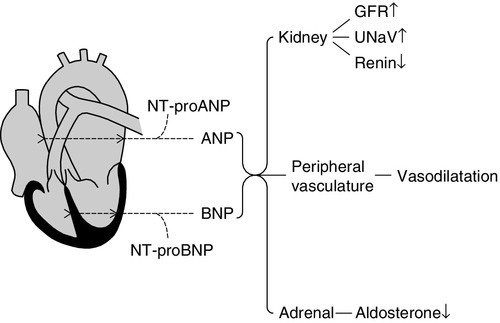
Glomerular filtration and the action of aldosterone do not constitute the complete control over renal sodium excretion within mammalian systems. The existence of a third factor (or factors) had been proposed for 20 years prior to the identification of a specific natriuretic factor in 1981. This factor was originally identified in rat cardiac atria and was termed atrial natriuretic factor (now known as atrial natriuretic peptide, ANP). Since then, a further two natriuretic peptides have been identified in humans. Circulating ANP is a 28 amino acid (AA) peptide with a 17 AA ring structure formed by a disulphide bridge between cysteines at positions 7 and 23: the gene for the ANP precursor molecule is located on the short arm of chromosome 1. Three exons code for a 151 AA peptide (preproANP) which, following removal of the signal peptide, results in a 126 AA peptide (proANP) – the main storage form. On secretion into the circulation, proANP is cleaved into the N-terminal 1–98 peptide (NT-proANP), and the biologically active 99–126 peptide (ANP). The major stimulus to the secretion of ANP is atrial stretch and the major sites of synthesis are the atria.
In 1988, a second natriuretic peptide was identified, in porcine brain, and termed brain natriuretic peptide (BNP). It was later shown to be produced predominantly in the ventricles of the heart. Circulating BNP is a 32 AA peptide, also with a 17 AA ring structure formed by a disulphide bridge between cysteines at positions 10 and 26. The ring structure has a high level of homology with the ring of ANP. Also like that of ANP, the gene for BNP is located on the short arm of chromosome 1. Three exons code for a 134 AA peptide (pre-proBNP) which, following removal of the signal peptide, results in a 108 AA peptide (proBNP). Further cleavage on release into the circulation results in the N-terminal 1–76 peptide (NT-proBNP) and the biologically active 77–108 peptide (BNP). Both ANP and BNP share a common receptor that mediates a natriuretic response in the kidneys by causing an increase in glomerular filtration rate and blocking sodium reabsorption in the inner medullary collecting ducts. They also influence sodium reabsorption through antagonism of the renin–angiotensin–aldosterone axis. Both ANP and BNP reduce sympathetic tone in the peripheral vasculature. The basic physiology of ANP and BNP is summarized in Figure 4.2.
FIGURE 4.2 The physiology of ANP and BNP. N-terminal pro-peptides are co-secreted with the active natriuretic peptides. Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) share a common receptor to increase glomerular filtration rate (GFR) and urine sodium excretion (UNaV), and to reduce renin and aldosterone secretion.
In 1990, a further ‘natriuretic peptide’ was described in porcine brain and termed C-type natriuretic peptide (CNP). The gene for CNP is coded on chromosome 4. Although CNP has been identified in human plasma, this peptide is mediated through a different receptor and does not have direct natriuretic function, but acts primarily as an antiproliferative regulator in the vascular cell system and as a neuropeptide.
There is no doubt that ANP, and to a lesser extent BNP, has important physiological functions in relation to sodium balance. Simple experiments can readily demonstrate changes in ANP and BNP concentrations in relation to dietary sodium intake, and the interaction of ANP and BNP with the renin–angiotensin–aldosterone axis results in a dual ‘fine-tuning’ system of sodium control, using afferent information obtained from the heart and kidneys simultaneously. However, unlike the situation with the renin–angiotensin–aldosterone axis, no primary disorders of natriuretic hormone excess or deficiency have yet been identified with certainty. Because the major stimulus to the release of ANP and BNP is cardiac wall stretch, the major current clinical utility of measuring these peptides, particularly BNP and NT-proBNP, is in the diagnosis and monitoring of cardiac failure.
Sodium appetite
The renal conservation of sodium is remarkably efficient, but when sustained, non-renal losses occur under physiological conditions, such as through sweating due to prolonged vigorous physical activity or as a result of prolonged exposure to high ambient temperature, then a mechanism for increasing sodium intake comes into play – the salt appetite. That such a mechanism exists in humans can be observed in pathological states of impaired sodium conservation such as Addison disease. However, accurately defining the sodium appetite in humans under physiological conditions is a subjective and difficult task. Salt intakes are largely conditioned by traditions of food intake and cultural habits of seasoning food with salt. The impulse to add salt to prepared food appears to be automatic in many people, who do so even before tasting. Animal experiments have implicated an entirely separate, brain-based, renin–angiotensin–aldosterone system as a controlling influence on active salt-seeking behaviour.
Intracellular fluid and water
Water crosses cell membranes by simple diffusion through the lipid bilayer and through specific water channels known as aquaporins. The existence of specific water channels had been suspected for many years, not least to explain the dramatic changes that can occur in water permeability in the epithelial cells of the renal collecting ducts during hydration or dehydration. However, it was not until 1992 that the first aquaporin was characterized in human red cells – aquaporin 1 (AQP 1). To date, 13 different mammalian aquaporins have been identified (AQP 0–12). Specific human disease has been associated with mutations in the genes coding for AQP 0 (congenital cataracts) and AQP 2 (autosomal recessive nephrogenic diabetes insipidus – see p. 41). Although mutations in the gene for AQP 1 have been described, affected individuals remain asymptomatic. In addition, the condition neuromyelitis optica (NMO), a rare demyelinating and inflammatory disease of the central nervous system (CNS), is caused by autoantibodies directed against the main water channel of the CNS – AQP 4. The regulation of the gene expression of aquaporins may have important pathophysiological consequences in disease states associated with water retention or cerebral oedema.
Under physiological conditions, the solute content of cells is constant and, therefore, cell volume is dependent on solvent, not solute, content. The majority of cells behave as ‘effective’ osmotic meters – swelling when body water increases and contracting when body water decreases. Normally, the ECF and hence ICF osmolality are maintained at about 285 mmol/kg.
Control of renal water output
Osmoregulation
There is a minimum obligatory loss of water by the kidneys each day that is dependent upon the maximum achievable urine concentration and the osmotic load for excretion. The maximum renal loss of water occurs when, for a given osmotic load, the minimum urine concentration is achieved.
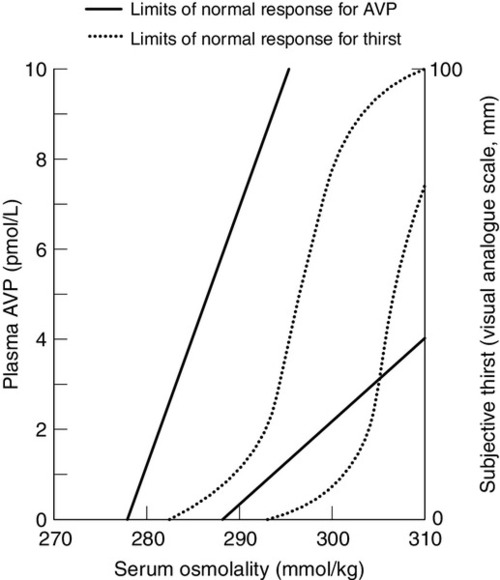
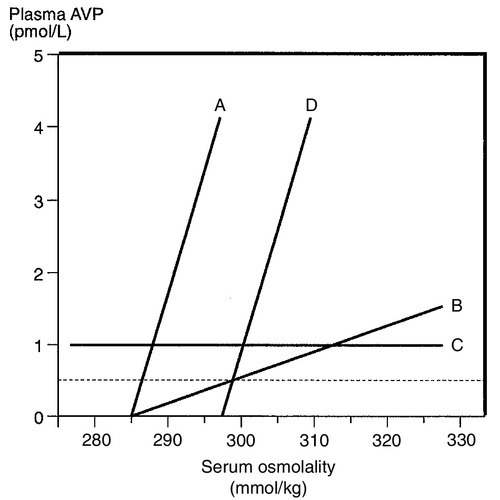
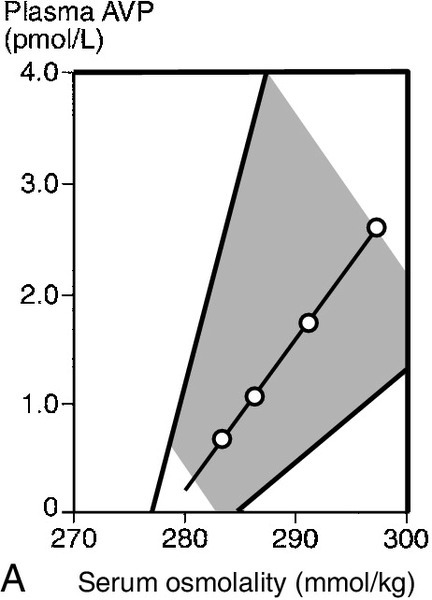
The control of water output by the body is through secretion of antidiuretic hormone (arginine vasopressin, AVP) and its renal action. Arginine vasopressin is a nonapeptide synthesized in magnicellular neurons within two paired nuclei in the hypothalamus – the supraoptic and paraventricular nuclei. The gene for AVP in humans is located on the short arm of chromosome 20. Three exons code for a pre-pro-vasopressin that, following removal of the signal peptide, results in pro-vasopressin, which is subsequently packaged into neurosecretory granules. The granules are then transported by axonal flow to nerve terminals in the posterior pituitary. During transport, pro-vasopressin is further cleaved to AVP, neurophysin II and a glycoprotein. The neurophysin II forms tetramers, with one AVP molecule bound to each neurophysin moiety and the whole possessing a further AVP binding site. The stimulus to release AVP into the circulation results in the simultaneous release of AVP, neurophysin II and the glycoprotein. Closely associated cells within the hypothalamus (the osmoreceptor cells), by virtue of their swelling or shrinking in response to changes in ECF osmolality, control the release of AVP from the posterior pituitary. The effect of changing plasma sodium concentration (and hence osmolality) on plasma AVP concentration is shown in Figure 4.3. Other solutes confined to the ECF, for example exogenously administered mannitol, have a similar effect. By contrast, urea produces no significant stimulation of AVP because it freely permeates cell membranes. The osmoreceptor response is often characterized by its set point (variously defined by the plasma osmolality at which a measurable AVP response commences or by the basal state osmolality) and by its responsiveness (gain or sensitivity as judged by the slope of the response). Thus, in situations of water depletion, ECF osmolality will increase, osmoreceptor cells will contract and plasma AVP secretion will increase. Hydration will reverse these events and suppress AVP. This system constitutes the osmoregulatory control of AVP release.
Non-osmotic control of arginine vasopressin
In addition to osmoregulation, certain non-osmotic controls over the secretion of AVP exist, including ECF hypovolaemia, hypotension, nausea and an oropharyngeal response. The AVP response to hypovolaemia and hypotension is relatively insensitive when changes are proportionally small (5–10% reductions), but increases exponentially as further reductions occur. Thus, a reduction in ECF volume or blood pressure of 20% or more will result in a plasma AVP concentration far in excess of that observed during normal osmoregulation. The influence of baroreceptor or volume receptor afferent input appears to modulate the osmotic response but does not abolish it: modulation occurs by decreasing the threshold for AVP release and increasing the gain of the systems.
Nausea is the single most powerful stimulus to AVP secretion. It overrides osmoregulatory control, and plasma AVP concentrations may increase 100-fold or more. A short-lived oropharyngeal suppression of AVP can occur following oral ingestion of fluid and prior to any reduction in serum osmolality, thus direct studies of osmoregulation of AVP need to avoid any such stimulus.
Renal responsiveness to arginine vasopressin
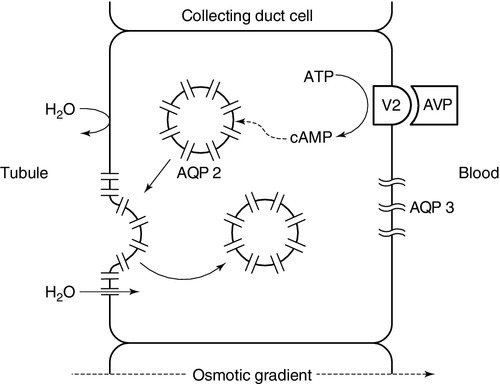
The apical (luminal) surface of the specialized cells lining the collecting ducts is essentially impermeable to water, except when AVP occupies its specific receptors on the basolateral (contraluminal) surface – the V2 receptor (AVPR2). Stimulation of AVPR2 results in AQP 2 channels, located in vesicles beneath the apical membrane, fusing with that membrane and rapidly delivering water channels to the cell surface. A schematic representation of this model is shown in Figure 4.4. Under these conditions, water is absorbed into the collecting duct cells as a result of medullary hyperosmolality and is thence absorbed into the bloodstream: concentrated urine is formed. During states of overhydration, when AVP secretion is suppressed, the luminal surfaces of the collecting duct cells remain impermeable to water. Luminal fluid rendered hypotonic in the diluting segments of the nephrons is not exposed to the medullary hypertonicity and, hence, no water is absorbed into the bloodstream; dilute urine is formed. The renal response to AVP is thus dependent on an intact receptor–effector mechanism within the collecting duct cells, resulting in an alteration in luminal membrane permeability, and upon the presence of a renal medullary osmotic gradient. Human urine osmolality varies between approximately 50 and 1400 mmol/kg.
FIGURE 4.4 Renal action of arginine vasopressin (AVP). Interaction of AVP with its V2 receptor located on the basolateral membrane results in the generation of cAMP, which subsequently causes the fusion of AQP 2 laden vesicles with the otherwise impermeable apical membrane. Under the influence of the medullary osmotic gradient, water is drawn across the cell and is taken up within the blood via AVP-independent AQP 3 water channels.
Control of water intake
Osmoregulation
The physiological stimulus to water intake is thirst. However, the act of drinking in human societies in temperate regions is predominantly a social or habitual act not dependent on thirst. The control of water balance under non-pathological conditions is thus, in many individuals and for much of the time, achieved by control of output.
The osmoregulation of water output by the kidneys can only modulate and restrict an established deficit, but to reverse such a deficit, a specific water input mechanism – thirst – is required. The osmoregulation of thirst is similar in principle to that of AVP and appears to be primarily governed by alteration in osmoreceptor cell size. The osmoreceptor cells controlling thirst are considered to be closely linked to, but distinct from, those controlling AVP secretion. A common method of study in human subjects is the measurement of subjective thirst during continuous osmotic stimulation with hypertonic saline infusion, and a typical range of normal responses is shown in Figure 4.3. Controversy exists as to the exact relationship between the onset of effective renal water conservation and the onset of effective thirst – that is, a sensation of thirst sufficient to cause active seeking and intake of water. However, physiologically, the onset of AVP secretion (and hence water conservation) differs from the onset of thirst and normally precedes it. The magnitude of this difference in water output and input effector controls will determine whether an individual controls physiological water balance primarily by thirst and water intake or by renal conservation of water.
Non-osmotic control of thirst
Non-osmotic thirst occurs when extracellular fluid is lost without corresponding cellular dehydration, the osmotic pressure of the extracellular fluid remaining unchanged. In this respect, the overall control of thirst parallels the control of AVP, with both osmotic and hypovolaemic stimuli. There is good evidence from animal experiments of both neural and hormonal mediators controlling non-osmotic thirst. Angiotensin II is the most potent human thirst stimulant and may act directly upon the brain, but even when the effects of angiotensin II are blocked, significant hypovolaemia will still stimulate thirst.
Thirst following haemorrhage is a commonly reported clinical observation but, like the AVP responses to extracellular hypovolaemia, often a considerable degree of haemorrhage (15–20% of total blood volume) is necessary before the sensation becomes strong. Thus for day-to-day water balance, the primary physiological control of thirst is osmotic.
Extracellular fluid, intracellular fluid and potassium
In health, plasma potassium concentrations range between 3.1 and 4.6 mmol/L, with values in serum approximately 0.3–0.4 mmol/L greater, owing to release of potassium during clot formation (mean serum concentration 4.0 mmol/L). The intracellular concentration of potassium is ~ 160 mmol/kg, and 98% of the total body potassium is present in the intracellular fluid. There are two aspects to the physiological control of potassium, namely, the total body content and its distribution between intra- and extracellular spaces.
Extracellular and intracellular fluid distribution of potassium
The potassium content of cells is determined by the balance of activity between uptake of potassium due to membrane-bound Na+,K+-ATPase and the passive loss or leakage of potassium out of the cell. Many factors can influence the distribution of potassium, for example acid–base status, hormones (insulin, catecholamines), osmolality and the cellular content of potassium. The influence of acid–base status is widely recognized as an important contributor to potassium distribution, with an association between hypokalaemia and alkalosis and between hyperkalaemia and acidosis, particularly when the acidosis is induced by mineral rather than organic acids.
Insulin promotes active uptake of potassium by cells, probably by direct stimulation of Na+,K+-ATPase, and this activity appears to be independent of the effect of insulin on glucose uptake. The importance of the effects of insulin in controlling plasma potassium under physiological conditions is not understood, but its action has an important therapeutic role in the treatment of hyperkalaemia.
Catecholamines have an effect on potassium distribution, with β-adrenergic actions essentially promoting cellular uptake and α-adrenergic actions resulting in increases in plasma potassium concentration, but again the significance of these effects under physiological conditions is not understood. The net effect of catecholamines on cellular uptake of potassium probably explains the transient hypokalaemia frequently observed in acutely ill patients.
An acute increase in extracellular tonicity, such as occurs following hyperosmotic infusions of saline or mannitol, results in an increase in plasma potassium concentration. This results from leakage of potassium from cells, a phenomenon that is not related to extracellular acidosis, but may be linked to cellular dehydration, altered cell membrane function or altered cell metabolism. An increase in extracellular tonicity is also observed in patients with hyperglycaemia in the absence of insulin and has important therapeutic relevance in the provision of potassium replacement during the treatment of hyperglycaemia. The effects of tonicity under physiological conditions are probably of no significance.
Potassium depletion results in a greater loss of potassium from the ECF than the ICF, and potassium excess results in a greater proportional rise of ECF than of ICF potassium concentration. The controlling influences over these changes are not defined, but the result is a significant alteration in membrane potential: this is increased with potassium depletion and decreased with excess. The effects on neuromuscular function of either condition constitute the most important clinical complications of disorders of potassium metabolism.
Renal control of potassium output
Intrinsic tubular control
The traditional understanding of potassium handling by the kidneys is that potassium is freely filtered by the glomeruli, but that up to 95% has been reabsorbed before the tubular fluid reaches the distal convoluted tubules. The predominant control of potassium excretion appears to reside in the control of distal tubular reabsorption and secretion.
Plasma potassium itself has a major effect on potassium secretion in the distal tubules, tending to correct any imbalance. Acute changes in sodium delivery to the distal tubules may also influence potassium excretion – restricted sodium delivery impairs potassium excretion, but a tendency to natriuresis is accompanied by a kaliuresis. However, chronic effects on potassium excretion as a result of changes in sodium intake are not seen because of the influence of the renin–angiotensin–aldosterone axis.
Aldosterone
Potassium directly influences aldosterone secretion from the adrenal cortex. A high plasma potassium concentration stimulates aldosterone secretion and a low concentration suppresses secretion. In addition to its effects on sodium reabsorption by the principal cells, aldosterone stimulates hydrogen ion secretion by the α-intercalated cells of the distal tubules and collecting ducts. Acidosis is associated with reduced potassium secretion and alkalosis with enhanced secretion. The net effect of aldosterone is to stimulate exchange of potassium and hydrogen ions for sodium ions. Therefore, the relative proportions of potassium and hydrogen ions within the cells of the distal tubules, together with the ability to secrete hydrogen ions, will determine the effect of systemic acidosis or alkalosis on potassium excretion. Acting alone, an acidosis will promote potassium retention and an alkalosis will promote a kaliuresis.
Urine potassium concentration can vary between about 5 mmol/L and 150 mmol/L. Adaptation of urinary excretion to a variation in input tends to be slow, taking a few days to achieve a new balance. In this respect, urinary control of potassium is less sensitive than the control of sodium.
DISORDERS OF SODIUM METABOLISM
As sodium is predominantly an extracellular cation, the control of sodium balance will control the volume of the ECF. The tonicity of body fluids is under osmoregulatory control, therefore sodium deficit or sodium excess presents clinically with primary changes in ECF volume rather than changes in sodium concentration within the ECF. Hyponatraemic and hypernatraemic states are discussed in the section on water metabolism.
Sodium deficiency
Clinical presentation
Sodium is always lost from the body in association with water. As the sodium concentration of all body fluids is equal to or less than plasma (except on occasions of high sodium intake, when urine sodium concentration may exceed plasma concentration), loss of any body fluid except plasma will generally result in an excess loss of water over sodium. Any loss of sodium, however, will result in a reduction of ECF volume, including a reduction in circulating plasma volume. Clinical presentation will depend on the severity of the decrease. When the changes are mild, patients are often described clinically as being dehydrated, a description that should be confined to pure water deficiency but, unfortunately, in general usage is not. Except in rare instances, truly dehydrated (water depleted) patients are extremely thirsty; patients with all but the most severe salt and water deficiency are not.
Reduced intravascular volume, when mild, will result in postural hypotension and a compensatory increase in pulse rate; central venous pressure is reduced and this can be assessed clinically by observation of neck vein filling or directly measured following insertion of a cannula into a central vein. When the volume reduction is more severe, hypotension and eventually shock will result in oliguria; the central venous pressure is further reduced. Reduction in interstitial fluid volume results in reduced skin turgor and transcellular fluid reductions result in dry mouth and reduced intraocular pressure.
Causes of sodium deficiency
The causes of sodium deficiency can be classified broadly into extrarenal, primary renal (resulting from renal disease) and secondary renal (resulting from disturbed hormonal control of renal sodium retention or from inappropriate use or abuse of diuretics). In addition, and somewhat difficult to classify, is the sodium deficiency that can occur when isolated jejunal segments are incorporated into urinary diversion operations (jejunal conduit, jejunal continent diversion, see p. 55). These procedures are now rarely performed, as alternative sources of donor intestine are preferred. When jejunum is used in such procedures, there is a risk of postrenal loss of sodium.
Extrarenal sodium loss
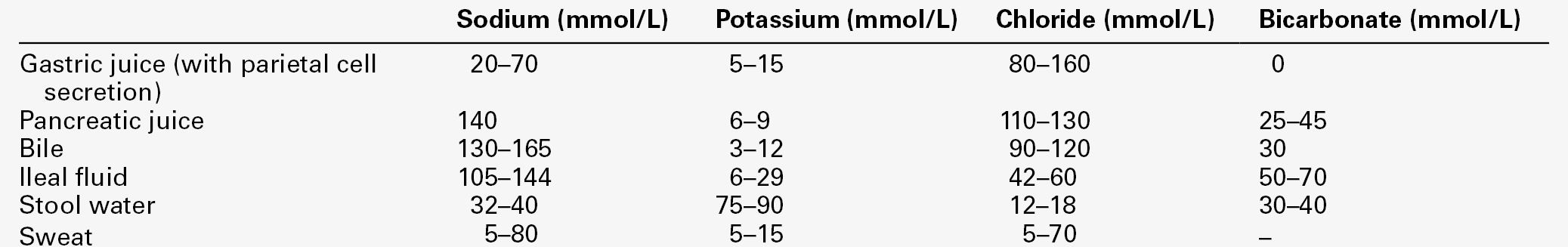
Extrarenal fluids have sodium concentrations that may approach the concentration in plasma (Table 4.3). The major causes of extrarenal sodium deficiency are summarized in Box 4.1. The commonest clinical presentations result from gastrointestinal disease. The clinical history may considerably underplay the degree of deficit, especially in chronic conditions or conditions resulting in sequestration of fluid.
Primary renal sodium loss
The major causes of primary renal sodium loss are summarized in Box 4.2. The recovery phase of acute kidney injury is often associated with a polyuria, kaliuresis and natriuresis. Usually this stage is short lived, lasting only a few days, but it may occasionally be prolonged. A natriuresis may occur following successful renal transplantation and this may be in part due to transient tubular dysfunction; the recovery phase usually lasts only a few days, but occasional patients show prolonged natriuresis.
Relief of urinary tract obstruction, most commonly seen in patients with prostatic enlargement, is often followed by a short period of diuresis and natriuresis. The exact mechanism of this is not fully understood, but is probably related to a urea-induced osmotic diuresis, together with elimination of excess sodium retained during the obstruction phase. The natriuresis in these cases is corrective and is not strictly a primary renal condition: it is unlikely to lead to sodium depletion. Normally, this phase of post-obstructive natriuresis and diuresis lasts between one and seven days, but occasionally a more prolonged natriuresis occurs, leading to sodium deficiency. This rare event is secondary to tubular damage occurring during obstruction. Salt wasting has been described in association with meticillin-induced acute interstitial nephritis. Full resolution of the condition may be delayed for several months, during which time minimum obligatory losses of sodium may exceed 100 mmol/24 h.
Chronic kidney disease is generally associated with a reduced capacity to excrete sodium. Considerable adaptation occurs to increase the natriuretic capacity of the remaining functioning nephrons, which paradoxically impairs the overall sodium conserving function. On a Western type diet, sodium intake normally exceeds minimum obligatory urine sodium loss, but if dietary salt is restricted or non-renal losses of sodium are increased, the obligatory loss may induce sodium deficiency. Minimum obligatory losses may be as little as 40 mmol/24 h and even lower if enough time is given for adaptation, but occasionally patients have minimum obligatory losses exceeding 150 mmol/24 h (3 mmol/kg body weight in children). The term salt-losing nephropathy is appropriate for this group of patients.
Salt-losing nephropathy is a clinical state, rather than a specific disease. It is normally associated with chronic kidney disease due to tubulointerstitial disease or to glomerulonephritis with significant interstitial abnormalities. A common example of a toxic nephropathy inducing a salt-losing state is analgesic abuse, in which there is typically also reduced concentrating ability and renal tubular acidosis.
Secondary renal sodium loss
The disruption of extrinsic controls over renal sodium handling can give rise to a secondary renal sodium loss. Patients present with a variety of symptoms, some specifically related to a contracted ECF volume, such as postural hypotension and an increased sodium appetite. Causes of secondary renal sodium loss, presented in Box 4.3, are essentially hormone or diuretic induced. In Addison disease, the synthesis and secretion of aldosterone are reduced because of adrenal gland destruction, leading to distal renal tubular sodium wasting. Congenital adrenal hyperplasia (CAH) with associated sodium wasting is a result of impaired mineralocorticoid synthesis, most commonly as a consequence of 21-hydroxylase deficiency, but also, less commonly, 3β-hydroxysteroid dehydrogenase deficiency. In either condition, the degree of sodium wasting is variable: it is present in about two-thirds of patients with 21-hydroxylase deficiency (see Chapter 18). Very rare forms also exist, including cholesterol desmolase deficiency (lipoid adrenal hyperplasia) and corticosterone methyl oxidase deficiency – a deficiency in the mixed-function oxidase catalysing the final steps of aldosterone synthesis. Corticosterone methyl oxidase deficiency is not strictly a type of CAH, as the synthesis of cortisol is unaffected and, consequently, the adrenals are not hyperplastic. In all these conditions, the associated hypovolaemia stimulates renin production, and all may be associated with hyperkalaemia. Treatment is primarily by glucocorticoid and mineralocorticoid replacement.
It might be predicted that deficient production of renin would also lead to renal sodium loss because of a secondary deficiency of aldosterone. The condition hyporeninaemic hypoaldosteronism is invariably associated with renal insufficiency, but the characteristic feature is hyperkalaemia rather than hypovolaemia. As in the majority of patients with renal insufficiency, inappropriate renal sodium loss will induce hypovolaemia if the patient is placed on a low sodium intake, but it is unclear if this is more severe in hyporeninaemic hypoaldosteronism. Although this condition is a cause of secondary renal sodium loss, its main importance is in the differential diagnosis of hyperkalaemia and it is discussed in more detail later in this chapter (see Syndromes of hypoaldosteronism, p. 60).
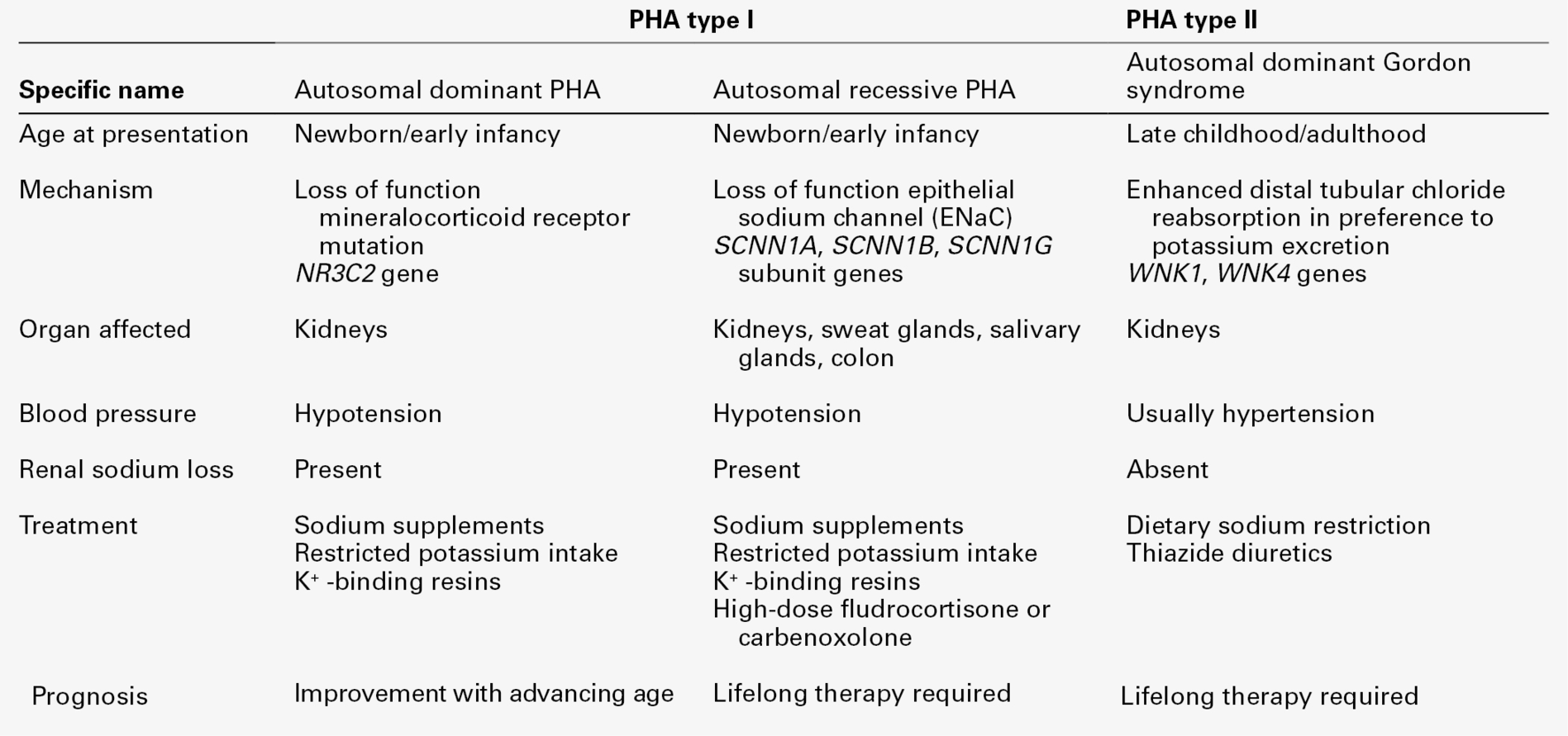
Pseudohypoaldosteronism (type 1) is a rare congenital disease existing in two forms, caused by an end organ failure of response to aldosterone within the principal cells lining the renal collecting ducts. It is caused by either a loss of function mutation in the mineralocorticoid receptor (MR) gene (autosomal dominant form) or a mutation resulting in loss of function in the epithelial Na+ channel (ENaC – autosomal recessive form). Infants present with dehydration, hyponatraemia, hyperkalaemia, metabolic acidosis, failure to thrive and weight loss. Renal sodium wasting is unresponsive to exogenous mineralocorticoids. Pseudohypoaldosteronism is also discussed in more detail later in this chapter (see Syndromes of hypoaldosteronism, p. 60).
Diuretic abuse, especially when surreptitious, may present a difficult diagnostic problem. The major potent diuretics, such as furosemide or bumetanide, may induce significant renal sodium loss and the differential diagnosis includes Bartter syndrome. Chronic abuse with thiazide diuretics may produce a clinical picture similar to Gitelman syndrome. In all cases, however, the major and persistent finding is significant hypokalaemia (see p. 55).
Laboratory investigation of sodium deficiency
No single laboratory finding is diagnostic of sodium deficiency, which, therefore, remains primarily a clinical diagnosis. There may be uraemia, particularly of a prerenal pattern (with urea concentration disproportionately elevated in relation to that of creatinine). Serum sodium concentration may be normal, decreased (usually in association with uraemia) or even increased when the sodium deficit is proportionately less than the water deficit. Serum sodium and urea concentrations cannot indicate accurately the degree of sodium depletion or reduction in ECF volume.
Measurement of the haematocrit may help to establish the degree of reduction in ECF volume, especially if the individual’s normal haematocrit is known (see Appendix 4.1a). Similarly, serum protein measurements may suggest ECF volume contraction. In a steady state, hyponatraemia in association with clinical evidence of sodium deficit can also be used to estimate the extent of sodium deficit (see Appendix 4.1b). Either method provides only a rough guide to subsequent replacement.
Urine electrolytes will help to establish whether the loss has been renal or extrarenal. If there has been gastrointestinal loss of sodium or another extrarenal loss, renal sodium retention should be maximized. Random urine sodium concentrations will show conservation of sodium with a concentration < 10 mmol/L. The mature, fully functioning renal system can reduce urine sodium concentration to < 1 mmol/L, but this capacity is less in the very young and old. Twenty-four hour urine sodium output will show significant sodium retention, with 24 h urine sodium excretion as low as 1 mmol and invariably < 15 mmol, even in the elderly. Serum osmolality measurements are of no value in the differential diagnosis of sodium depletion, adding nothing to the information obtained from serum electrolytes, urea and creatinine measurements. Urine osmolality will generally show an overall significant increase towards maximum, reflecting the oliguric state, especially when sodium depletion is sufficient to cause a non-osmotic increase in arginine vasopressin (AVP).
Only when a renal loss of sodium is present without evidence of renal impairment should secondary hormonal or diuretic-related sodium depletion be considered. For the majority of hypoaldosterone-related disorders, there will be associated hyperkalaemia (for specific investigation of hypoaldosteronism, see Chapter 18). Diuretic abuse can provide an intriguing puzzle as, along with Bartter syndrome and Gitelman syndrome, it provides the major differential diagnosis of renal sodium loss in association with hypokalaemia.
Management of sodium deficiency
The essential steps in treating sodium deficiency and ECF volume depletion are to attempt to treat the causes of sodium and water loss and to adequately replace the fluid already lost. The amount of fluid replacement must be balanced against measured or estimated losses, both intra- and extracorporeal. The measurement of body weight is a useful adjunct to monitoring; an increase in weight may indicate accumulation of interstitial or transcellular fluid. Accurate fluid balance charts (see Table 4.4) should indicate a positive or negative balance, but even the most assiduously prepared charts (many are not) provide only an approximate estimate of balance, especially when confounding problems such as pyrexia (increasing insensible losses) or hypermetabolic states (increasing metabolic water) intervene. Vital signs: pulse, blood pressure and central venous pressure (if measured), all provide additional useful information to improve the practice of the art.
TABLE 4.4
Ideal components of a fluid balance chart
| Input | Output | Extravasation |
| Oral fluid | Urine output | Body weight |
| Intravenous fluid | Gastrointestinal losses | |
| Water generated in metabolism (~ 500 mL/24 h) | Surgical drains etc. | |
| Insensible losses (~ 900 mL/24 h) |
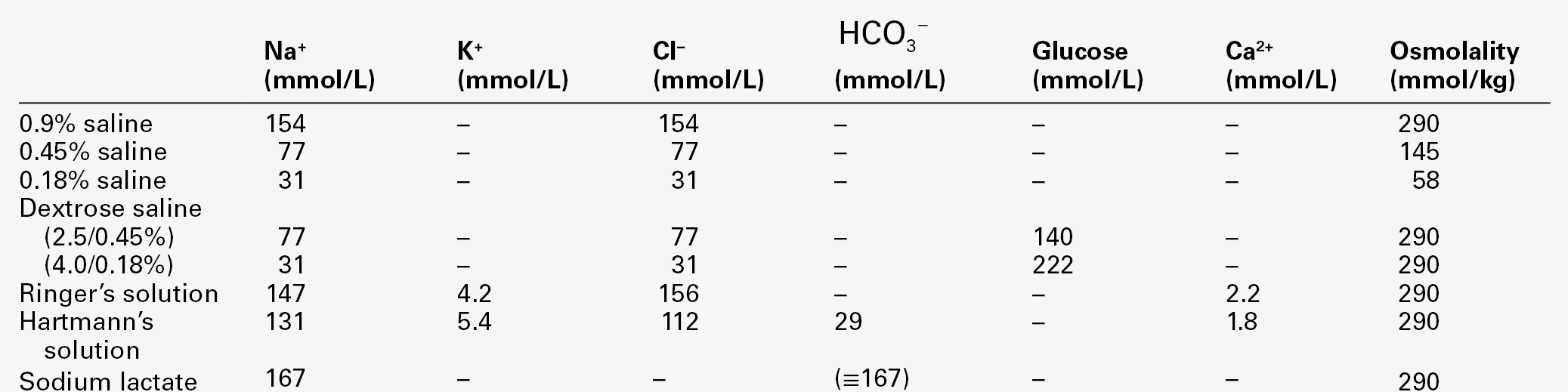
The type of replacement will be dependent on cause and severity. Mild forms of sodium deficit, such as caused by overtreatment with diuretics or chronic salt-losing nephropathy, may be adequately treated with oral sodium and water supplementation. For more severe forms of sodium deficit, intravenous infusion is required; the types of fluids available are shown in Table 4.5. All of them are iso- or hypoosmolal to normal plasma. Hyperosmolal fluids, such as 1.8% or 5% sodium chloride or 2.74% or 8.4% sodium bicarbonate, are not appropriate treatments for sodium depletion with clinical evidence of reduced ECF volume, even in the presence of hyponatraemia, as infusions of such fluids will increase ECF volume in part by shifting fluid from the ICF volume.
Hypoosmolal fluids may be used when sodium deficit is associated with a greater degree of water deficit, that is, sodium deficit in association with hypernatraemia.
Sodium excess
Clinical presentation
Sodium excess is almost invariably associated with water excess (and is usually an iso-osmolal sodium excess). The clinical presentation will depend on the severity of the expansion of ECF volume and its relative distribution within the ECF volume compartments. In practice, the clinical presentation is with oedema (peripheral or pulmonary) or effusions (pleural, ascites) or with hypertension.
Peripheral oedema, due to sodium retention, is characteristically ‘pitting’ when digital pressure is applied to the affected part (lymphoedema, due to lymphatic obstruction, is characteristically non-pitting, while in myxoedema, the swelling is brawny in nature). Measurement or clinical observation of the central venous pressure may suggest an associated increase in intravascular volume, as may the demonstration of hepatic congestion or the presence of pulmonary crepitations.
Hypertension is a common clinical finding, but hypertension specifically due to chronic iso-osmolal sodium excess is rare. Causes of this form of hypertension are discussed later, but all are associated with an increased mineralocorticoid effect in the initial stages, promoting renal sodium conservation and renal loss of potassium. The association of muscle weakness (caused by hypokalaemia) with hypertension is an important clinical pointer to such a cause of sodium retention.
Causes of sodium excess
Sodium excess with oedema
The causes of sodium excess in association with oedema formation are shown in Box 4.4. Congestive cardiac failure (CCF) is a common clinical example of sodium excess, presenting with dependent oedema, congested liver, increased jugular venous pressure and pulmonary crepitations due to oedema. The ‘backward failure’ hypothesis suggests that increased transcapillary pressure resulting from increased central venous pressure causes oedema and hence reduces intravascular volume. Reduced intravascular volume will in turn stimulate sodium retention. Although readily understandable, this theory is inadequate because patients generally have increased intravascular volumes. The ‘forward failure’ hypothesis suggests inadequate perfusion of the kidneys because of reduced cardiac output, which in turn promotes sodium retention. However, high cardiac output failure demonstrates that sodium retention can be independent of cardiac output. The reduced ‘effective circulating volume hypothesis’ lacks precise definition, but suggests that only a proportion of the volume is ‘effective’, that is, circulating volume appears to be reduced in that the renin–angiotensin–aldosterone mechanism is activated, as is the release of AVP. The onset of CCF causes ANP and, particularly, BNP, release, leading to markedly elevated plasma concentrations. However, the natriuretic effect of the hormones is severely blunted and corrective natriuresis does not occur without therapeutic intervention, with diuretics or with exogenous natriuretic peptide administration in pharmacological doses.
The nephrotic syndrome is characterized by severe glomerular proteinuria, hypoalbuminaemia and oedema. The classic hypothesis explained the oedema and secondary sodium retention, on the basis of reduced oncotic pressure owing to hypoalbuminaemia. However, the rare condition congenital analbuminaemia is not associated with significant oedema, and nephrotic patients infused with albumin do not consistently respond with a natriuresis and reduction of oedema. Sudden remission of minimal-change glomerulonephritis may result in clinical resolution prior to an increase in plasma albumin, and the presumed reduced circulating volume in nephrotic syndrome is less commonly found when sought than normal or increased circulating volume. These observations imply an intrarenal mechanism of sodium retention in the nephrotic syndrome. Sodium retention does not appear to be primarily driven by the renin–angiotensin–aldosterone system; moreover, ANP concentrations are moderately increased, not decreased, and the natriuretic effect of the hormone is maintained.
Chronic liver disease frequently results in a state of sodium retention leading to oedema and ascites. Again, the physiology of sodium retention is multifactorial, resulting from decreases in glomerular filtration, enhanced proximal tubular reabsorption and increased renin–angiotensin–aldosterone production, presumably from decreased ‘effective’ intravascular volume. As in CCF, the plasma ANP concentration is significantly increased, but again the natriuretic response is blunted. The concentration of AVP may also be significantly increased.
Pregnancy
Pregnancy demands a retention of between 700 and 1000 mmol of sodium in order to expand the maternal plasma volume and interstitial space and to provide the fetus with sodium. Sodium retention occurs progressively during pregnancy, even though the glomerular filtration rate is greatly increased, so that the reabsorption of filtered sodium must be substantially increased above the non-pregnant level. The renin–angiotensin–aldosterone system is stimulated during pregnancy, a finding that supports the theory of renal sodium retention as a response to reduced ‘effective’ vascular volume (due to redistribution of ECF volume to the growing fetus and its maternal circulatory support). However, many studies have shown increased plasma ANP concentrations during pregnancy, a finding somewhat at odds with the presumed reduced ‘effective’ vascular volume. Thus, the kidneys may retain sodium in response to some other stimulus (such as the effect of oestrogens), and the increase in ANP may represent the response to the resultant increased atrial pressure from expanded vascular volume. However, these two apparently conflicting theories are not mutually exclusive and it seems likely that the resultant increase both in the activity of the renin–angiotensin–aldosterone system and the secretion of ANP forms the basis for an enhanced control over sodium balance. Enormous differences in daily sodium intake (from < 10 mmol to > 300 mmol) can be adequately accommodated during pregnancy.
Dependent leg oedema is extremely common during pregnancy, especially during the third trimester, and much of this is attributable to the mechanical pressure of the uterus upon the venous return from the lower limbs. However, there is a general tendency to an increased interstitial fluid content because of reduced oncotic pressure (as a result of reduced plasma protein concentration) and because of the effects of oestrogens, which enhance the hydration of the mucopolysaccharide ground substance of connective tissue. This general tendency to increased interstitial fluid content may also produce a more generalized oedema.
Excessive sodium retention occurs in pre-eclampsia. Total body sodium is increased, but plasma volume is usually decreased, with shifts of fluid to the interstitium. The reduced plasma volume is the result of intense vasoconstriction, which also results in hypertension. In pre-eclampsia, renin activity and aldosterone concentrations in plasma in the third trimester are less than those observed during normal pregnancy. Furthermore, ANP concentrations are significantly increased above those found in normal pregnancy, or indeed those found in pregnancy associated with essential hypertension. Atrial natriuretic peptide appears to be released into the maternal circulation in response to vasoconstriction and an increased volume load to the cardiac atria. Thus in pre-eclampsia, as compared with normal pregnancy, renin, aldosterone and ANP all act as if to promote natriuresis, but, for reasons which are not understood, natriuresis is impaired.
Menstrual cycle
Despite intensive study over 50 years or so, the question of whether the normal menstrual cycle is associated with sodium retention remains unresolved. There is widespread belief that the majority of women retain sodium premenstrually, giving rise to premenstrual oedema in some. Plasma aldosterone and renin concentrations are elevated during the luteal phase of the ovulatory cycle, but this elevation is coupled with an increase in progesterone, which is thought to antagonize the renal actions of aldosterone. Studies have shown that plasma ANP concentrations remain the same during the follicular and luteal phases. It has also been demonstrated that, in healthy females, the ANP response to volume expansion is no different in the follicular and luteal phases of the menstrual cycle, and the proportional suppression of renin and aldosterone is also identical between the two phases of the cycle. When these findings are coupled with the observations that body weight, creatinine clearance and basal sodium excretion do not alter during the phases of the cycle, it is apparent that there is little evidence of renal sodium retention. This remains an area for further research, particularly as inappropriate treatment of perceived symptoms may lead to further complications of sodium balance (see below).
Idiopathic oedema
Idiopathic oedema, sometimes known as cyclical oedema, is a condition that occurs in females postpuberty. Oedema of the face, hands and legs can develop rapidly, and weight gains up to 4 kg in 24 h have been described. The aetiology of this condition is unknown, but one suggestion is that diuretics may be used to produce rapid weight loss and that cessation of this self-medication leads to rebound sodium retention. The diuretic is subsequently recommenced for cosmetic reasons to reduce oedema formation and weight gain. Thus, a vicious cycle of sodium depletion followed by sodium retention and oedema is set in place. However, in some patients prior diuretic use cannot be demonstrated.
Many patients with idiopathic oedema show exaggerated sodium retention on assuming an upright posture with a marked reduction in renal blood flow and GFR. Oestrogens do not appear to play an important role, as the condition has been demonstrated in a patient who had previously undergone bilateral oophorectomy. The role of renin–angiotensin–aldosterone is not fully understood, with a proportion of patients having increased plasma renin and aldosterone concentrations, particularly when in an upright posture, but with suppression of concentrations by a high salt diet. Limited studies of ANP have been performed, but no abnormalities have been demonstrated: normal basal values are found and are stimulated by volume expansion.
Sodium excess without oedema
The causes of sodium excess without oedema are shown in Box 4.4. Acute sodium loading is a rare event, invariably caused by inappropriate sodium administration to highly dependent individuals, for example those in intensive therapy units, especially the very young, the old and the highly incapacitated. Acute hypernatraemia is a powerful stimulus for thirst and thus, to maintain the condition, the intake of fluid must be prevented by an inability, for whatever reason, to express or act upon the desire to drink. Examples of acute sodium loading include administration of high sodium concentration oral feeds to infants (either accidentally or as a form of abuse), the use of oral hypertonic sodium chloride solutions as emetics, excessive administration of intravenous hypertonic sodium bicarbonate and the voluntary consumption of excessive quantities of table salt. In the past, the accidental introduction of hypertonic sodium into the circulation during therapeutic abortion has been a cause of acute sodium excess. Acute sodium loading associated with hypernatraemia is a life-threatening condition and should be treated promptly with free water administration.
Mineralocorticoid excess has a variety of causes (see Box 4.4). Although this is a condition of excess sodium retention, the usual clinical presentation is hypertension with hypokalaemia. The severity of hypokalaemia is in turn dependent on sodium intake, being reduced in severity if sodium intake is reduced. All of these conditions are discussed elsewhere in this book, either as part of the pathology of the adrenal glands (see Chapter 18) or in relation to hypokalaemia (see p. 55). The fascination of these conditions with respect to sodium is why sodium retention does not usually progress to increase the volume of all the ECF spaces sufficiently to cause oedema.
As the ECF volume expands, the filtered load of sodium increases and the fraction reabsorbed by the renal tubules decreases. This mechanism constitutes the ‘escape’ for sodium (but not potassium) from mineralocorticoid effects. The underlying mechanism of escape has been the subject of investigation since it was first described over 50 years ago. The mechanism appears multifactorial with a haemodynamic response to increased intravascular volume resulting in reduced proximal reabsorption of sodium. There is good evidence that natriuretic peptides may account for a part of the escape mechanism – ANP concentrations are elevated in primary hyperaldosteronism and return to normal when the effects of hyperaldosteronism are reversed or antagonized. Evidence also exists of a decrease in the thiazide-sensitive Na+-Cl− co-transporter (NCCT) within the distal convoluted tubules. In addition intrarenal substances such as prostaglandins, kinins and nitric oxide may also promote natriuresis.
Laboratory investigation of sodium excess
The investigation of sodium excess is confined almost exclusively to the investigation of oedema and of hypertension; hypernatraemia is not a feature of sodium excess except in rare instances of acute sodium loading, when the cause is usually clear from the clinical history.
In practice, the purpose of the investigation of oedema is primarily to differentiate reduced oncotic pressure from other causes, usually by the measurement of serum protein concentration and the confirmation of any route of protein loss, for example the measurement of urine protein excretion. The diagnosis of oedema secondary to CCF and liver disease is primarily clinical; detailed sodium balance studies are not normally justified or, indeed, practicable. Idiopathic oedema is also a condition that is initially diagnosed from the clinical history, although periods of continuous sodium loading with simultaneous sodium balance studies may reveal a propensity for sodium retention.
The laboratory investigation of sodium excess as a contributor to hypertension involves the demonstration of increased mineralocorticoid action, the simplest pointer being the association of hypokalaemia and hypertension. Investigation of primary hyperaldosteronism, conditions of increased glucocorticoid secretion and conditions of sodium retention due to congenital adrenal hyperplasia are covered in Chapter 18. Other causes of hypokalaemia and hypertension are discussed in the section on potassium metabolism (see p. 55).
Management of sodium excess
For those conditions associated with oedema, the management will be aimed primarily at ameliorating the primary cause. This primary treatment, when applicable, needs to be coupled with attempts to control sodium and water retention by the restriction of dietary sodium and, when appropriate, the use of diuretics. The laboratory’s role in management is to monitor the potential complications of diuretic treatment, notably hypokalaemia (especially with the use of loop diuretics), hyperkalaemia (with the use of potassium-sparing diuretics such as spironolactone) and hyponatraemia (when over-diuresis results in non-osmotic stimulation of AVP with secondary water retention). In addition, the laboratory’s function is to monitor the effects of natriuresis on renal function by monitoring serum urea or creatinine concentrations.
For conditions of sodium excess without oedema, the initial management is to increase renal sodium excretion, either by attention to iatrogenic causes (such as exogenous mineralocorticoid treatment) or by treatment with diuretics such as spironolactone. This treatment is combined, when appropriate, with antihypertensive therapy. The laboratory’s role in management is to monitor for possible complications, particularly with respect to potassium homoeostasis. For those patients with surgically correctable conditions, such as primary hyperaldosteronism due to an adrenal adenoma, the definitive treatment is surgery.
DISORDERS OF WATER METABOLISM
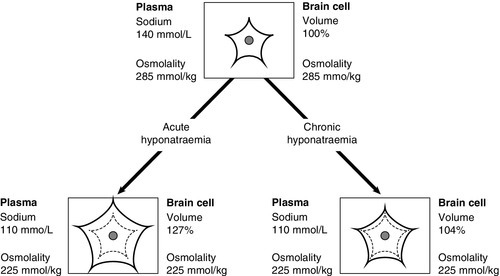
As water is distributed throughout all body spaces, the effects of pathological excess or deficiency will be reflected in the relative functional sensitivities of each space. For example, a 10% excess or reduction in total body water (TBW) is unlikely to result in clinical features of alterations in ECF volume. However, ICF volume changes may considerably impair cellular function; in particular, rapid changes may significantly impair brain cell function.
Disorders of water metabolism include three categories of conditions. First are conditions in which polyuria is the major feature. Polyuria is defined as a urine output in adults in excess of 50 mL/kg body weight/24 h. In polyuria, one aspect of water homoeostasis is defective but may be compensated by another: either a reduced ability to concentrate the urine is compensated by a secondary increase in thirst, or an excessive water intake is compensated with a secondary increase in urine output. In either case, patients are usually normonatraemic unless the compensatory mechanism is compromised.
Second are those of water deficiency in which homoeostasis is defective and normal body water content cannot be maintained: patients present with hypernatraemia (serum sodium above 145 mmol/L).
Third are conditions of primary water excess in which homoeostasis is defective: patients present with hyponatraemia (usually defined as a serum sodium < 130 mmol/L) or clinical features of water intoxication.
Polyuria
Primary polyuria with secondary polydipsia
Polyuria is an early feature of diabetes mellitus as a result of the osmotic effects of an increased filtered load of glucose. Polyuria can also be a feature of renal failure due to the loss of medullary hypertonicity and the reduction in the production of AQP 2. Thus polyuria due to diabetes mellitus or renal failure should be differentiated at the outset. A primary inability to concentrate urine with secondary polydipsia is known as diabetes insipidus (DI), and may be due to either impaired release of AVP from the posterior pituitary (cranial diabetes insipidus, CDI) or to impaired renal response to AVP (nephrogenic diabetes insipidus, NDI).
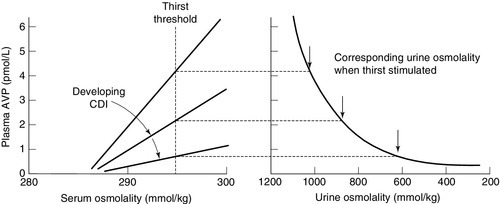
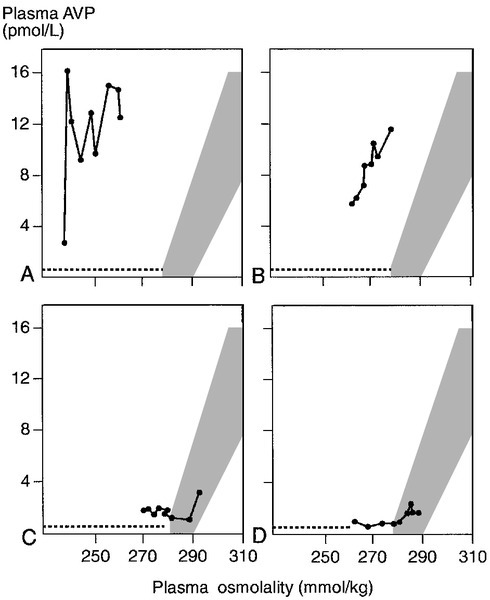
The major causes of CDI and NDI are shown in Box 4.5. There are four causes of inherited CDI, all extremely rare. Autosomal dominant CDI is caused by mutations involving the pre-pro-vasopressin gene excluding the region coding for AVP itself. The disorder is due to a progressive degeneration of magnicellular neurons, probably due to the accumulation of abnormal AVP-neurophysin II complexes. Presentation may be in the neonatal period or delayed until later in childhood. An even rarer autosomal recessive form exists in which the coding for AVP is mutated; presentation is in the early neonatal period. The two other causes of inherited CDI are Wolfram syndrome and congenital septo-optic dysplasia, with about one-third of patients with each condition exhibiting overt symptoms of CDI. Wolfram syndrome may present with a combination of diabetes insipidus, diabetes mellitus, optic atrophy and deafness – hence the alternative name of DIDMOAD syndrome. Patients with either CDI or NDI may retain a partial ability to concentrate the urine, which can lead to diagnostic confusion. The osmoregulatory set point for AVP release and thirst is maintained in both conditions. Thus, in developing CDI, as the number of active cells releasing AVP from the posterior pituitary diminishes, the slope of the osmoregulatory response will decrease (see Fig. 4.5). With a plasma osmolality for thirst normally set slightly above that for AVP release, the urine concentration that can be achieved at the onset of thirst will diminish. When CDI is severe, thirst will be stimulated even though the urine is close to maximum dilution. Intermediate stages can, therefore, provide confusion, as an increase in plasma osmolality due to water deprivation despite thirst can be associated with a normally concentrated urine. Diagnostic confusion may be further compounded by two additional features not shown in Figure 4.5:
FIGURE 4.5 The relationship between the osmoregulatory control over arginine vasopressin (AVP) secretion and the renal response to its action. For illustrative purposes, thirst is shown as a single point threshold. Normally, thirst does not become active until urine concentration approaches maximum. In developing cranial diabetes insipidus (CDI), as the number of cells releasing AVP diminishes, so thirst becomes active at correspondingly reduced urine osmolality. Polyuria leads to polydipsia.
• a secondary loss of urine-concentrating ability once the sustained polyuria has resulted in medullary washout, with a reduction of the concentration gradient within the medulla of the kidneys.
The former phenomenon may mask a developing CDI. The latter feature may result in a very blunted response to endogenous and exogenously administered AVP (or analogue), thus suggesting a primarily nephrogenic basis to the polyuria, whereas in fact the loss of renal concentrating ability is secondary to the severe polyuria.
Congenital NDI is a rare condition. About 90% of cases are X-linked and due to a loss of function mutation within the gene coding for the V2 AVP receptor (AVPR2). Affected males present within the first six months of life with failure to thrive, irritability, severe thirst and excessive wetting. Often the diagnosis is made during the first acute hospital admission because of impaired consciousness or convulsions, due to severe water depletion leading to significant hypernatraemia. The kidneys are completely unresponsive to AVP or AVP analogues. The female carriers of the condition are of interest, as many show evidence of polyuria, especially during pregnancy. Originally, this polyuria was considered to be due to the mimicking of affected males within the family, but this explanation is now disproven, with many females having demonstrably impaired renal responses to AVP – a partial form of congenital NDI – but some being almost as severely affected as males with congenital NDI. Of the remaining 10% of congenital NDI, inherited as autosomal recessive conditions, about 7% are due to mutations in the AQP 2 gene and the remaining 3% currently have no identified cause.
Both lithium and demeclocycline may produce clinically severe NDI with marked degrees of polyuria. Patients receiving lithium should be monitored strictly, to ensure effective but safe plasma concentrations of lithium to reduce the incidence of unwanted effects, including polyuria. Demeclocycline, once used to treat acne vulgaris, is no longer used as an antibiotic in man, but has, in the recent past, occasionally been used to control water-retaining states (see p. 51). The NDI of hypokalaemia and hypercalcaemia is generally less severe, with polyuria rarely greater than 4 L/24 h in adults. Recent work has suggested that NDI secondary to lithium, hypokalaemia and obstructive uropathy may all be related to alterations in AQP 2 expression.
Pregnancy and polyuria
There are several subtle alterations to normal osmoregulation in pregnancy, with the osmotic thresholds for both thirst and AVP secretion falling ~ 10 mmol/kg around the 6th week of gestation and remaining at these lower levels throughout pregnancy, so that a new steady state is achieved. The postpartum rise of these thresholds to normal occurs within approximately three weeks.
The major potential complication of osmoregulation in pregnancy is polyuria with secondary polydipsia. Pregnancy is discussed separately from other causes of polyuria because, although partial forms of CDI and NDI may be unmasked, one underlying cause is unique to pregnancy. This is due to the massive increase in the clearance of AVP, in both the placenta and the circulation, as a result of the release from the placenta of vasopressinase (cystine aminopeptidase, oxytocinase). In addition, there is evidence of increased clearance of AVP by the liver and kidneys related to increased blood flow to both organs. The increased clearance of AVP produces a form of diabetes insipidus that is unresponsive to exogenous pharmacological doses of AVP, but is responsive to the now commonly prescribed vasopressin analogue desamino- 8-D-arginine vasopressin (dDAVP, desmopressin), which is resistant to enzymic degradation by vasopressinase. The response to a standard water deprivation test is thus identical to that found in CDI. Diabetes insipidus related to excessive vasopressinase activity resolves quickly following birth, with normal osmoregulation resuming within two weeks.
Polyuria secondary to primary polydipsia
Primary polydipsia is found in association with a variety of psychiatric and other CNS disorders. The commonest form is the ‘compulsive water drinking’ found in up to 7% of psychiatric inpatients. One possibility is that these patients have a reduced osmoreceptor setting for thirst, which is at or below the setting for AVP release. However, a subgroup of compulsive water drinkers use excessive water intake as a form of purging or self-therapy, and thus formal assessment of thirst thresholds using subjective visual analogue scales may be difficult, if not impossible, to interpret. This latter group has sometimes been classified as having psychogenic polydipsia, although the terms ‘compulsive water drinking’ and ‘psychogenic polydipsia’ are often used synonymously. The mechanisms are further complicated by the fact that many of the drugs used in psychiatry have powerful anticholinergic effects and may cause dryness of the mouth with a subsequent desire for oral fluids.
Primary polydipsia due to a hypothalamic disorder is rare, but can be dramatic. Even immediately following ingestion of large volumes of water, patients may possess such cravings for further water intake that they will go to extreme lengths to obtain it, such as consuming bath water or even lavatory water. Hypothalamic disorders can occasionally give rise to a water-retaining state and, if excessive thirst is also present, severe acute hyponatraemia may result (see p. 46).
Excessive thirst is a rare feature of conditions in which the renin–angiotensin system is stimulated, possibly because the concentration of angiotensin II, a powerful central thirst stimulant, is increased. Thus, pathological thirst has been described in renal artery stenosis, Wilms tumour, end-stage renal disease and even congestive cardiac failure. There is some evidence that when thirst occurs in these conditions, it can be controlled by angiotensin-converting enzyme (ACE) inhibitors. The mechanisms, however, are not well understood, as paradoxically, some animals develop increased thirst in response to ACE inhibitors; this is presumed to be because of increased angiotensin I delivery to thirst-stimulating areas of the brain that are inaccessible to ACE inhibitors, and at which conversion to angiotensin II subsequently occurs.
Laboratory investigation and treatment of polyuria
The first step is to confirm polyuria. Patients (and doctors) may confuse frequency of micturition with polyuria. True polyuria is invariably associated with polydipsia, and nocturnal polydipsia is an important pathological symptom. Fasting plasma glucose, serum potassium, calcium and urea measurements in the basal state may reveal diabetes mellitus, hypokalaemia, hypercalcaemia or renal impairment. Serum sodium may provide a pointer to the final diagnosis, tending towards the upper reference limit in secondary polydipsia and DI, but towards the lower reference limit in primary polydipsia. However, overlap of serum sodium concentrations between the diagnostic groups means that a water deprivation test is often necessary.
Water deprivation test
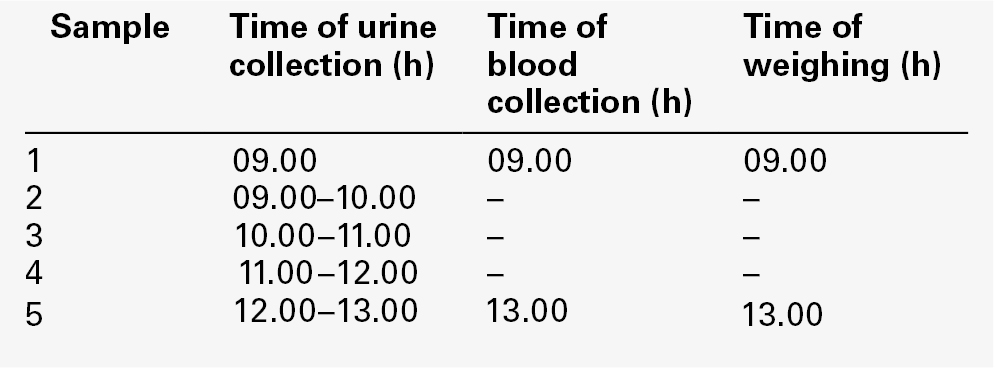
A standard protocol that can be performed on outpatients or day cases in the majority of patients is provided in Appendix 4.2a. The test is conceptually simple to understand but is often incorrectly performed. Weighing the patient prior to and during water deprivation is a most important component of the test. Weighing allows the detection of surreptitious water intake – weight loss during the test should approximate to urine output (1 L = 1 kg). Weighing is also necessary to prevent gross dehydration in patients with severe DI; a weight loss of 3% body weight is normally the maximum that should be allowed, and if this is achieved, the test is concluded with dDAVP administration. However, care should be applied with respect to the weight loss limit as certain patients, often but not invariably with primary polydipsia, may prepare for the test by considerable water loading. Alternatively, patients with severe DI may start the test grossly dehydrated. Basal serum and urine osmolality should be measured before the test is started, as a low serum sodium and/or osmolality ([Na]s < 135 mmol/L, [Osm]s < 275 mmol/kg) may imply prior water loading and therefore the limit on weight loss can be extended, whereas an elevated serum sodium and/or osmolality ([Na]s > 145 mmol/L, [Osm]s > 295 mmol/kg) with dilute urine at the start of the test should preclude initiation of water deprivation.
If the water deprivation part of the test is concluded without adequate urine concentration being achieved (urine osmolality < 600 mmol/kg or urine:serum osmolality ratio < 2:1), dDAVP should be administered intravenously or intramuscularly. A sample of urine is collected after 2 h and, if necessary, at intervals during the next 16 h to determine maximum achievable urine concentration. Patients are allowed to drink during this time, but intake should be limited: a total input over 16 h of not more than 1000 mL is recommended. This is particularly important in patients who have primary polydipsia, as otherwise water intoxication could ensue. For patients with severe NDI, however, this limit may be inadequate and some extension may be required, matching output to input. Problematic patients should, therefore, be admitted to hospital and kept under strict observation.
Some care is required in interpretation of the final results of urine concentration achieved following dDAVP. Any patient with a history of prolonged polyuria of whatever cause will have some impairment of urine concentrating ability because of renal medullary washout. Attempts have been made to establish diagnostic criteria based upon the percentage increase of urine concentration following exogenous AVP preparations and that achieved by water deprivation alone. Such fixed criteria do not take into account serum osmolality measurements during or after water deprivation and may lead to misclassification. The majority of patients under investigation will be patients who have CDI or primary polydipsia. These patients may only achieve urine concentrations of 400 mmol/kg following dDAVP but, in the case of CDI, serum osmolality at the end of water deprivation would be at or above the physiological range. If diagnostic confusion exists, then further information may be achieved by plasma AVP measurements during a repeat water deprivation test. Alternatively, a hypertonic saline infusion with serial plasma AVP measurements may be necessary.
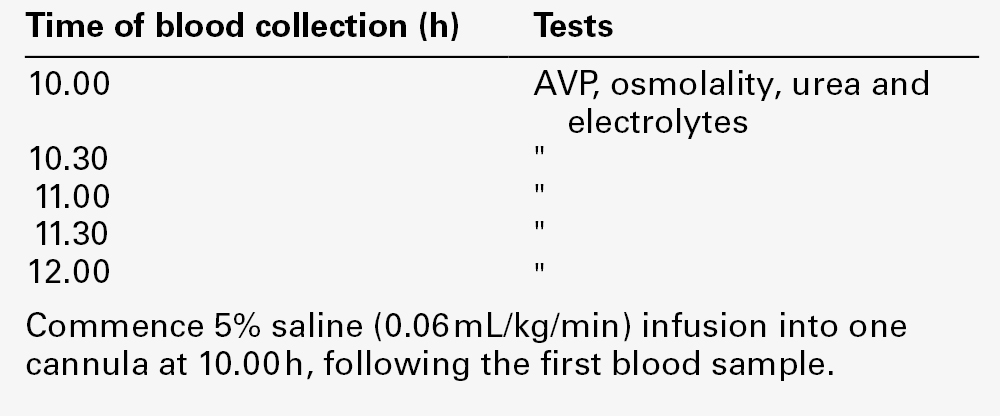
Hypertonic saline infusion
A protocol for hypertonic saline infusion is given in Appendix 4.2b. This test involves producing an acute elevation in serum sodium (~ 10 mmol/L) with frequent monitoring of plasma AVP concentration. Although acute extracellular osmotic changes should generally be avoided, the degree of change induced by the procedure is sufficient to induce significant and easily detectable increases in plasma AVP without complications; the morbidity of the test is extremely low. The major function of this test is to differentiate partial forms of CDI from primary polydipsia and from partial NDI. In CDI, the slope of the relationship between the plasma osmolality and the plasma AVP is reduced, but thresholds for thirst and AVP release are normal (see Fig. 4.5). The slope of this relationship remains normal in primary polydipsia and NDI.
Management of polyuria
The treatment of CDI has been revolutionized following the introduction of dDAVP. This analogue of AVP has a prolonged action and virtually no pressor activity. It is simple to administer either orally or nasally (by metered dose nasal spray). Patients tolerate the treatment extremely well and soon become practised in altering doses to meet their social requirements. Dilutional hyponatraemia is a potentially serious side-effect, but, in practice, is rarely encountered.
Because of the efficacy and patient acceptance of dDAVP, other forms of treatment have virtually disappeared. Lysine vasopressin (porcine antidiuretic hormone, available in oil as Pitressin) suffers the major disadvantage of native AVP in that, to achieve reasonable duration of action, a dose high enough to produce pressor effects may be required. Other drugs, which act by either enhancing renal responsiveness to AVP, for example chlorpropamide, or inhibiting renal diluting capacity, for example thiazide diuretics, are no longer used for CDI.
Primary polydipsia is a difficult condition to treat, but, in many instances, requires no primary treatment other than reasonable restriction of access to water. The major problem in this condition is the avoidance of antidiuresis and thus the potential for acute water intoxication.
The treatment of NDI is dependent on the cause. Acquired forms, such as those resulting from hypokalaemia or hypercalcaemia, require correction of the primary metabolic disturbance. Lithium treatment is probably the commonest cause of NDI, although the prevalence has fallen considerably since the recommended therapeutic range for serum lithium in the treatment of bipolar affective disorder has been reduced. Some reduction of polyuria can often be attained by reduction of lithium dose or by dividing doses of lithium to avoid very high peak serum concentrations. The renal resistance to AVP is related to serum lithium concentration. For intractable cases of lithium-induced polyuria, alternatives to lithium therapy should be sought.
Congenital NDI poses considerable problems of management. The severe polyuria may lead to enlarged bladder, hydroureter and hydronephrosis, and eventually to renal impairment, which ironically will reduce the symptoms. In addition, any failure to respond to the secondary polydipsia, such as may occur during illness or any degree of incapacity, will lead rapidly to hypernatraemia. The main treatment available until now has been thiazide diuretics, which inhibit the function of the diluting segment of the distal tubule, coupled with sodium restriction (< 1.5 mmol/kg per 24 h). This treatment can be used in combination with amiloride in older children, but is less well tolerated in the infant. Alternative combinations include a thiazide with a non-steroidal anti-inflammatory drug such as indometacin or a combination of thiazide with a cyclooxygenase-2 inhibitor. Treatments such as these can reduce urine flow rates to about one-third of pretreatment levels. New treatments are now under active investigation. Many of the loss of function mutations of AVPR2 lead to intracellular retention of the receptor (impaired trafficking to the basolateral membrane) rather than actual reduced affinity for AVP. Molecular chaperones, also known as ‘pharmacochaperones’, show promise as future therapies for such mutations. In addition, AVP-independent targeting of AQP 2 insertion into the apical membrane by selective E-prostanoid receptor agonists, may also prove to be a useful therapy.
Nocturnal polyuria
The study of renal diurnal rhythms of water and electrolyte excretion consistently demonstrates that, in health, both urine flow and electrolyte excretion are attenuated during sleep. Thus, it is expected that, unless significant water and/or electrolyte loading occurs immediately prior to retiring to bed, physiologically there will be no interruption to sleep due to the need to urinate. The terms nocturia and nocturnal polyuria are poorly defined. Nocturia as a descriptive symptom simply means the voluntary voiding of an indeterminate volume of urine at night (as opposed to enuresis, which is involuntary voiding). As a symptom, it can be extremely debilitating, as the frequent and persistent interruption of sleep can lead to severe fatigue. Nocturia is a common complaint of increasing prevalence with age in both men and women. One reason for the lack of consistency in the definition or investigation of nocturia, and in particular nocturnal polyuria, is that patients can present to a variety of medical and surgical specialties dependent on age, gender and on associated conditions, symptoms and subjective assessment.
Any disease process that reduces the functional capacity of the bladder or reduces the ability of the kidney to concentrate urine may give rise to daytime frequency and/or polyuria and nocturia. For some patients, however, nocturia is not associated with either daytime frequency or polyuria, but is associated with relative polyuria only at night. Box 4.6 lists the common causes of nocturnal polyuria.
Laboratory investigation and treatment of nocturnal polyuria
To diagnose nocturnal polyuria, it is first necessary to exclude daytime polyuria and urodynamic causes of frequency. The investigation of the osmoregulation of a patient with suspected nocturnal polyuria is difficult, not least because there are no universally accepted investigation protocols. Moreover, it is likely now that investigations would be expected to take place in an outpatient setting, rather than on a fully staffed and equipped clinical investigation unit. The simplest demonstration of the existence and severity of nocturnal polyuria is to perform a 12 h-split 24 h urine collection, with one 12 h session incorporating the whole period of sleep, for example collection one 09.00–21.00 h, collection two 21.00–09.00 h. A 12 h urine collection overnight normally contains approximately one-half of the total 24 h creatinine excretion, but much less than half the volume of overall solute excretion. More detailed study is possible but requires a high degree of patient cooperation in the outpatient setting. Patients can be instructed to collect a complete 24 h urine collection, while maintaining an accurate diary of voiding times, collecting individual aliquots of each voiding and, if possible, an accurate volumetric measurement of each voiding. Alternatively, estimates of the volume of each voiding can be made from the relative creatinine concentration of each timed voiding, particularly if the patient is willing to reduce diurnal variations in creatinine excretion by avoiding meat consumption over the period of study. Providing the patient is willing and capable of following such a protocol, detailed diurnal patterns of excretion of volume, sodium and osmoles (or, indeed, any measurable urine constituent) can be constructed. Figure 4.6 demonstrates the diurnal pattern of sodium excretion in a man presenting with severe nocturnal polyuria and a 12 h split urine volume of 0.57 L (daytime) to 1.75 L (night-time). This man was subsequently shown to suffer from severe obstructive sleep apnoea. Following treatment with continuous positive airways pressure (CPAP), the nocturnal polyuria resolved completely as the nocturnal natriuresis was reversed. Other causes of nocturnal polyuria, for which treatment of the primary cause is not possible or is insufficient to ameliorate symptoms, have been managed either with a loop diuretic taken 6 h prior to retiring to bed (to induce a relative sodium depletion at night) or in combination with night-time dDAVP.
FIGURE 4.6 Diurnal pattern of urine sodium excretion in a man presenting with severe nocturnal polyuria due to obstructive sleep apnoea. The shaded area shows the mean and 95% confidence limits of normal cumulative 24 h sodium excretion (… …, pre-treatment; …
…, pre-treatment; … …, post-treatment with continuous positive airways pressure).
…, post-treatment with continuous positive airways pressure).
Hypernatraemia
Mild hypernatraemia (serum sodium > 145 mmol/L) is not uncommon in hospital patients, especially in the elderly, but severe hypernatraemia (> 154 mmol/L) has an incidence of less than 0.2% of hospital admissions per annum. The causes of hypernatraemia are shown in Box 4.7.
Spurious hypernatraemia may occur due to sampling error, for example sampling from the same limb receiving a hypertonic sodium infusion such as 8.4% sodium bicarbonate. Spurious hypernatraemia may also occur because of post-collection contamination of the sample with sodium salts or because of evaporative losses after collection.
Acute sodium poisoning may also result in severe hypernatraemia (see p. 38), which is not initially due to water depletion, but will induce water depletion through natriuresis and diuresis, and will require correction with free water administration.
The major clinical causes of hypernatraemia are due to water deficit, either physiologically, when the desire to drink cannot be expressed, e.g. in neonates, the old or incapacitated or unconscious patients, or pathologically, e.g. with a history of polydipsia and thirst that is, for whatever reason, not satisfied.
Water deficiency with thirst
Adult patients with water deficiency, whose ability to ingest water is impaired by incapacity, will be oliguric (< 400 mL/24 h). The urine will be concentrated (osmolality > 1000 mmol/kg), although the capacity for urinary concentration is restricted in the neonate to ~ 500 mmol/kg and, in the adult, urine concentrating capacity declines in the elderly and may fall to < 700 mmol/kg over the age of 70.
In patients whose water deficiency is due to complete DI (cranial or nephrogenic), the urine is not concentrated. However, if the water deficiency is sufficient to reduce ECF volume and thus renal function significantly, the urine may not be maximally dilute, but will approximate to the osmolality of serum. In addition, in partial forms of DI, severe water deficiency may result in near maximally concentrated urine. In order to avoid diagnostic confusion, it is, therefore, advisable to correct any water deficiency prior to establishing the diagnosis with a water deprivation test.
Water deficiency without thirst
Patients rarely may present with severe hypernatraemia but no thirst or polyuria. The cause is a group of conditions known collectively as adipsic hypernatraemia and hypodipsic or essential hypernatraemia. The hypothalamic disorder causing hypodipsic hypernatraemia may be due to trauma, a primary or secondary tumour, a granuloma (e.g. sarcoid or histiocytosis) or vascular impairment, or the condition may be idiopathic. The aetiological similarity to those conditions causing CDI underlines the close proximity of osmoreceptors for AVP and thirst control within the hypothalamus. Four subtypes of hypodipsic hypernatraemia have been recognized (Fig. 4.7).
FIGURE 4.7 Relationship between plasma arginine vasopressin (AVP) and serum osmolality in patients with hypodipsic hypernatraemia: (A) normal regulation of AVP release; (B) partial defect in AVP release; (C) total defect; (D) reset osmostat. The dashed line represents the limit of detection of current AVP assays. Adapted from Robertson et al. 1982.
In type 1 (Fig. 4.7, line A), normal osmotic control over AVP release is retained but thirst appreciation is absent – primary adipsia. This disorder is rare, but demonstrates the separate osmoregulation of thirst and AVP. An example of this defect in a male patient with a history of chronic hypernatraemia is shown in Figure 4.8. During hypertonic saline infusion, he retained normal osmotic control over plasma AVP release, but subjective thirst assessment remained absent throughout. He had undergone hypothalamic surgery for severe behavioural disturbance and his serum sodium was found to be consistently > 150 mmol/L without any experience of thirst. Adipsic hypernatraemia has been described in up to 20% of patients surgically treated for craniopharyngioma.
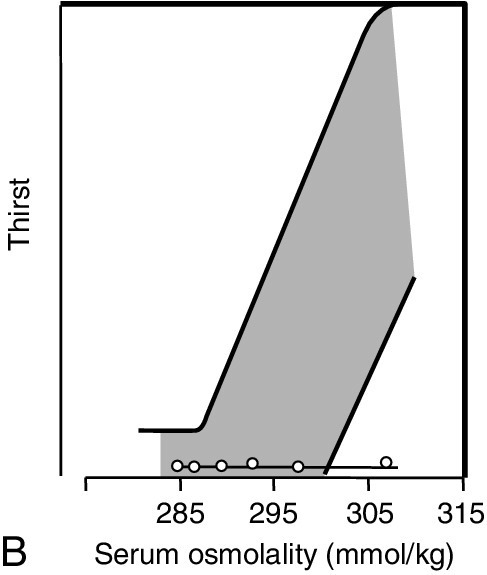
FIGURE 4.8 Osmoregulation in a patient with type 1 hypodipsic hypernatraemia syndrome. (A) Normal osmotic control of arginine vasopressin (AVP) release. (B) Complete absence of thirst stimulation despite significant osmotic stimulus. Shaded areas represent 95% confidence limits of normal response.
In type 2 hypodipsic hypernatraemia (Fig. 4.7, line B), the plasma AVP response is similar to that seen in patients with severe CDI, with a substantial loss of incremental gain of plasma AVP as serum osmolality rises. Unlike the situation in CDI, however, the osmotic thirst response is severely blunted. This group of patients is of interest, as the renal responsiveness to AVP is often considerably enhanced and patients are thus not polyuric. Urine concentration may approach the maximum when hypernatraemia is severe and patients retain the ability to dilute urine.
In type 3 (Fig. 4.7, line C), plasma AVP concentration is unresponsive to changes in serum osmolality, but is fixed at a low level. Osmotic thirst is entirely absent. Patients are, therefore, at risk of developing either hypernatraemia or hyponatraemia, depending on the prevailing fluid intake.
Type 4 hypodipsic hypernatraemia (Fig. 4.7, line D) represents true resetting of the osmostat for both plasma AVP response and thirst: the incremental gain for both remains normal and patients can concentrate and dilute urine normally, but around a higher setting of plasma sodium and hence serum osmolality. Severe hypernatraemia is generally not a feature, as dehydration does result in thirst. Thus, hypodipsia in these patients is only relative to the degree of thirst seen in normal subjects with corresponding degrees of hypernatraemia; the full range of thirst appreciation is maintained.
Management of hypernatraemia
Hypernatraemia in the incapacitated patient is invariably due to water depletion and should be corrected. Care should be exercised in the rate of correcting severe hypernatraemia in which the duration is greater than 48 h, and the rules on the rate of correction are directly comparable with those applicable to the correction of chronic dilutional hyponatraemia (see p. 51). The response of brain cells to persistent severe dehydration is to generate intracellular solutes, sometimes termed idiogenic osmoles or osmolytes. These are organic solutes that can be synthesized without apparent detrimental effect on cell structure or function. Recent nuclear magnetic resonance spectra of hypernatraemic human brains in vivo have identified myoinositol, scylloinositol, N-acetylaspartate and choline as osmolytes. Oral hydration with plain water is the simplest regimen (for estimate of deficiency, see Appendix 4.1c), but if oral or enteral fluids cannot be given, then hypotonic sodium or isotonic glucose solutions should be infused. It is strongly recommended that serum sodium is corrected at a rate of no greater than 12 mmol/L per 24 h; once corrected, any underlying polyuria should then be investigated as outlined in the previous section. A conscious patient with hypernatraemia, but without expressed thirst, is by definition suffering from one or other form of hypodipsic hypernatraemic syndrome. Although it is possible to differentiate pure adipsia (type 1) from other types of the syndrome by the use of tests of water deprivation and water loading (see Appendix 4.2), in practice the diagnosis can only be confirmed by the study of plasma AVP secretion and thirst in response to hypertonic saline infusion.
Management of hypodipsic hypernatraemia syndromes
The treatment of hypodipsic hypernatraemic syndromes varies considerably with type. Type 1, in which AVP release is normal but thirst is absent, and type 2, with a reduced pituitary release of AVP, enhanced renal response to AVP and blunted thirst response, both require a fluid intake regimen of more than 2 L/24 h for an adult to avoid hypernatraemia. Concentration of the urine should be avoided and at least one dilute urine should be passed each day. Type 3 poses the greatest problem of management, as fluid output and urine concentration cannot be used by the patient to gauge fluid balance. A standard regimented fluid intake is required and body weight needs to be accurately monitored each day to monitor fluid status. Frequent plasma monitoring is required to avoid the progressive development of hyper- or hyponatraemia. Type 4 requires no specific treatment, as alterations in fluid regimens will simply be handled in a physiologically normal way except at a higher osmotic setting than in normal individuals.
Hyponatraemia
Hyponatraemia (serum sodium concentration < 130 mmol/L) is common in hospital inpatients, with a prevalence of 2–3%. The lower reference limit for serum sodium is reduced by ~ 5 mmol/L in hospitalized patients as opposed to healthy controls. The majority of cases of hyponatraemia are mild, self-limiting and require neither treatment nor extensive investigation.
By definition, hyponatraemia implies an excess of water in comparison with sodium, although primary water excess is not always responsible for hyponatraemia. For example hyperglycaemia or the infusion of mannitol may induce significant fluid shifts from the ICF to the ECF. An estimate of the expected fall in serum sodium in relation to hyperglycaemia is given in Appendix 4.1d. However, this section is primarily concerned with states of water excess and with acute and chronic dilutional hyponatraemia: dilutional hyponatraemia is also defined as hyponatraemia with clinically normal ECF volume or normovolaemic hyponatraemia. The full classification of hyponatraemia is shown in Box 4.8.
Spurious hyponatraemia is due to in vivo or in vitro contamination of the specimen with water or fluid containing sodium at a concentration that is less than that of plasma. The commonest example seen in hospital practice is venesection from a limb concurrently receiving a dextrose infusion.
Pseudohyponatraemia (molar concentration) is due to the replacement of a portion of the plasma water space with either lipid or protein. This can occur in patients with severe endogenous or exogenous hypertriglyceridaemia and in patients with high plasma protein concentrations (usually due to paraproteinaemia). The concentration of sodium in plasma water (molal concentration) is normal, but each litre of plasma now contains less water. Serum osmolality (molal concentration) is normal.
Patients with hypovolaemic or hypervolaemic hyponatraemia present with signs of ECF volume depletion or excess. These conditions are discussed under disorders of sodium metabolism; they are usually differentiated clinically from dilutional hyponatraemias and also by the presence of biochemical evidence of the underlying cause, for example renal or hepatic dysfunction.
Although acute and chronic hyponatraemia may share certain common causes and the definition is somewhat arbitrary (acute hyponatraemia is of < 48 h duration), these conditions present and are managed in quite separate ways. This is because of the adaptation of cells, in particular brain cells, to chronic hypotonicity. The nature of this adaptation is summarized in Figure 4.9 and can, in part, be attributed to the reduction of cerebral osmolytes (see p. 45).
FIGURE 4.9 The major differences in brain cell volume in acute hyponatraemia and chronic hyponatraemia. In chronic hyponatraemia, cell osmotic content has been reduced.
Acute dilutional hyponatraemia
The main causes of acute dilutional hyponatraemia are shown in Box 4.9. Self-inflicted acute hyponatraemia has been described, but is extremely difficult to induce due to the enormous human capacity for renal water excretion – urine flow rates of 27.5 mL/min (equivalent to 39.6 L of urine in 24 h) have been described, although low osmotic loads for excretion or renal impairment can severely reduce water clearing capacity.
Psychogenic polydipsia (and the much rarer hypothalamic polydipsia) may result in acute hyponatraemia. However, the commonest cause of acute dilutional hyponatraemia seen in hospitalized patients is inappropriate postoperative fluid regimens involving the intravenous infusion of excessive volumes of low-sodium fluid (e.g. ‘dextrose saline’). The acute hyponatraemia following transurethral prostatectomy (TURP), known as the transurethral resection syndrome (TURS), is also worthy of specific mention, as it is the most serious acute hyponatraemia likely to present at the current time. In the development of TURS, irrigant fluid (1.5% glycine) is absorbed into the circulation via the open venous sinuses of the prostatic bed; glycine is used as it provides a non-electrolytic medium that will not dissipate the energy of cautery used in the resection, and the slightly hypotonic concentration provides an ideal optical interface. Risk factors for the development of the syndrome include a prolonged operative procedure, high irrigant hydrostatic pressure and significant blood loss. Strict control over these risks has resulted in a marked reduction in the incidence of this complication in recent years. If, however, sufficient irrigant is absorbed, this fluid will reduce plasma sodium concentration by simple dilution, but osmolality will not initially be reduced by an equivalent extent. An osmolal gap (see Appendix 4.1e,f) will initially develop as a result of high plasma glycine concentrations (up to 15 mmol/L), but unless the resultant free water is subsequently excreted, the osmolal gap will disappear as the glycine is metabolized. High plasma concentrations of both glycine and ammonia (which may result from the rapid metabolism of glycine) have been proposed to contribute to the severe deterioration in mental state that occurs in full-blown TURS. However, patients with hypoosmolal hyponatraemia respond rapidly to measures designed to treat acute water intoxication alone.
Acute hyponatraemia of sufficient severity to present clinically, with symptoms of headache, confusion and drowsiness, is a medical emergency, as the condition may lead to coma and death. Progressive clinical symptoms may be associated with signs of raised intracranial pressure, decerebrate posturing, fixed dilated pupils, bradycardia, hypertension and convulsions. All cells swell in size when placed in hypotonic medium: the change in size is particularly important with respect to brain cells confined within the fixed volume of the cranium (see Fig. 4.9). In acute hyponatraemia, adaptation of brain cell size cannot occur quickly enough to prevent the effects of cellular swelling and subsequent tentorial herniation with dramatic clinical sequelae. Females of reproductive age appear to be particularly prone to the effects of acute hyponatraemia.
Chronic dilutional hyponatraemia
Chronic dilutional hyponatraemia is common and has many possible causes (Box 4.10). The majority (excluding chronic hyponatraemia associated with beer potomania; see p. 49) can be related to a failure of water excretion, presumed to be due to a failure to suppress AVP secretion. There is good evidence that certain tumours (e.g. small cell (oat cell) carcinoma of the bronchus) possess the capacity to synthesize and secrete AVP, but in the majority of cases, the origin of the AVP is presumed to be the hypothalamic-posterior pituitary axis.
Patterns of plasma AVP response to increasing plasma sodium concentration induced by hypertonic saline infusion have been studied in chronic hyponatraemia. Four patterns of response have been described, as shown in Figure 4.10.
FIGURE 4.10 The four patterns of plasma arginine vasopressin (AVP) responses to changes in plasma osmolality induced by hypertonic saline infusion in patients with chronic dilutional hyponatraemia. (A) Random variation. (B) Resetting of osmostat. (C) Constant low-level release. (D) Hypovasopressinaemic antidiuresis. Shaded areas represent the inter-subject variation limits of normal response. The dotted line represents the limit of detection of current AVP assays. Data from Zerbe R L, Stropes L, Robertson G L. Vasopressin function in the syndrome of inappropriate antidiuresis. Annual Review of Medicine 1980; 31:315–327.
2. In type B, there is a normal response of AVP release to osmotic stimulation but it occurs at a much lower setting – a resetting of the osmostat. Theoretically, this condition could result from the adaptive response to chronic hyponatraemia as the osmotic content of brain cells falls. This condition has been described in association with tumours and various neurological disorders.
3. In type C, there is a constant low-level release of AVP, even when osmolality is suppressed below the normal threshold, but a qualitatively and quantitatively normal response above the threshold. This pattern may be seen transiently following pituitary/hypothalamic trauma.
4. In type D, there is suitable suppression of AVP concentration when the patient is hypoosmolal, suggesting either that immunologically distinct antidiuretic material is being secreted or that the renal tubules are somehow rendered more sensitive to extremely low concentrations of circulating AVP. This pattern of response is seen in patients with gain of function mutations of the AVPR2 gene.
Although this classification of AVP responses in chronic dilutional hyponatraemia assists pathophysiological understanding, no reliable relationship has been found between the aetiology of the condition and any one pattern of response, save for the gain of function mutations in the AVPR2 gene. The measurement of AVP in chronic dilutional hyponatraemia, therefore, for the vast majority of patients has no current diagnostic or prognostic function.
The syndrome of inappropriate antidiuretic hormone secretion
The term ‘syndrome of inappropriate antidiuretic hormone secretion’ (SIADH) was first coined by Bartter and Schwartz in 1957, to describe patients with severe hyponatraemia who were without evidence of renal failure, adrenal failure or saline depletion, but who had indirect evidence of persistent AVP secretion. The criteria for diagnosing the syndrome are shown in Box 4.11. Before this description, such patients were often misclassified as suffering from renal salt wasting because of the associated hyponatraemia and natriuresis. The original description thus provided a useful explanation of the biochemical findings and led to more rational therapeutic regimens.
Unfortunately, the term SIADH tends to be used universally to describe any acute or chronic dilutional hyponatraemia. Apart from certain causes of acute water intoxication and rare forms of chronic hyponatraemia, plasma AVP is detectable in the vast majority of patients with hyponatraemia of whatever cause. In addition, the criteria for diagnosis may produce anomalies, for example chronic dilutional hyponatraemia with reset osmostat (Fig. 4.10B) could fit the criteria when plasma osmolality is above the new threshold for AVP release, but fail to comply when plasma osmolality falls below this threshold, when maximally dilute urine is excreted. The term SIADH implies physiological understanding when often very little exists, and is a diagnosis that does not indicate immediate management or prognosis. Syndrome of inappropriate antidiuretic hormone secretion is a term that should now be replaced with more descriptive terminology, for example chronic dilutional hyponatraemia secondary to small cell carcinoma of the lung.
Sick cell syndrome
The association of sick cells and hyponatraemia was first explored by Flear and Singh in 1973, and it was an attempt to explore critically the assumption of common pathophysiology of the vast number of conditions grouped under SIADH, as well as to explain the hyponatraemia of seriously ill patients with conditions such as congestive cardiac failure (CCF), cirrhosis or serious pulmonary or CNS infections. One hypothesis was that sick cells leak normally non-diffusible solutes, but gain sodium, leading to extracellular hyponatraemia, but maintenance of serum osmolality. This combination of events may be observed in extremely ill patients after major surgery or burns, but has not been detected in the vast majority of patients with chronic hyponatraemia. However, a type of sick cell syndrome that is relevant to the development of chronic hyponatraemia is that resulting from a primary overall reduction in cellular osmotic content, through either increased loss or reduced production. Osmoreceptor cells so affected would result in control of AVP around a lower osmotic setting – a reset osmostat (SIADH type B). In practice, however, it is difficult to distinguish between a primary loss of cellular osmotic content (sick cells) or a secondary adaptive loss in response to a primary chronic dilutional hyponatraemia. Like SIADH, the term ‘sick cell syndrome’ should now be replaced by descriptive terminology, for example ‘chronic dilutional hyponatraemia secondary to chest infection’.
Low osmotic load hyponatraemia
One unusual cause of hyponatraemia requiring special mention is that associated with a low osmotic load for renal excretion. Beer potomania is an example of such a condition. It is caused by the consumption of a large volume of beer containing only very low quantities of electrolytes, coupled with a diet poor in protein and other minerals. Unlike starvation, in which osmoles such as ketones and urea are generated and excreted in the urine, the daily osmolal load for excretion in individuals at risk for beer potomania can be extremely low (< 250 mmol/24 h). Thus, if the minimum achievable urine osmolality is normal at about 50 mmol/kg, then, allowing for insensible fluid loss, a consumption of more than 6 L of such beer over the 24 h period will result in water retention independent of AVP secretion or responsiveness. Binges can result in acute hyponatraemia, although the more usual presentation is of a severe chronic hyponatraemia, occasionally complicated by plasma electrolyte changes secondary to alcoholic liver disease. The condition is unusual in the UK, probably because of the higher mineral content of traditional beers compared with continental beers, together with the tradition of serving bar snacks rich in sodium chloride. An association with excess cider consumption (cider potomania) has also been described, although this condition can only occur in an individual with normal urine diluting capacity if factory-type cider is consumed, rather than traditional craft cider. The former may have only the equivalent of 25% apple juice content, whereas the latter has 100% with a corresponding mineral content providing non-metabolizable osmoles in excess of 70 mmol/kg.
The concept of beer potomania as a cause of dilutional hyponatraemia is that of a mismatch between fluid intake and the capacity for excretion, independently of AVP secretion or responsiveness. This concept may have other parallels, for example in elderly patients treated with thiazide diuretics and with diets of low mineral content, adequate calories and relatively high fluid loads – the so-called ‘tea and toast’ diet. Thiazide diuretics act by blocking the reabsorption of sodium and chloride in the cortical diluting segment of the nephron. Thus, the capacity to generate maximally dilute urine is impaired and a concomitant ‘tea and toast’ diet can result in the development of severe dilutional hyponatraemia.
Cerebral salt wasting
The association of cerebral pathology, hyponatraemia and failure to retain urine sodium is termed cerebral salt wasting (CSW). This term was coined before the description and understanding of SIADH, in which there is also hyponatraemia and, during access to free water or following infusion of saline, a continued renal loss of sodium. Recent reports have attempted to re-implicate a primary renal sodium loss in some patients with cerebral pathology. Natriuretic peptide excess has been suggested as a mechanism. Caution is required, however, as many of such case reports invariably describe a euvolaemic, as opposed to hypovolaemic, hyponatraemia indistinguishable from hyponatraemia associated with SIADH. Differentiation of CSW from SIADH will become increasingly important as selective AVPR2 antagonists (vaptans) become more widely available and affordable (see p. 51). Vaptans are contraindicated in hypovolaemic hyponatraemia.
Laboratory investigation of hyponatraemia
Hyponatraemia is, by definition, a laboratory diagnosis, but the differentiation of hypovolaemic and hypervolaemic hyponatraemia is largely clinical, as is the differentiation between acute and chronic dilutional hyponatraemia.
Acute dilutional hyponatraemia may constitute a medical emergency, yet its laboratory investigation is often cursory and incomplete. The diagnosis is by clinical history of acute water loading without evidence of corresponding diuresis, and rapid deterioration in mental state, possibly coupled with signs of cerebral oedema. It is most important to confirm the serum sodium measurement, preferably with a fresh sample, ensuring that contamination of the specimen is avoided. The most important additional laboratory investigation is the measurement of serum osmolality, which will validate the serum sodium measurement or indicate the extent of any osmolal gap. Measurement of urine osmolality is superfluous, even if a sample is available.
The laboratory investigation of chronic dilutional hyponatraemia is predominantly to confirm the water-retaining state that has produced the condition and to exclude correctable metabolic causes. The serum electrolyte pattern in chronic dilutional hyponatraemia will usually show normokalaemia, but a hypochloraemia of corresponding degree to the hyponatraemia. The serum urea concentration is often low and is reduced not only by dilution, but also because of reduced tubular reabsorption. Serum urate concentration may also be low because of changes in tubular function, but this is not invariable and renal handling of urate may be directly affected by the cause of the hyponatraemia, for example a thiazide diuretic.
Urine measurements may be of confirmatory help in chronic dilutional hyponatraemia, but can be misleading. The urine is inappropriately concentrated, that is, it is anything other than maximally dilute. There is widespread belief that urine osmolality must exceed serum osmolality for the diagnosis of a water retaining state, but this is not the case. The concentration of urine is dependent on both water and osmolal content. An anorexic patient may have a total osmolal load of only 400 mmol/24 h to excrete, but if fluid intake is sufficient to produce 2 L of urine, then the overall urine concentration at balance is 200 mmol/kg. A urine concentration of 220 mmol/kg would produce a positive water balance, even though the urine is hypoosmolal to plasma.
One of the remarkable features of chronic dilutional hyponatraemia, especially when it is clearly demonstrated that plasma AVP concentrations are grossly elevated, is that patients do not form maximally concentrated urine. Patients with chronic dilutional hyponatraemia reach new steady states and then remain in sodium and water balance. Thirst thresholds appear to be downregulated to match the reduced plasma osmolality. Sudden increases or decreases in fluid intake may upset the steady state until a new one is reached. The mechanism of renal adaptation is probably related to downregulation of the expression of AQP 2 in the renal collecting ducts coupled to a reduction in the concentration gradient within the renal medulla.
The urine sodium concentration may also be of diagnostic value to differentiate hypovolaemic hyponatraemia if clinical signs are absent or misleading, but only if the sodium deficit is not renal in origin. Again, some care is needed, as chronic dilutional hyponatraemia is classically associated with a natriuresis, which can be defined essentially as anything other than maximal sodium retention. Often, when the patient is on free fluids, the natriuresis is very marked, with a urine sodium concentration > 100 mmol/L. But as a new steady state is approached, urine sodium excretion falls, and when fluid and sodium intake are restricted, urine sodium may fall to very low concentrations (< 10 mmol/L); this then indicates the total body sodium deficit that has developed secondarily to the natriuresis induced by initial positive water balance.
Other laboratory investigations recommended when the cause of the water-retaining state is not clear include thyroid function tests and a short tetracosactide stimulation test. There is a recognized, but variable, association of chronic hyponatraemia with hypothyroidism, and occasionally patients with primary or secondary adrenal failure present with nothing more specific than chronic dilutional hyponatraemia.
As previously stated, measurements of plasma AVP concentration are not normally of any assistance in the diagnosis of chronic dilutional hyponatraemia, but may be of value to determine if normal osmoregulation has been restored following a period of treatment. An indirect assessment of water handling using a water load test can also occasionally be used for this purpose (see Appendix 4.2c).
Management of hyponatraemia
The management of hypovolaemic hyponatraemia is primarily to restore blood volume and ECF volume to normal and to correct the underlying disorder. The management of hypervolaemic hyponatraemia is primarily to treat the underlying disorder and to use diuretic therapy when appropriate. Management of both these conditions is covered within the sections on sodium deficiency and excess.
The management of euvolaemic or dilutional hyponatraemia has created considerable controversy in recent years. This has its origins in the morbidity and mortality of uncorrected severe dilutional hyponatraemia (serum sodium ≤ 115 mmol/L), whether it is acute or chronic in nature. In the USA, a regimen of partial correction of hyponatraemia was adopted in the past, which resulted in patients’ serum sodium concentrations being increased rapidly to ~ 125 mmol/L. The rate of increase in sodium was usually in excess of 0.5 mmol/L per hour and often several-fold greater. Unfortunately, this regimen has become increasingly associated with the development of the neurological disorder known variously as central pontine myelinolysis (CPM), pontine and extrapontine myelinolysis or osmotic demyelination syndrome. The usual course of events in such cases is that the patient presents clinically with hyponatraemic symptoms, which can be either mild, such as weakness and confusion or severe, such as convulsions or coma. During the rapid correction of the plasma sodium concentration, these symptoms improve, but in the following few days the patient’s neurological condition deteriorates and further symptoms develop, including behavioural disturbances and convulsions and these, in turn, may lead on to the full-blown condition of CPM, including pseudobulbar palsy and quadriparesis. Further confusion as to the origin of the condition has been a continued, but unsubstantiated, association in the literature of CPM with uncorrected hyponatraemia.
Recommended management of symptomatic acute dilutional hyponatraemia
The biggest danger in this condition is failure to treat promptly. Water restriction has a supportive role, but must not be used alone. Cerebral oedema is the major complication, and acute elevation of plasma sodium using 5% sodium chloride infusion to produce a maximum increase of 12 mmol/L per 24 h or a serum sodium concentration of approximately 125 mmol/L (whichever is the lesser), is recommended. If an osmolal gap exists, then the infusion should increase serum osmolality to no greater than 255 mmol/kg. A formula to calculate the amount of sodium required is given in Appendix 4.1g. Short-term rates of infusion resulting in an increase of serum sodium up to 5 mmol/L per hour, have, in acutely symptomatic patients, been used successfully with dramatic clinical improvement and with no long-term sequelae. A simple general rule for patients falling within the definition of acute hyponatraemia and who are symptomatic is to match the rate of correction with the presumed rate of onset. Diuretics such as furosemide and mannitol may be of benefit, but lack the precision of control afforded by hypertonic saline infusion, and when used in combination with hypertonic saline infusion may make controlling the rate of correction more difficult.
Recommended management of chronic dilutional hyponatraemia
The cornerstones of management in chronic dilutional hyponatraemia are to prevent further falls in plasma sodium concentration, to treat any underlying condition, to alleviate any symptoms attributable to hyponatraemia and to avoid any therapeutic complications. Many patients who are in a steady state with mild hyponatraemia require no specific treatment. For those at risk of further falls in plasma sodium, water should be restricted to a degree sufficient to induce negative water balance (usually 500–800 mL of water per 24 h). Water restriction will increase plasma sodium concentration in anyone, but the rate of increase may be very slow, and if any degree of sodium depletion exists, an increase in plasma sodium will only be achieved at the expense of a contraction in ECF volume and a potential deterioration in renal function. Certain patients cannot tolerate fluid restriction, and for these, until recently, treatment with the tetracycline antibiotic demeclocycline (DMC) may have been considered. Arginine vasopressin receptor antagonists (collectively termed ‘vaptans’) are now commercially available. Tolvaptan is a selective vasopressin V2-receptor antagonist with an affinity for the V2-receptor greater than that of native arginine vasopressin. Treatment of chronic dilutional hyponatraemia commences with a single 15 mg oral dose and can be titrated up to a single daily dose of 60 mg, depending on response. Regular monitoring of serum electrolytes and renal function is strongly recommended. Tolvaptan should not be used in the treatment of acute dilutional hyponatraemia, which requires hypertonic saline infusion. The introduction of selective AVPR2 antagonists has rendered previous treatment options of DMC or lithium, obsolete.
In the very rare instances in which severe chronic hyponatraemia produces neurological symptoms, it may be considered prudent to raise the plasma sodium concentration acutely with hypertonic saline. If this is considered, the rate of infusion should be most carefully controlled with frequent monitoring of serum sodium. As in the management of acute hyponatraemia, it is recommended that serum sodium is raised to a concentration no greater than 125 mmol/L by this method. In this instance, however, the rate of infusion should produce a change in serum sodium of no greater than 0.5 mmol/L per hour to minimize the risk of the development of CPM: this is in contrast to the treatment of acute symptomatic hyponatraemia, in which the recommended 24 h rate and maximum concentration are the same, but the maximum rate for short periods may be much more rapid – up to 5 mmol/L per hour.
DISORDERS OF POTASSIUM METABOLISM
Potassium is predominantly an intracellular cation, but disorders of potassium metabolism are generally identified by measurements of extracellular potassium. Dramatic changes in plasma potassium concentration may occur as a result of transcellular shifts without necessarily any alteration of total body potassium. Clinical disorders are therefore classified on the basis of serum potassium concentration, rather than of potassium depletion or excess.
Hypokalaemia
The definition of hypokalaemia is not strict, but persistent serum potassium concentrations < 3.4 mmol/L (plasma 3.1 mmol/L) require investigation. The immediate clinical effects of hypokalaemia are on neuronal and muscular function: they result from an increase in the ratio of the intracellular to extracellular potassium concentrations. In addition, hypokalaemia, when associated with severe potassium depletion, affects the function of a wide variety of organ systems. Box 4.12 lists the possible clinical effects of hypokalaemia.
Causes of hypokalaemia
Hypokalaemia can be caused by redistribution of potassium (in vitro or in vivo), inadequate intake or excessive loss (renal or extrarenal).
Redistribution hypokalaemia in vitro
This form of hypokalaemia, sometimes known as spurious hypokalaemia, has been described in association with two clinical situations (Box 4.13). First, it may occur in patients with leukaemia and very high white blood cell (WBC) counts; if the blood is taken and allowed to stand at room temperature without separation, the WBCs take up extracellular potassium. The second situation in which this phenomenon may occur is if blood is taken from a diabetic patient who received intravenous insulin a few minutes prior to venesection; erythrocytes take up extracellular potassium, and if blood is subsequently left to stand at room temperature unseparated for 2 h or so prior to analysis, serum potassium concentration will decrease.
Redistribution hypokalaemia in vivo
The major causes of transcellular shift of potassium occurring in vivo are also shown in Box 4.13. Both alkalosis and increased plasma bicarbonate concentration without alkalosis will induce increased cellular uptake of potassium, as does endogenous or exogenous insulin. Catecholamines and β-adrenergic agonists such as adrenaline (epinephrine), salbutamol and fenoterol can all promote cellular uptake of potassium, and many patients admitted as acute medical emergencies may have significant, but transient, hypokalaemia, probably secondary to endogenous catecholamine release. Toxic chemicals such as toluene (involved in some forms of glue sniffing) and the ingestion of soluble barium salts (such as barium carbonate, which is used in certain pottery glazes, but has occasionally been mistaken for flour in cooking) have been implicated in redistribution hypokalaemia. It should be noted that barium salts used as contrast material for radiological investigations are not water soluble and do not pose a risk of toxicity.
Hypokalaemic periodic paralysis
This type of periodic paralysis occurs in two forms.
Familial hypokalaemic periodic paralysis (FHPP) is a rare autosomal dominant condition found most commonly in male Caucasians (male to female ratio 3:1 because of reduced penetrance and expression in females). The condition is characterized by attacks of flaccid paralysis affecting the limbs and trunk but rarely the facial and respiratory muscles; attacks commonly commence at night and patients present with weakness or paralysis on awakening. Attacks can last for up to 24 h and other acute manifestations of hypokalaemia such as cardiac arrhythmias may also be present. Spontaneous remission occurs with the re-establishment of normokalaemia. The attacks can begin in childhood, but often the onset may be delayed until the second decade of life. Periodicity of attacks is extremely variable between patients, with daily to yearly intervals (median four to six weeks). The attacks may be provoked by exercise followed by rest, a high carbohydrate intake, a glucose and insulin infusion, high sodium intake, adrenaline, glucagon administration and hypothermia. The total body potassium is unchanged during the attack, but potassium moves rapidly from the extracellular space into muscle. Mutations in three separate genes have now been implicated. The commonest mutations found are in the gene encoding the skeletal muscle voltage-gated calcium channel α-subunit (CACNA1S), which accounts for the majority of cases. Also described are mutations in the gene coding for the skeletal muscle voltage-gated sodium channel α-subunit (SCN4A) and the skeletal muscle voltage-gated potassium channel (KCNE3).
The management of FHPP is similar to that of any other hypokalaemia (see later), with either oral potassium supplements (up to 120 mmol/day) or, if necessary, intravenous administration, though particular care must be taken to avoid infusion of solutions containing glucose. Monitoring of serum potassium is important following the attack to ensure rebound hyperkalaemia does not occur. Prophylaxis against attacks includes a low carbohydrate diet, together with oral potassium supplements and the daily administration of spironolactone (100–200 mg), but probably most effective is the daily administration of acetazolamide (250–750 mg), although in patients with SCN4A mutations, acetazolamide may be contraindicated.
Hypokalaemic periodic paralysis with thyrotoxicosis (THPP) is a condition which primarily occurs in individuals of Chinese or Japanese extraction, but has been described in other races, including Caucasians and black people. The male preponderance is even more marked than in familial hypokalaemic periodic paralysis (FHPP) (20:1), and the age of onset is later (generally in the third decade). Links with mutations in the KCNE3 gene are described, but not in Chinese populations for whom links with mutations in CACNA1S have been made.
The clinical presentation of THPP is indistinguishable from the familial form, but the condition completely remits when the patient becomes euthyroid.
Extrarenal causes of potassium depletion
Box 4.14 lists the causes of extrarenal potassium depletion.
A low dietary intake of potassium as the sole cause of hypokalaemia is rare. Renal conservation can reduce urine potassium in normal subjects to < 5 mmol/L, so that without severe polyuria, a considerable lead-in period is necessary before clinically apparent hypokalaemia develops. Anorexia nervosa may, because of associated vomiting, speed the onset of clinically significant deficiency (see p. 55). One interesting cause of hypokalaemia is seen occasionally when patients with severe anaemia are treated with haematinics, resulting in a considerable and rapid increase in reticulocyte count; it has been speculated that, in certain patients, the degree of hypokalaemia that develops may induce cardiovascular death.
Excessive sweating is a potential cause of hypokalaemia. Sweat has relatively low potassium concentration (normally < 10 mmol/L), but subjects who undergo severe physical exertion in hot climates, or who exercise vigorously in saunas, can lose considerable volumes of sweat (up to 12 L in a day). Subjects undergoing such ordeals may be aware of the potential for severe sodium depletion without necessarily compensating for the associated potassium depletion.
The commonest causes of extrarenal potassium depletion are those involving loss of gastrointestinal fluid rich in potassium, in particular diarrhoea. Stool water is rich in potassium with concentrations of up to 90 mmol/L, although the normal daily volume of water lost by this route results in stool potassium losses of < 10 mmol/24 h. Diarrhoea of whatever cause results in an increase in stool weight and fluid content, so that severe diarrhoea may lead to stool volumes up to 2 L/24 h. Although potassium concentration in stool water tends to decrease as the volume increases, there is a limited colonic capacity for sodium/potassium exchange, and the daily losses of potassium in severe diarrhoea may exceed 100 mmol. As the stool water contains significant concentrations of bicarbonate, diarrhoea is often accompanied by a metabolic acidosis with hyperchloraemia. Thus, common causes of diarrhoea, such as bacterial causes, inflammatory bowel disease and diarrhoea associated with malabsorption syndromes, as well as less common causes, such as the watery diarrhoea–hypokalaemia achlorhydria (WDHA) syndrome – due to a pancreatic adenoma secreting vasoactive intestinal peptide (VIPoma) – are typically associated with a metabolic acidosis. Certain causes of diarrhoea, however, such as chloride-losing diarrhoea, Zollinger–Ellison syndrome (gastrinoma) and laxative abuse, are associated with a metabolic alkalosis. Typically, these conditions are associated with hypokalaemia because of associated renal, rather than gastrointestinal, potassium loss. Villous adenoma of the rectum results in the loss of large volumes of mucus, often rich in sodium, potassium and chloride. The acid–base association can, therefore, be variable depending on predominant losses and replacement.
Potassium may be lost from the gastrointestinal tract as a result of the use of cation exchange resins such as sodium polystyrene sulphonate (Resonium A). This material is used primarily to treat clinically important hyperkalaemia. Geophagia (soil-eating) can, under certain circumstances, result in the development of hypokalaemia, as clay in the soil may bind potassium in the gut, which then passes into the stool and is lost from the body.
Renal causes of potassium depletion
The classification of renal potassium depletion adopted here is based on the associated acid–base disorder.
Renal hypokalaemic acidosis
The causes of renal hypokalaemic acidosis with normotension are summarized in Box 4.15. Acidosis, with renal potassium loss, hypokalaemia and hyperchloraemia, is found in both distal (type 1) and proximal (type 2) renal tubular acidosis (RTA), as well as in RTA induced by the carbonic anhydrase inhibitor acetazolamide.
The need for urinary diversion from the bladder, as may occur following severely impaired capacity due to fibrosis or following cystectomy for carcinoma, presents the urological surgeon with four options.
2. Transplantation of the ureters into the intact sigmoid colon (ureterosigmoidostomy) was the original diversion operation involving the gastrointestinal tract, and is occasionally still performed when medical or religious objections preclude the fashioning of an alternative exit: the urine is passed with faeces via the rectum and anus. Potentially, ureterosigmoidoscopy is associated with significant metabolic complications (see below).
3. The commonest diversions now performed are those utilizing an isolated segment of bowel to form a conduit leading to the skin surface, which require a stoma bag or a constructed bladder fashioned to contain a valve to maintain continence.
4. Finally, if enough of the bladder is salvageable, then the wall may be augmented with opened segments of bowel (augmentation cystoplasty).
To understand the potential metabolic complications of potassium and other electrolytes that might arise from such procedures, it is necessary to know in some detail how the bowel normally handles the absorption of fluid and electrolytes. The jejunum normally rapidly absorbs fluid and electrolytes, but the electrolyte components, including potassium, will be absorbed only if there is a favourable concentration gradient; if the gradient is reversed then secretion of electrolytes into the lumen can occur. In the normal ileum, active sodium absorption occurs by sodium and chloride co-transport, which is coupled to bicarbonate excretion; potassium transport is passive. In the normal colon, there is a mixture of electrogenic sodium absorption with passive chloride absorption, together with sodium and chloride co-transport coupled to bicarbonate excretion, as occurs in the ileum. However, potassium is actively secreted into the lumen until the concentration rises to approximately 20–30 mmol/L.
As may be predicted, if bowel wall is exposed to urine, the prevalence of metabolic complications due to electrolyte redistribution will be based on the exposed surface area of bowel, the duration of contact and the type of bowel wall exposed. The diet and fluid intake will alter the urinary constituents and their concentrations, which can influence passive transport and concentration-limited active secretion. Any associated renal disease may have a direct metabolic effect. Thus, predictions in individual patients may be difficult but, in general, ureterosigmoidostomies give the greatest complication rate overall with lower rates for the other techniques. Diversions involving the sigmoid colon result in loss of bicarbonate and may result in loss of potassium, depending on the urine concentration of potassium entering the colon. Thus there is the potential for the development of significant hyperchloraemic acidosis with hypokalaemia.
Diversions involving ileal segments usually have low complication rates and, although hyperchloraemic acidosis may occur, hypokalaemia is rare. Diversions using jejunal segments are mentioned here for completeness, but the potential effect on potassium is quite different. Complications include sodium, chloride and bicarbonate depletion, with hyperkalaemia. For that reason, jejunal segments are rarely used for urinary diversion.
Diabetic ketoacidosis is associated with severe potassium depletion, but patients are usually hyperkalaemic or normokalaemic at presentation; if hypokalaemia is present, this is associated with extreme potassium depletion.
Renal hypokalaemic alkalosis
Potassium depletion with hypokalaemia and metabolic alkalosis can be further classified into normotensive and hypertensive conditions.
The causes of renal hypokalaemic alkalosis with normotension are summarized in Box 4.15. Loss of gastric fluid through prolonged vomiting, or drainage of fluid via nasogastric aspiration, results in hypochloraemic alkalosis with renal excretion of bicarbonate and potassium. The associated chloride depletion results in enhanced potassium secretion by the distal tubule (see Non-respiratory alkalosis, Chapter 5, p. 82).
Diuretics that act primarily on the thick ascending limb of the loops of Henle (furosemide, ethacrynic acid) or on the distal collecting ducts (thiazides, chlorthalidone) can result in chloride depletion, which can further enhance the direct diuretic induced urinary potassium loss. Laxative abuse is often associated with metabolic alkalosis and renal chloride wasting; it may also be associated with simultaneous, but unrecognized, diuretic abuse or with self-induced vomiting.
Congenital chloride-losing diarrhoea is a rare condition in which ileal chloride absorption is defective. Patients present within the first decade of life with watery diarrhoea and metabolic alkalosis. The diagnosis is made from the association of hypochloraemia (serum chloride usually < 90 mmol/L), alkalosis, low urine chloride concentration (< 20 mmol/L), but a high stool water chloride concentration (130–147 mmol/L). A similar clinical picture is seen in some patients with Zollinger–Ellison syndrome and in the rare condition of systemic mastocytosis secondary to basophil leukaemia; in the latter condition there is hypersecretion of gastric fluid due to histamine release, with diarrhoea, intestinal loss of chloride and secondary renal loss of potassium.
Rarely, cystic fibrosis (CF) may result in hypokalaemia in association with hypochloraemic alkalosis because of the high sweat chloride concentration in CF.
Bartter syndrome, a recessive autosomal condition, presents with hypokalaemic alkalosis in association with hyperreninaemic hyperaldosteronism. There is renal wasting of potassium and chloride, but patients are resistant to the pressor effects of angiotensin II and, thus, are normotensive or mildly hypotensive. Patients present in childhood with failure to thrive, unexplained hypokalaemia and, occasionally, renal impairment. Renal biopsy typically reveals hyperplasia of the juxtaglomerular apparatus. Occasionally, presentation is delayed until adulthood, at which time the major differential diagnosis is of diuretic abuse.
Bartter syndrome is caused by a loss of function mutation in one of the genes coding for three transport proteins located in the epithelial cells of the thick ascending limb of the loops of Henle – respectively the Na+-K+-2Cl− co-transporter (NKCC2), the potassium-secreting channel (ROMK1), which are both located on the apical membrane and the basolateral chloride channel (CLC-K6). Variations in phenotypic expression of Bartter syndrome have been explained by association with specific transport protein mutations, for example early presentation, associated hypercalciuria and impaired urine-concentrating ability are typically associated with apical transport proteins, whereas later presentation and normal urine calcium excretion are associated with CLC-K6 mutations.
Gitelman syndrome is an autosomal recessive condition presenting with hypokalaemic alkalosis, but, unlike Bartter syndrome, is associated with hypomagnesaemia and hypocalciuria. Gitelman syndrome is caused by loss of function mutations in the gene coding for the thiazide-sensitive Na+-Cl− co-transporter (NCCT), situated in the distal convoluted tubules. This disorder may be asymptomatic in childhood and then present in adult life with weakness, fatigue, paraesthesia and, rarely, tetany.
A detailed classification of conditions causing renal hypokalaemic alkalosis with hypertension is given earlier in this chapter (see Box 4.4, p. 37), but these are summarized in Box 4.15.
The association of hypokalaemic alkalosis with hypertension is due to functional mineralocorticoid excess, resulting in sodium retention and distal tubular potassium wasting with net acid excretion. Excess production of aldosterone may be primary (hyporeninaemic) due to adenoma, hyperplasia or, rarely, carcinoma of the zona glomerulosa of the adrenal cortex (see Chapter 18). Three forms of familial hyperaldosteronism are currently described. Familial hyperaldosteronism type I (FH-I), a rare autosomal dominant condition, is also known as glucocorticoid suppressible hyperaldosteronism. It is due to genetic recombination, resulting in the adrenocorticotrophic hormone (ACTH)-responsive region of 11-hydroxylase being incorporated into aldosterone synthase (hybrid CYP11B1/CYP11B2 gene mutation). FH-II is not glucocorticoid suppressible, appears to be up to five times more prevalent than FH-I and is clinically indistinguishable from apparent sporadic primary hyperaldosteronism. Family studies reveal linkage at chromosome 7p22. Candidate genes involved in cell cycle control are implicated as adrenal hyperplasia and adrenal adenomas are reported as common in FH-II patients. FH-III, also not glucocorticoid suppressible, has been described in a single affected family in which all three members had hypertension, hyperaldosteronism and high plasma concentrations of the hybrid steroids 18-hydroxycortisol and 18-oxocortisol. Only bilateral adrenalectomy could normalize blood pressure.
Secondary (hyper-reninaemic) hyperaldosteronism with hypertension is seen in patients with accelerated (malignant) hypertension and renal artery stenosis. Renin-producing tumours, such as benign haemangiopericytoma of the juxtaglomerular apparatus, are rare causes of hyper-reninaemic hyperaldosteronism.
Cortisol possesses only weak mineralocorticoid activity, but when plasma concentrations are sufficiently elevated, a mineralocorticoid effect results. Cushing syndrome, ectopic ACTH syndrome or the administration of exogenous glucocorticoid with mineralocorticoid activity can result in significant hypokalaemic alkalosis and hypertension.
Two forms of congenital adrenal hyperplasia result in excess production of deoxycorticosterone (DOC), which has mineralocorticoid action. These are 11β-hydroxylase deficiency and 17α-hydroxylase deficiency.
Pseudohyperaldosteronism can present in two forms. Liddle syndrome is a rare autosomal dominant condition that clinically resembles primary hyperaldosteronism, but in which aldosterone and renin are suppressed. The onset of hypertension and hypokalaemia occurs typically in childhood, but, even within a single family, penetrance can be extremely variable. The condition is caused by mutation within the gene coding for the collecting duct amiloride-sensitive epithelial sodium channel (ENaC), resulting in an ‘open’ channel, unregulated by the mineralocorticoid receptor (compared with loss of function mutations causing pseudohypoaldosteronism type 1, see p. 35).
Apparent mineralocorticoid excess has been described in children and, as in Liddle syndrome, there is hyporeninaemic hypoaldosteronism, but unlike Liddle syndrome, patients are responsive to spironolactone. This rare autosomal recessive condition is caused by impaired activity of the enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD) due to mutations within the HSD11B2 gene. This enzyme normally inactivates cortisol in the kidney by conversion to cortisone (see p. 29). The mineralocorticoid receptor has equal affinity for cortisol, and selectivity for aldosterone is normally maintained by 11β-HSD activity. An acquired form of this syndrome can be produced by excess ingestion of glycyrrhizic acid found in natural liquorice root, as well as glycyrrhetinic acid, the active component of the peptic ulcer drug carbenoxolone.
Renal hypokalaemia without specific acid–base disorder
There remains a miscellaneous group of conditions that result in renal loss of potassium, but without a specific acid–base disorder: these conditions are summarized in Box 4.15.
Several drugs can result in renal potassium loss. Penicillins, especially those administered in large molar quantities, e.g. carbenicillin, are excreted in anionic form in the urine and are associated with kaliuresis. Both cisplatin and the aminoglycoside group of antibiotics have been associated with hypokalaemia, probably because of associated magnesium depletion (see below).
Acute myelomonocytic leukaemia is associated with renal loss of potassium, especially in association with renal excretion of lysozyme released from the leukaemic cells.
Both the diuretic phase of acute kidney injury and that following the relief of urinary tract obstruction can result in a negative potassium balance with associated hypokalaemia.
Finally, there is increasing evidence that magnesium depletion, of whatever aetiology, is associated with an increase in renal potassium loss. Intracellular magnesium deficiency affects potassium excretion by reducing Na+,K+-ATPase activity proximally within the nephron, thus increasing distal sodium delivery and hence potassium secretion. In addition, magnesium deficiency reduces magnesium dependent inhibition of ROMK channels within the collecting duct principal cells, which also increases potassium secretion. In patients who are both potassium and magnesium depleted, supplementation with potassium will not result in correction of hypokalaemia unless magnesium is also supplemented.
Laboratory investigation of hypokalaemia
In the majority of patients presenting with hypokalaemia, the cause is clear from the clinical history. The role of the laboratory is then to provide a monitoring service for serum potassium and no further specific investigation is required. However, on occasion, patients presenting with inexplicable, consistent, severe hypokalaemia provide a considerable diagnostic challenge.
The first stage in differentiating the cause of hypokalaemia is to ensure that the serum potassium is a true reflection of in vivo concentration and not due to in vitro redistribution (see Box 4.13). A fresh sample of blood should be obtained for immediate separation and measurement of sodium and potassium concentration, and at the same time, renal function should be assessed by the measurement of serum urea or creatinine concentrations (or both), and magnesium should be measured to exclude concomitant magnesium depletion. If possible, blood should also be obtained for assessment of acid–base status. The clinical history, including a detailed past and present drug history, is essential. The next step in laboratory investigation is to differentiate renal from extrarenal causes of hypokalaemia by measuring urine potassium. The definition of renal potassium conservation is not absolute, but, in the presence of hypokalaemia, a 24 h potassium excretion of < 15 mmol and a random urine potassium/creatinine ratio of < 1.5 mmol/mmol provide strong evidence of extrarenal loss. The transtubular potassium gradient (TTKG) can also be used. TTKG is calculated by dividing the urine/serum potassium concentration by the urine/serum osmolality ratio (see Appendix 4.1h). The TTKG in renal conservation of potassium is expected to be < 2.
When potassium is not conserved by the kidneys, the presence of a metabolic acidosis would suggest one of the causes listed in Box 4.15 under renal hypokalaemic acidosis, although the majority of such conditions, excepting certain RTAs, should be apparent from the clinical history.
Hypokalaemia with metabolic alkalosis can be further differentiated by measurement of urine chloride. Again, no absolute criteria are available, but a random urine chloride < 20 mmol/L (or, with polyuria, 2 mmol/mmol creatinine) indicates renal chloride retention and implies chloride depletion. Those conditions listed in Box 4.15 as chloride-depleting include certain conditions that frequently provide a diagnostic challenge, such as diuretic abuse and surreptitious vomiting. If the urine chloride concentration exceeds 20 mmol/L, then Bartter syndrome, Gitelman syndrome and diuretic abuse (with recent diuretic ingestion) should be considered; in the presence of hypertension, those conditions associated with glucocorticoid or mineralocorticoid excess should be considered, although it should be remembered that hypertension is common, but mineralocorticoid-induced hypertension is not. (For full investigation of states of glucocorticoid or mineralocorticoid excess, see Chapters 18 and 38). Particular care should be exercised when interpreting urine electrolyte concentrations in hypokalaemic states. As mentioned previously, the concentration of potassium or chloride may be reduced if polyuria secondary to hypokalaemia is present. Therefore, it may be necessary to express concentrations in terms of creatinine or some other measure of urine concentration. The excretion or retention of electrolytes may be time-dependent; for example, diuretics may induce a kaliuresis and chloruresis following ingestion, which reverts between doses to a kaliuresis with chloride retention. If stopping the diuretic, with subsequent chloride, but not potassium replacement, induces urine potassium conservation, the implication is that there is extrarenal loss of potassium. In addition, two or more conditions may coexist, for example anorexia and vomiting, laxative abuse and diuretic abuse. Finally, severe potassium depletion may of itself induce renal chloride wasting, thus potentially, a severe extrarenal loss of potassium may result in renal potassium wasting.
Management of hypokalaemia
The decision to treat hypokalaemia will depend on the severity of the condition, the presence of symptoms, particularly muscle weakness, electrocardiographic evidence of cardiovascular effects and concomitant therapies, for example with cardiac glycosides, which increase cardiac sensitivity to hypokalaemia.
The magnitude of any potassium deficit can only be estimated. For hypokalaemia associated with a deficit rather than redistribution, a rough guide is that a serum potassium of 3.0 mmol/L equates with a total body deficit of 300 mmol and a serum potassium of 2.0 mmol/L, with a total deficit of 700–800 mmol.
Unless hypokalaemia is severe and potentially life-threatening (serum potassium < 2.5 mmol/L), then in general, oral replacement is preferred. Although certain fruit juices contain relatively high concentrations of potassium, the volumes required to provide sufficient potassium for replacement may be impracticable (see Table 4.6). Potassium salts are available in various formulations, including syrups, effervescent preparations and sustained-release tablets. Potassium chloride is usually the preferred salt, and potassium bicarbonate should only be used in hyperchloraemic states. Potassium citrate is usually prescribed to alkalinize the urine and reduce the discomfort of urinary tract infections, but can be substituted for potassium bicarbonate in the treatment of hypokalaemia. Currently, the preferred sustained-release formulation is a microencapsulated form, which spreads the release of the potassium salt within the gastrointestinal tract and minimizes the risk of ulceration and stricture formation. Oral replacement is usually prescribed in doses that equate with a normal adult dietary intake of between 40 and 120 mmol/day, although up to 200 mmol/day may be required and tolerated.
TABLE 4.6
Potassium content of oral preparations
| Preparation | Potassium content |
| Salt | (mmol/g) |
| Potassium chloride | 13.4 |
| Potassium bicarbonate | 9.9 |
| Potassium citrate | 9.2 |
| Fruit juice | (mmol/100 mL) |
| Tomato juice | 8.2 |
| Orange juice | 3.0 |
| Grapefruit juice | 3.0 |
| Apple juice (and craft cider) | 3.2 |
In states of severe hypokalaemia, or when oral replacement is not possible, potassium can be given intravenously into peripheral veins. The maximum rate of infusion should not usually exceed 20 mmol/h, although this limit can, with cardiac monitoring, be increased to 40 mmol/h. The maximum concentration of potassium in the infusion should not usually exceed 40 mmol/L. If it is necessary to minimize fluid intake, or if hypokalaemia proves resistant to such replacement, concentrations up to 80 mmol/L may be infused into a central vein.
Hyperkalaemia
There is no strict definition of hyperkalaemia, but persistent serum potassium concentrations > 5.3 mmol/L (plasma > 5.0 mmol/L) warrant further investigation. The most significant clinical effect of hyperkalaemia is on cardiac function, most importantly to cause cardiac arrest. This risk does not become appreciable until serum potassium is > 6 mmol/L, but is considerable at concentrations > 8 mmol/L, particularly if the increase is rapid. Electrocardiographic changes become apparent at lower concentrations of potassium and are more prominent if the hyperkalaemia is associated with hypocalcaemia, hyponatraemia, hypermagnesaemia or acidosis. Occasionally, patients present with a form of ascending muscular weakness resembling the Guillain–Barré syndrome. Box 4.16 shows the major clinical effects of hyperkalaemia.
Causes of hyperkalaemia
Hyperkalaemia may reflect an inappropriate retention of potassium within the body or an alteration in the distribution of potassium intra- and extracellularly. This alteration in distribution may occur in vivo, or in vitro, when there has been redistribution of potassium from blood cells to serum during the time between venesection and separation of serum.
Redistribution hyperkalaemia in vitro
This form of hyperkalaemia is sometimes known as spurious or pseudohyperkalaemia (Box 4.17). The most common form is due simply to in vitro loss of potassium from erythrocytes, and may or may not be associated with visible haemolysis. This type of spurious increase in serum potassium is also seen in certain patients with leukaemia with extremely high WBC counts (> 100 × 109/L), and in patients with thrombocythaemia when platelet counts exceed 1000 × 109/L; in both situations the cells are fragile and lyse during blood clotting. To exclude these causes in patients with leukaemia or thrombocythaemia, blood should be collected into a heparinized container and the plasma separated quickly from the cells at room temperature.
Whole blood samples stored at 4°C will eventually release potassium from red cells into the plasma, without necessarily any evidence of haemolysis. In certain families, this tendency is greatly accentuated, giving rise to the condition familial pseudohyperkalaemia, which is due to disordered cation transport in the erythrocyte membrane.
Redistribution hyperkalaemia in vivo
The major causes of transcellular shift of potassium occurring in vivo are also shown in Box 4.17. As previously discussed, acidosis, particularly that induced by mineral acid, will result in increased extracellular potassium concentration. Acidosis and hypertonicity due to hyperglycaemia and insulin deficiency lead to the hyperkalaemia frequently seen in potassium-depleted diabetic patients with ketoacidosis.
Various drugs may promote or accentuate hyperkalaemia. Succinylcholine, a depolarizing muscle relaxant, results in some increase in plasma potassium in all subjects, but this is particularly evident in patients with increased total body potassium. Thus succinylcholine should be avoided in all patients who are hyperkalaemic. β-Adrenergic blocking agents have been associated with minor increases in plasma potassium concentration, but this may be greatly accentuated during vigorous exercise.
The release of potassium from cells during strenuous exercise is well described, and a similar phenomenon may occur locally in an ischaemic limb, such as may happen during prolonged venous stasis prior to venesection. Hyperkalaemia has been described in patients undergoing chemotherapy for malignancy, which causes massive lysis of neoplastic cells. This phenomenon has been described in the treatment of chronic lymphocytic leukaemia, acute lymphoblastic leukaemia and lymphosarcoma. Its occurrence emphasizes the need for maintenance of hydration and for careful electrolyte monitoring during aggressive chemotherapy. Acute haemolytic disorders can give rise to hyperkalaemia by a similar mechanism.
Hyperkalaemic periodic paralysis
Hyperkalaemic periodic paralysis (HYPP) is a rare autosomal dominant condition that presents with attacks of muscular weakness, paralysis (usually sparing the respiratory muscles) and an associated acute rise in plasma potassium (up to 8.0 mmol/L). The periodicity of attacks is variable, ranging from daily to only a few attacks a year. This type of periodic paralysis is provoked by a high potassium intake, glucocorticoids, hypothermia and during the recovery phase after vigorous exercise. During attacks, electrocardiographic monitoring shows tall T waves but cardiac arrhythmias are rare. The disorder is due to gain of function mutations in the skeletal muscle voltage-gated sodium channel X subunit (SCN4A) gene. The management of HYPP is administration of the β2-agonist salbutamol, which can easily be taken by inhalation. Salbutamol has also been used prophylactically.
Potassium retention
The major causes of potassium retention are listed in Box 4.18. An increased potassium intake alone, without associated renal impairment, in practice only occurs as an iatrogenic complication of inappropriate intravenous loading. Excessively high oral loads of potassium are counteracted by a combination of reduced gastrointestinal absorption, vomiting and diarrhoea.
A reduction in glomerular filtration rate (GFR), of whatever cause, increases the risk of development of hyperkalaemia. However, on a normal diet containing approximately 100 mmol of potassium per day, GFR may fall to less than 10 mL/min before a significant risk of hyperkalaemia arises, and increased colonic secretion of potassium may further protect the patient when GFR is reduced further. It is important, however, that patients with significantly reduced GFR avoid high intakes of potassium or any condition that results in endogenous shifts of potassium.
Decreased tubular secretion of potassium may occur in response to treatment with the potassium-sparing diuretics: spironolactone, triamterene or amiloride. Hyperkalaemia is a particular risk when such a drug is used in a patient with impaired renal function or in a patient with a high potassium intake. Urinary diversions that involve jejunal segments (see p. 55) result in a significant incidence of hyperkalaemia due to reabsorption of potassium from the urine while contained within the segment.
Syndromes of hypoaldosteronism
This broad group of conditions is summarized in Table 4.7. Primary hyper-reninaemic hypoaldosteronism is found in Addison disease (see Chapter 18) and the rare condition corticosterone methyl oxidase deficiency, in which aldosterone synthesis is impaired. Heparin given in continuous high doses can also inhibit aldosterone synthesis through inhibition of 18-hydroxylase and possibly by inducing atrophy in the zona glomerulosa.
TABLE 4.7
Syndromes of hypoaldosteronism with relative plasma renin activities and aldosterone concentrations (↑, increased; ↓, decreased; N, normal)
| Syndrome | Renin | Aldosterone |
| Primary hypoaldosteronism | ||
| Addison disease | ↑ | ↓ |
| Isolated aldosterone deficiency | ↑ | ↓ |
| Heparin treatment | ↑ | ↓ |
| Congenital adrenal hyperplasia | N to ↑ | N to ↓ |
| Angiotensin-converting-enzyme inhibition | N to ↑ | N to ↓ |
| Hyporeninaemic hypoaldosteronism | ↓ | ↓ |
| Secondary tubular disorders | N | N |
| Pseudohypoaldosteronism | ||
| type I | ↑ | ↑ |
| type II | ↓ | N to ↓ |
Two forms of congenital adrenal hyperplasia (CAH) result in impaired mineralocorticoid synthesis. 21-Hydroxylase deficiency is the most common form of CAH. Virilization is characteristic, but not all patients exhibit features of hypoaldosteronism; up to two-thirds develop renal sodium wasting with hyperkalaemia. 3β-Hydroxydehydrogenase deficiency is a rarer condition, in which the majority of patients have renal sodium wasting and hyperkalaemia.
Hypoaldosteronism may occur in patients receiving angiotensin-converting enzyme (ACE) inhibitors such as captopril. Patients at particular risk of developing hyperkalaemia include those with associated renal impairment or with high renin concentrations, for example resulting from congestive heart failure or renal artery stenosis.
Hyporeninaemic hypoaldosteronism is an increasingly recognized syndrome in which patients present with hyperkalaemia out of proportion to a reduction in GFR. The majority of such patients develop a hyperchloraemic metabolic acidosis and fall, therefore, into the classification of type 4 renal tubular acidosis (see Chapter 5). The syndrome is particularly prevalent in elderly patients with type 2 diabetes, but is also found in many other diseases in association with interstitial nephritis, including systemic lupus erythematosus, multiple myeloma, chronic obstructive uropathy, gout, sickle cell disease, lead nephropathy, following renal transplantation and in association with treatment with prostaglandin synthetase inhibitors and treatment with ciclosporin. Plasma renin activity is reduced, as is aldosterone concentration. Renin response to upright posture or to salt depletion is also reduced. The pathogenesis is not fully understood, but includes structural damage to the kidney, including the juxtaglomerular apparatus, and there is evidence in diabetes of impaired conversion of renin precursor to active renin. One intriguing aspect of hyporeninaemic hypoaldosteronism is why hyperkalaemia occurs, as aldosterone secretion is known to be stimulated by hyperkalaemia. However, it is likely that this direct stimulation of aldosterone can only occur in the presence of angiotensin II. An important aspect of this syndrome is that, because it occurs more frequently in the elderly, drugs that suppress both renin and aldosterone, such as β-blockers and calcium channel blockers as well as prostaglandin synthetase inhibitors, should generally be avoided in this age group.
Interstitial nephritis may give rise to hyperkalaemia in which aldosterone secretion is not suppressed. The pathogenesis in these conditions is presumed to be a direct impairment of tubular secretion of potassium. Many of the diseases associated with tubular dysfunction are those that are also associated with the development of hyporeninaemic hypoaldosteronism and include obstructive uropathy, sickle cell disease and systemic lupus erythematosus. The main clinical difference between direct tubular dysfunction and hyporeninaemic hypoaldosteronism is the failure of the former condition to respond adequately to mineralocorticoid replacement, and alternative treatments such as thiazide diuretics must be used.
Pseudohypoaldosteronism (PHA) is a term applied to a heterogeneous group of rare disorders linked by association with hyperkalaemia, metabolic acidosis, normal glomerular function and apparent renal tubular unresponsiveness or resistance to mineralocorticoids. The conditions are broadly segregated into PHA types I and II.
Pseudohypoaldosteronism type I can be inherited either as an autosomal dominant or autosomal recessive trait. Pseudohypoaldosteronism type II, alternatively known as Gordon syndrome, is inherited as an autosomal dominant condition. The major characteristics of these syndromes are summarized in Table 4.8.
Laboratory investigation of hyperkalaemia
The first stage in any laboratory investigation of hyperkalaemia is to ensure that the serum potassium is a true reflection of in vivo concentration. The storage of whole blood specimens in a refrigerator at 4°C is widely practised by clinicians in the belief that this will aid the preservation of a specimen, but this practice can greatly increase serum potassium without any evidence of haemolysis. This effect is particularly noticeable in familial pseudohyperkalaemia, but can affect any blood sample if storage is prolonged for 8–12 h. As previously mentioned, hyperkalaemia in patients with high WBC counts or platelet counts should be confirmed in freshly separated plasma, rather than serum.
Having confirmed true hyperkalaemia, the clinical history is required with particular emphasis on drug and dietary regimens, with information being sought regarding possible causes of in vivo redistribution.
Assessment of blood acid–base status and glucose concentration may be valuable. Serum potassium is usually measured with other electrolytes and markers of renal function, including urea and creatinine. When GFR is reduced to < 10 mL/min, hyperkalaemia is likely to develop unless dietary potassium is restricted. The measurement of urine potassium output is of marginal value except possibly in steady-state conditions when a 24 h urine potassium will provide evidence of excessive ingestion.
If GFR is not reduced sufficiently to explain the hyperkalaemia, a syndrome of hypoaldosteronism should be considered. The measurement of TTKG may provide useful information (see Appendix 4.1h). The expected physiological response in hyperkalaemia would result in a TTKG of > 10. A value for TTKG of < 7 is compatible with hypoaldosteronism. Normally, the clinical presentation of Addison disease is sufficiently characteristic to require only studies of cortisol response to exogenous ACTH preparations, but other forms of hypoaldosteronism require measurement of renin and aldosterone. Unfortunately, the various combinations of findings do not fit snugly with clinical classification of disorders. Hyper-reninaemic hypoaldosteronism is found in primary hypoaldosteronism, the exceedingly rare condition of corticosterone methyl oxidase deficiency, some forms of CAH, and with ACE inhibitor treatment. Hyporeninaemic hypoaldosteronism is found in the conditions grouped under the syndrome of the same name and is also found in the rare PHA type II (Gordon syndrome). In contrast, high renin and high aldosterone are found in PHA type I. However, normal renin concentrations and aldosterone activity may be found in those conditions causing interstitial nephritis with direct tubular inhibition of potassium excretion. Renin and aldosterone values are not always easy to interpret, as high plasma potassium directly stimulates aldosterone and suppresses renin activity. In addition, aldosterone concentration increases as the GFR falls without a corresponding change in renin activity. In situations in which diagnostic difficulty may exist, for example in hyporeninaemic hypoaldosteronism, it may be necessary to reassess aldosterone concentrations when plasma potassium has been reduced to within the reference range; alternatively a trial of mineralocorticoid replacement will differentiate hyporeninaemic hypoaldosteronism from direct tubular dysfunction.
Management of hyperkalaemia
Hyperkalaemia, particularly when severe (> 6.0 mmol/L), is a serious condition requiring immediate treatment because of the risk of sudden death. Emergency management is intravenous 10% calcium gluconate – 10 mL injected over 60–120 s and repeated every 15 min or so until the electrocardiographic changes improve (maximum dose 50 mL). This does not correct the hyperkalaemia, but is directly cardioprotective. In patients receiving digoxin, calcium gluconate should be infused more slowly – 10 mL over 30 min – to avoid digoxin toxicity induced by hypercalcaemia.
Two therapeutic regimens are available to lower plasma potassium rapidly. Glucose (50 mL of 50% glucose) can be infused over 15 min together with ten units of soluble insulin. This regimen may be repeated at hourly intervals and should be accompanied by serum potassium and plasma glucose monitoring. Alternatively, plasma potassium may be reduced by the infusion of 50–100 mL of 4.2% sodium bicarbonate (500 mmol/L) over a 15–30 min period (provided that the patient is not sodium overloaded).
For haemodialysis patients with hyperkalaemia, a regimen that has been shown to be of equal benefit to glucose and insulin in reducing plasma potassium is treatment with the β2-adrenergic agonist salbutamol. Inhalation of nebulized salbutamol (10–20 mg) will reduce plasma potassium by approximately 1 mmol/L within 30 min.
If hyperkalaemia is the result of increased body stores of potassium, then this excess must be removed. The relationship of plasma potassium to excess body potassium is highly variable and is not reliably predictable. As an approximation, elevation of plasma potassium by 1 mmol/L above normal without evidence of redistribution will roughly equate with a 200 mmol total excess. Polystyrene sulphonate resins (sodium or calcium salts) may be given by mouth (15 g, 3–4 times daily in water) or as an enema (30 g in methylcellulose, retained for 9 h). As an approximation, each gram of resin removes 1 mmol of potassium, so that up to 60 mmol may be removed in each 24 h period. For more dramatic reductions in body potassium, renal replacement treatment is required. Peritoneal dialysis is capable of removing 10–15 mmol of potassium each hour, whereas the efficiency of haemodialysis will allow up to 30 mmol to be removed each hour.
If a reversible cause of hyperkalaemia cannot be identified, then therapy for chronic hyperkalaemia should be designed to minimize the recurrence of severe hyperkalaemia. This should include a reduction in dietary potassium intake (< 50 mmol/day) and avoidance of volume contraction and drugs that cause redistribution hyperkalaemia. Sodium bicarbonate and thiazide diuretics may be useful and, in some cases, treatment with mineralocorticoids.
CONCLUSION
The physiological control over sodium, water and potassium within the human body is a complex interrelated series of systems of extreme precision and sensitivity. These systems regulate the extracellular fluid volume, the extra- and intracellular solute content, the intracellular volume and neuromuscular function, and, therefore, indirectly influence myriad functional and metabolic processes essential for life. The pathological causes and consequences of recognized abnormalities in the control of sodium, water and potassium have been explored in this chapter, together with the details of diagnosis and treatment.
Further reading
APPENDIX 4.1 FORMULAE
Formulae (a)–(e) provide approximations only and are supplied as illustrative guidelines. Any corrective procedure based upon any of these formulae should be accompanied by detailed clinical and laboratory monitoring.
[Concentrations]: serum (s), plasma (p) or urine (u) in mmol/L or mmol/kg (osmolality).
(a) Estimate of reduction in ECF volume from rise in haematocrit (HCT) when no blood loss has occurred
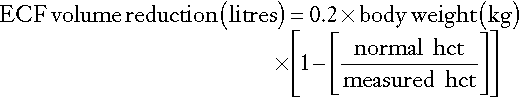
(b) Estimate of sodium deficit in patients with hypovolaemic hyponatraemia
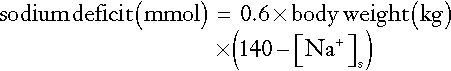
(c) Estimate of water deficit in hypernatraemia
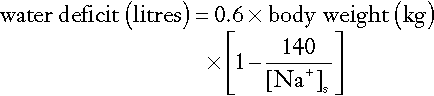
(d) Estimate of the expected sodium depression in hyperglycaemia-induced hyponatraemia
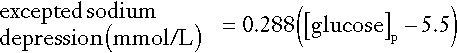
Reference
Adapted from Katz MA. Hyperglycemia-induced hyponatremia – calculation of expected serum sodium depression. N Engl J Med 1973;289:843–4.
(e) Calculation of serum osmolality
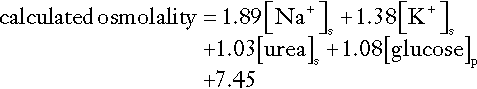
Reference
Adapted from Behagat CI, Garcia-Webb P, Fletcher E, Beilby JP. Calculated vs measured osmolalities revisited. Clin Chem 1984;30:1703–5.
(f) Calculation of osmolal gap
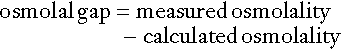
(g) Estimate of sodium required in acute water intoxication
(To increase serum sodium to 125 mmol/L):
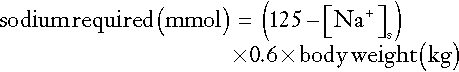
(Hypertonic saline (5%) = 855 mmol/L)
(h) Calculation of the transtubular potassium gradient (TTKG)
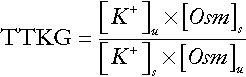
Reference
Adapted from Ethier JH, Kamel KS, Magner PO, Lemann Jr J, Halperin ML. The transtubular potassium concentration in patients with hypokalemia and hyperkalemia. Am J Kidney Dis 1990;15:309–15.
APPENDIX 4.2 DYNAMIC FUNCTION TESTS
(a) Water deprivation test
This test is used to differentiate between cranial diabetes insipidus, nephrogenic diabetes insipidus or primary polydipsia as causes of polyuria.
The patient is denied fluid and sloppy food from 08.30 h onwards. All patients should be observed closely to prevent covert access to fluid. The following protocol should be followed. Urine should, if possible, be collected hourly, the volume recorded and an aliquot tested for osmolality. Blood should be obtained for serum sodium and osmolality measurements. Accurate weight recordings are required to monitor loss and, in certain cases, detect surreptitious fluid consumption.
| Time of urine collection (h) | Time of blood collection (h) | Time of weighing (h) |
| 09.00–10.00 | 09.00 | 09.00 |
| 10.00–11.00 | – | – |
| 11.00–12.00 | – | – |
| 12.00–13.00 | 12.00 | 12.00 |
| 13.00–14.00 | – | – |
| 14.00–15.00 | – | 14.00 |
| 15.00–16.00 | – | – |
| 16.00–17.00 | 17.00 | 17.00 |
Notes
• If serum osmolality exceeds 295 mmol/kg and/or serum sodium exceeds 145 mmol/L, the test should be discontinued and the vasopressin test performed.
• If the test runs to completion and the urine osmolality remains below 600 mmol/kg, the supplementary vasopressin test should be performed.
Vasopressin test
Give 2 μg of dDAVP i.v. The patient is allowed to drink, but total fluid intake should be restricted to 1000 mL until 09.00 h the next morning, unless the patient’s weight loss continues above 3%, when free fluids should be allowed. Further urine collections are made the same day at 19.00 and 22.00 h, and on the following day at 07.00 and 09.00 h.
Interpretation
A normal subject will concentrate urine to > 600 mmol/kg during the period of water deprivation, and the serum osmolality will remain within the physiological range (or, more strictly, the urine:serum osmolality ratio will be greater than 2:1). If urine osmolality fails to increase to > 600 mmol/kg, but increases following dDAVP by > 20%, then cranial diabetes insipidus (CDI) is likely. The weight loss recorded should always equate with total urine volume passed.
Reference
This protocol is modified from the original description of the ‘short’ water deprivation test by Dashe AM, Cramm RE, Crist AC, Habener JF, Solomon DH. A water deprivation test for the differential diagnosis of polyuria. J Am Med Assoc 1963;185:699–703.
(b) Hypertonic saline infusion
This test is used to directly assess the osmoregulatory control of the release of plasma AVP and/or to assess the control of subjective thirst.
The patient is food-fasted overnight (12 h), but is allowed free access to water. Smoking is not allowed during this 12 h period or during any part of the test. No fluid of any kind should be consumed during the test, including sips, mouthwashes or ice cubes: all of these oropharyngeal stimuli can suppress the release of arginine vasopressin (AVP) from the posterior pituitary.
Pre-infusion preparation
| 09.00 h | Weigh | |
| Supine position | ||
| Indwelling cannulae with both antecubital veins kept patent with heparinized saline | One for eventual infusion of hypertonic saline, one for blood sampling | |
| 09.00–10.00 h | Rest hour |
Infusion protocol
For interpretation of results, see Figure 4.3 and text.
Notes
• Information concerning the handling of blood samples for AVP should be obtained from the laboratory. In general, samples should be collected into pre-chilled heparin tubes, transported on ice to the laboratory immediately, centrifuged rapidly at 4°C and the plasma stored at a maximum temperature of − 20°C (preferably − 70°C). The time from collection to storage should not usually exceed 20 min.
• Patients with a history of congestive cardiac failure should be closely monitored and, if necessary, the test curtailed and furosemide (40 mg i.v.) administered.
Reference
Adapted from Robertson GL, Athar S. The interaction of blood osmolality and blood volume in regulating plasma vasopressin in man. J Clin Endocrinol Metab 1976;42:613–20.
(c) Water load test
This test is used to assess osmoregulation indirectly by determining the renal response to water loading.
The patient is allowed free access to fluid 12 h prior to the test to ensure adequate patient-determined hydration at commencement. No smoking is allowed during the test period or for the 12 h prior to this. Adequate glucocorticoid replacement is required for patients with hypoadrenalism.
At 09.00 h, the bladder is emptied and an aliquot of urine (10–15 mL) saved for osmolality measurement. Blood (for urea and electrolytes, and osmolality) is collected and the patient weighed. An oral water load (20 mL/kg) is then consumed within 20 min. Further samples and weighings are obtained according to the following schedule. Urine volume output is measured accurately each hour.
Interpretation
In a normally hydrated subject, over 80% of a standard water load is excreted within 4 h of ingestion. Urine osmolality will typically fall to < 100 mmol/kg. Weight changes should confirm the observed difference between the water volume ingested and the urine volume loss. Difficulties in micturition may make the test difficult or impossible to interpret.
Reference
Penney MD, Murphy D, Walters G. Resetting of the osmoreceptor response as a cause of hyponatraemia in acute idiopathic polyneuritis. Br Med J 1979;2:1474–6.