Embryology
Early Embryonic Development
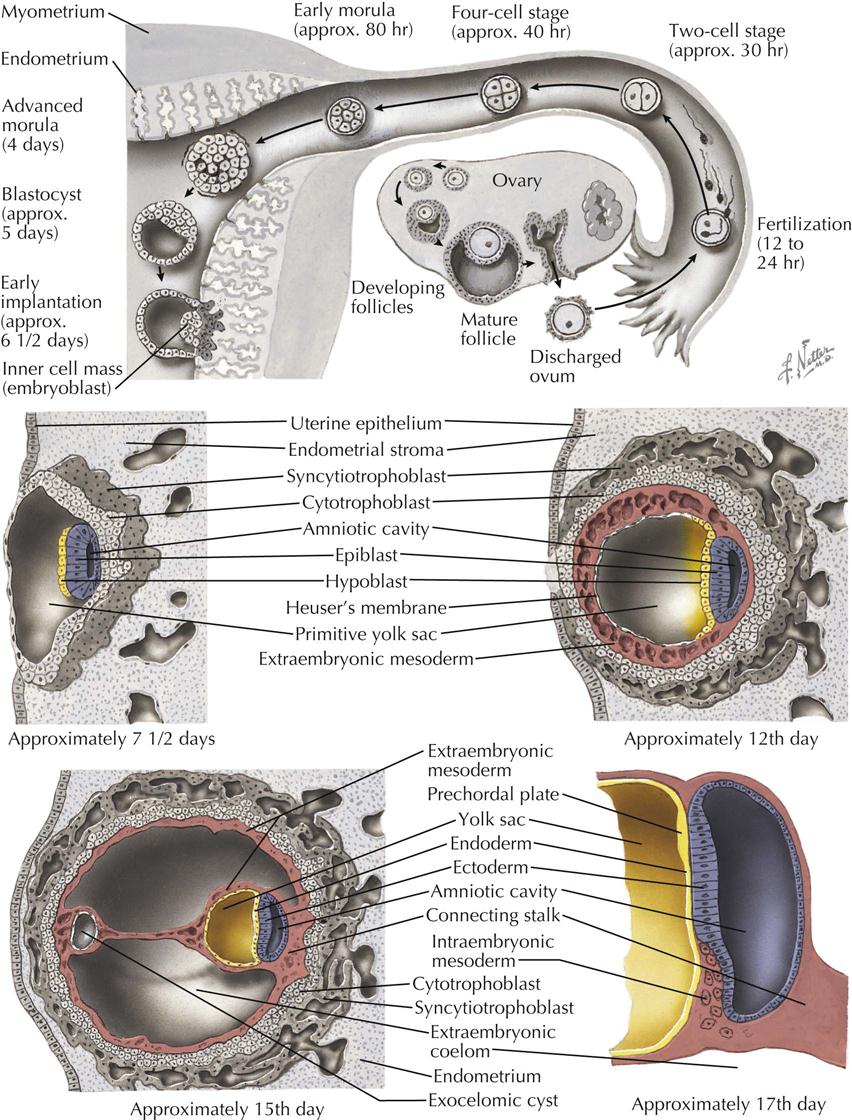
In humans, as in most other primates, fertilization takes place in the distal part of the uterine tube, near its fimbriated end, about 12 to 24 hours after ovulation. The fertilized ovum, or zygote, is transported to the uterus by rhythmic contractions of the tube, aided by the action of the cilia of the epithelium. During this passage down the uterine tube, which takes about 4 days, the zygote executes a number of cell divisions and, on reaching the uterus, consists of a clump of blastomeres, the morula, which has not increased appreciably in size from the zygote.
After entering the uterus, a blastocyst is formed as a fluid-filled cavity develops in the enlarging morula. The wall of the blastocyst consists of a single layer of flattened cells, the trophoblast, and an eccentrically placed mass of cells, the inner cell mass or embryoblast. The trophoblast of the blastocyst attaches to the uterine epithelium to begin the process of implantation into the endometrium during the second week. Its cells soon differentiate into an inner cytotrophoblast and an outer syncytiotrophoblast (syntrophoblast) and will eventually form the outer embryonic membrane (the chorion) and the fetal portion of the placenta. The embryo develops from the inner cell mass, which also contributes to the formation of the amnion and umbilical vesicle.
Soon after implantation, the cells of the inner mass differentiate into two layers: an inner hypoblast (future endoderm), and an outer epiblast (future ectoderm). Together, these two layers form the embryonic disc. At the same time, a space appears within the inner cell mass, the primitive amniotic cavity, lined by the cells that are continuous with the epiblast cells of the embryonic disc. The cavity of the blastocyst becomes lined by hypoblast cells from the embryonic disc migrating along the trophoblast to form the primitive umbilical vesicle (yolk sac). Cells of the primitive umbilical vesicle also give rise to extraembryonic mesoderm, loose connective tissue that separates the umbilical vesicle from the trophoblast. The embryo proper now is a disc made of two layers of cells and from which all the intraembryonic tissues will be derived: the ectoderm, consisting of a simple columnar epithelium that is also the floor of the amnion, and the cuboidal endodermal cells that form the roof of the yolk sac. Small cavities appear in the extraembryonic mesoderm and coalesce to form the extraembryonic coelom (chorionic cavity), except for a mesodermal stalk that connects the amnion to the trophoblast. This remaining mesodermal stalk later forms the connecting stalk (future umbilical cord).
With the formation of the extraembryonic coelom, the extraembryonic mesoderm becomes separated into two thin layers, completing formation of the three extraembryonic membranes. The trophoblast, with its inner coating of extraembryonic mesoderm, is now called the chorion. The primitive amnion with its outer coating of extraembryonic mesoderm is the amnion proper; and the primitive umbilical vesicle (yolk sac) becomes the umbilical vesicle proper with its outer coating of mesoderm.
At the future caudal end of the embryonic disc, ectodermal cells form in the midline a thickened ectodermal primitive streak with a primitive node at the cephalic end of the streak. The primitive streak and node give rise to a third germ layer, the intraembryonic mesoderm, situated between the ectoderm and endoderm. This creation of intraembryonic mesoderm is through the process of gastrulation, occurring during the third week. The mesodermal cells migrate laterally and cranially until ectoderm and endoderm are separated from each other by intraembryonic mesoderm, except at the cephalic oropharyngeal membrane and the caudal cloacal membrane, where ectoderm remains in contact with endoderm. The primitive streak mesoderm cranial to oropharyngeal membrane in the midline is the cardiogenic mesoderm. Laterally, all along the margin of the embryonic disc, the intraembryonic mesoderm is continuous with the extraembryonic mesoderm. The embryo, now trilaminar, becomes more elongated and pear shaped when viewed from its dorsal (ectodermal) or ventral (endodermal) side. The embryo is attached at its narrow caudal end to the chorion by the connecting stalk.
The ovulation age of the embryo at this stage of development is about 20 days, and its length is almost 1.5 mm. The time is now rapidly approaching when simple diffusion of oxygen and nutrients cannot adequately provide for the greatly increasing metabolic needs of the embryo, and a functional circulatory system becomes necessary.
Early Intraembryonic Vasculogenesis
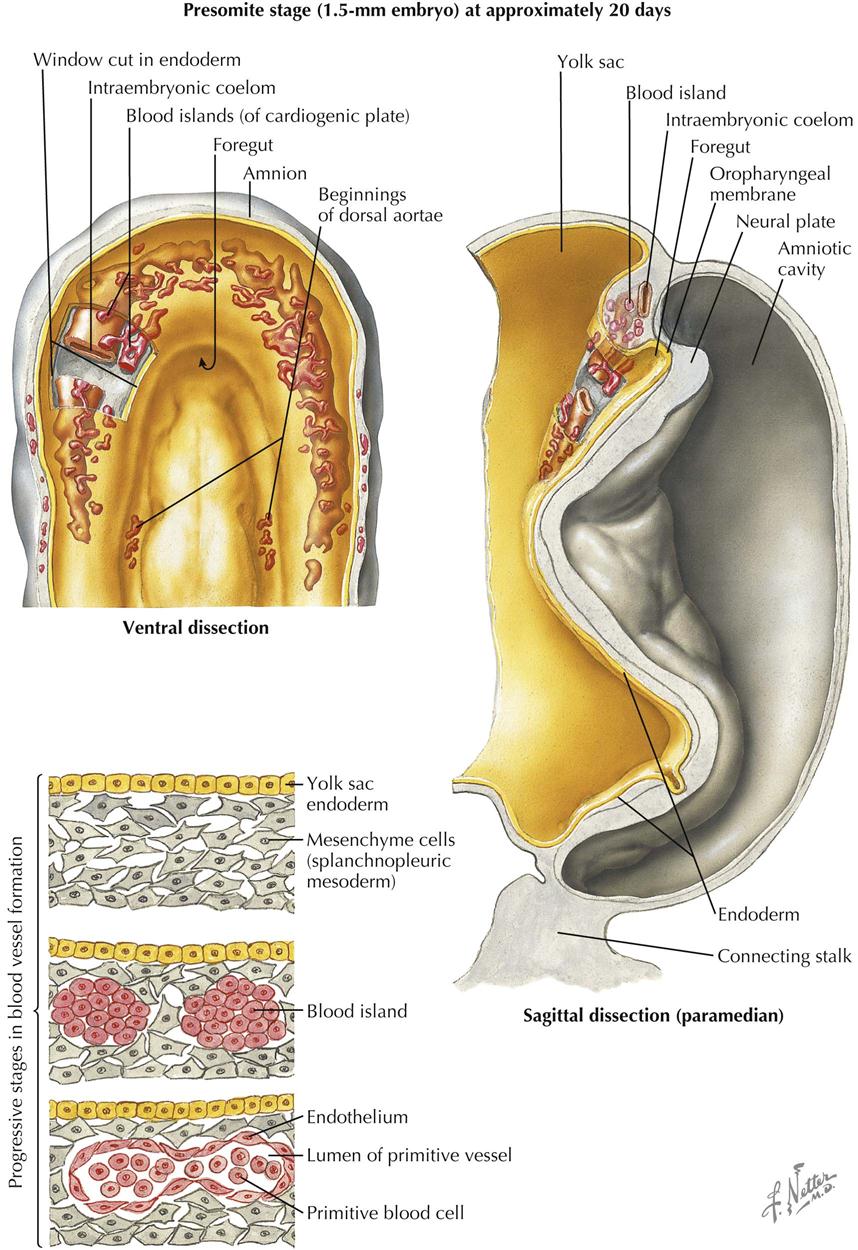
Presomite Stage
Although not the first organ system to make its appearance in the embryo, the cardiovascular system reaches a functional state long before the other systems, and doing so while still in a relatively primitive state of development. The vascular system grows from a simple, bilaterally symmetric plexus into an asymmetric, complex system of arteries, veins, and capillaries—a necessarily dynamic process involving the formation of new vessels and temporary detours, rerouting of the bloodstream, and the disappearance of previously dominant channels or even of entire vascular subsystems. The vascular system needs to enlarge as the embryo grows, adapting to marked changes in embryonic shape and developmental changes in other organ systems. While hard at work, the heart also must grow and differentiate from a simple tube into a complex, four-chambered organ with sets of valves. Finally, because the very young embryo is tiny compared to the mass of extraembryonic (placental) tissue, which the young heart also supplies with blood, this heart is relatively enormous compared with its relative size in the adult.
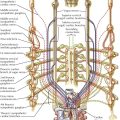
Describing the development of the cardiovascular system first requires review of the intraembryonic coelom (“body cavity”) formed by the confluence of small, initially isolated spaces that appear in the lateral mesoderm and cardiogenic mesoderm. The spaces fuse together and form the single, horseshoe-shaped intraembryonic coelom that extends the length of the embryo in the lateral mesoderm on each side, communicating across the midline cranially in the cardiogenic mesoderm. Later in development, a communication develops on each side between the caudal ends of the intraembryonic coelom and the extraembryonic coelom.
The formation of the coelom separates the lateral mesoderm into two layers: the parietal layer in contact with the ectoderm and the visceral layer in contact with the endoderm. The ectoderm with its parietal layer of lateral plate mesoderm is called somatopleure; endoderm with its visceral mesodermal layer is called splanchnopleure.
In the late presomite embryo, scattered masses of angiogenic cells differentiate in the cardiogenic mesoderm and the splanchnopleure mesoderm ventral to the entire extent of the horseshoe coelom. These cells also appear earlier in the wall of the umbilical vesicle (yolk sac). The angiogenic cell clusters, called blood islands, rapidly increase in number and size, acquire a lumen surrounded by a simple squamous endothelium, and unite to form a plexus of vessels. The angiogenic clusters can also form angioblastic cords that canalize to form vessels. In the cardiogenic mesoderm, paired endothelial heart tubes develop from paired angioblastic cords. The tubes are ventral to the central part of the U-shaped coelom that will form the pericardial cavity. The paired heart tubes begin to beat by day 21 or 22 and fuse into a single heart tube a few days later.
Another pair of angioblastic cords appear bilaterally, parallel and rather close to the midline of the embryo. These cords also acquire a lumen and form a pair of longitudinal vessels, the dorsal aortae (aortas). These vessels connect with the dorsocranial aspect of the endothelial (endocardial) heart tubes to establish the arterial pole of the developing heart. The caudal ends of the endothelial heart tubes make contact with vessels arising in the yolk sac mesoderm (vitelline veins) and later with the developing umbilical veins and common cardinal veins. Thus the venous pole of the heart, still paired, is determined.
Formation of the Heart Tube
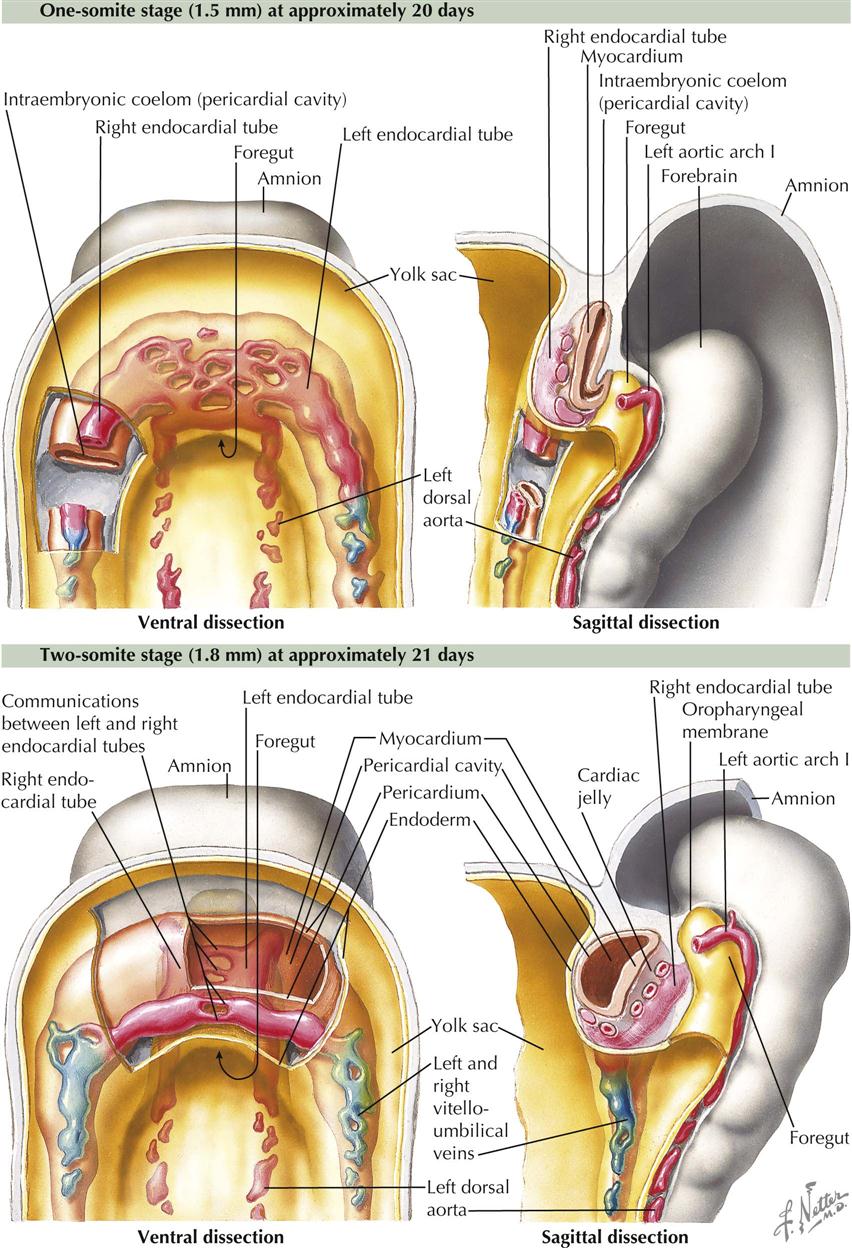
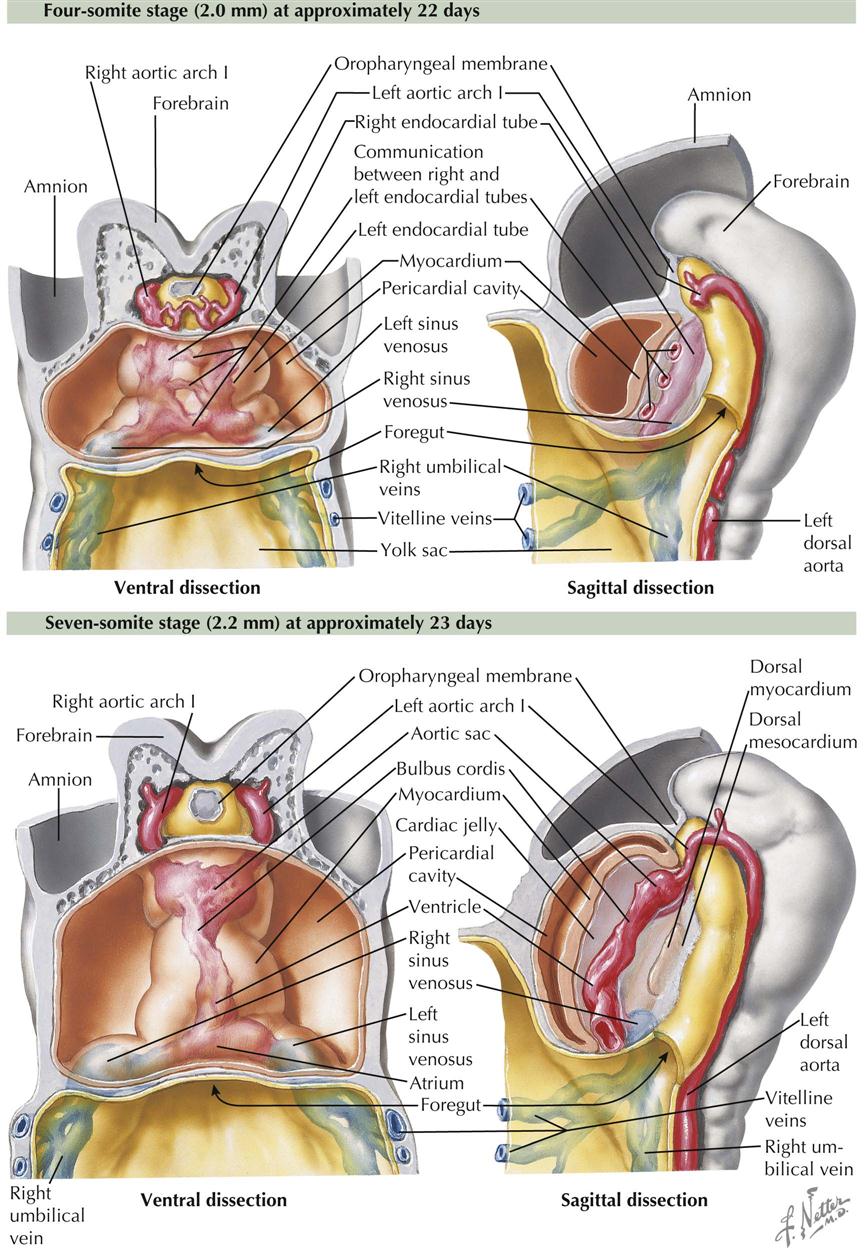
One-Somite and Two-Somite Stages
As the primitive, bilaterally symmetric cardiovascular system appears, shaping of the embryo during the fourth week profoundly influences the relative position of the cardiac portion of this system. The trilaminar embryonic disc folds into a cylinder, and the amnion tucks around the embryo on each side. The amnion also envelops the head end of the embryo as the ectodermal tube of the forebrain rapidly increases in size in a cranial and ventral direction. The result is a 180-degree sagittal plane rotation of the cardiogenic mesoderm and oropharyngeal membrane, which were originally cranial to the neural plate and the developing neural tube. The heart is now caudal to the oropharyngeal membrane rather than cranial, and the heart locates dorsal to the developing pericardial cavity (see Plate 4-3).
The folding of the gastrula also results in (1) formation of an endodermal gut tube consisting of a foregut, midgut, and hindgut and (2) each dorsal aorta leaving the cranial end of the endocardial tubes and curving dorsally around the foregut. Thus the first pair of aortic arches, or pharyngeal (branchial) arch arteries, appear.
With the folding of the trilaminar embryonic disc, the endoderm is shaped into a tube within the embryo. The midgut is continuous with the umbilical vesicle (yolk sac) that extends ventrally from the embryo. The foregut is an extension into the head region of the embryo, and the hindgut is a caudal extension. The umbilical vesicle is pressed against the connecting stalk, and the intraembryonic part becomes a narrow duct connected to the midgut. With the rotation of the cardiogenic mesoderm and oropharyngeal membrane from growth of the forebrain, the developing heart swings to a position ventral to the foregut, a relationship retained in the adult. The dorsal aortae are dorsal to the foregut, and pairs of pharyngeal (branchial) arch arteries flank the foregut to connect the heart tubes with the aortae.
As a result of all these changes, the endothelial heart (endocardial) tubes lie closer and parallel to each other. They quickly fuse into a single tube in a craniocaudal direction.
Four-Somite and Seven-Somite Stages
As indicated earlier, the heart tube is dorsal to the developing pericardial cavity. As the tube enlarges and bends, it bugles into the underlying coelom. As the heart tube comes to rest entirely within the pericardial cavity, it is suspended by the two opposing epithelial layers of the pericardial sac, the dorsal mesocardium. A ventral mesocardium never develops.
The mesodermal tissue surrounding the endothelial heart (endocardial) tube, meanwhile, has differentiated into three layers. The inner layer immediately around the endothelium is initially thick, gelatinous connective tissue called the cardiac jelly. The next layer is the cellular primitive myocardium. The third (outer) layer consists of flat mesothelial cells that also line the remaining pericardial cavity. The cardiac jelly disappears. The endocardial tubes persist as the inner lining of the heart chambers, the endocardium. The primitive myocardium elaborates and matures to become the muscular wall of the heart, the myocardium. The simple squamous epicardium, although continuous with the rest of the pericardial sac, derives from cells overlying the sinus venosus of the heart tube that migrate over the heart (see Plate 4-4).
The embryo now has seven somites, is about 2.2 mm long, and is approximately 23 days old. About 3 days have elapsed between the appearance of intraembryonic vasculogenesis and the formation of the endocardial tube. About this time, or slightly earlier, the heart begins to beat. No known cardiac anomaly can be attributed to the developmental phases described thus far, except the rare cases of acardia seen infrequently in twins with a common placental circulation.
Formation of the Heart Loop
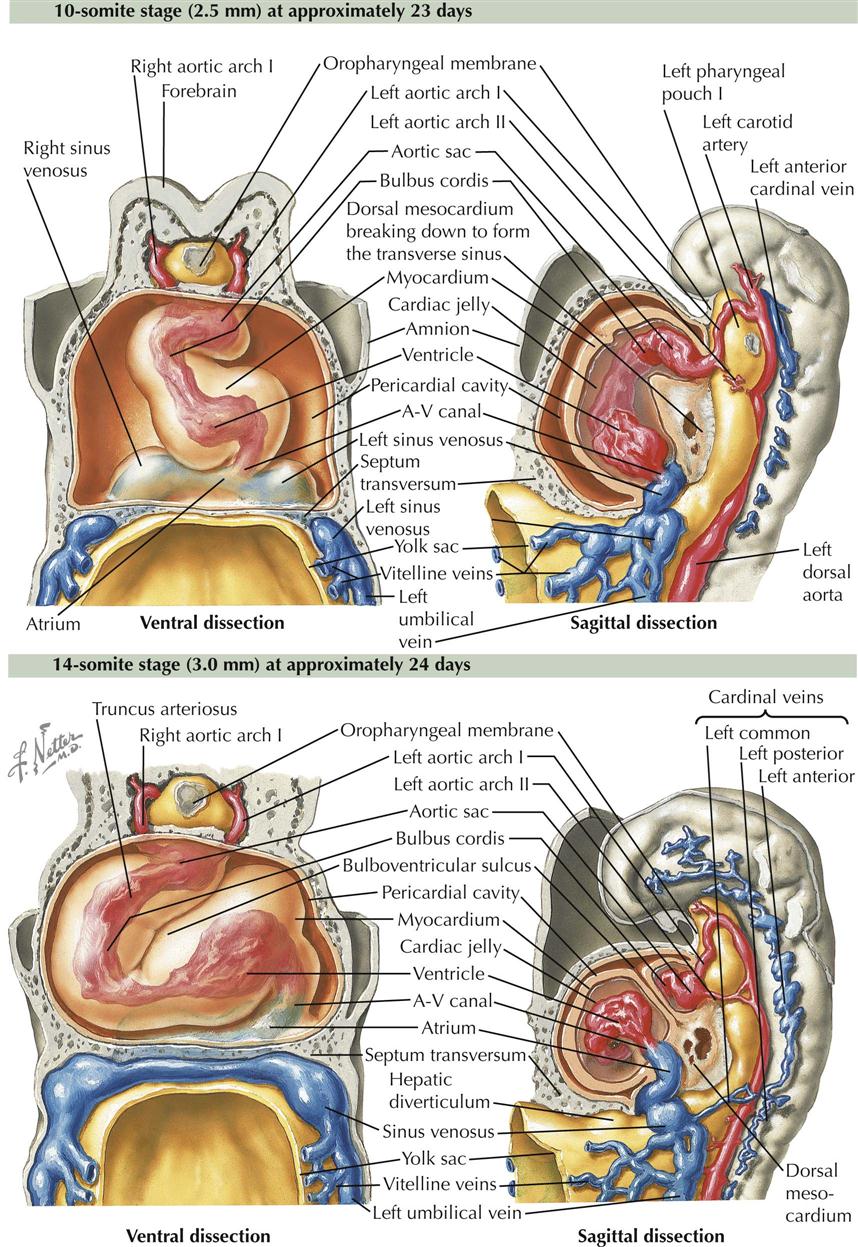
At the beginning of the next phase of development, the heart, as described earlier, is essentially a straight tube with a caudal venosus end and cranial arterial end. It lies within the pericardial cavity and is attached posteriorly only by the dorsal mesocardium.
10-Somite and 14-Somite Stages
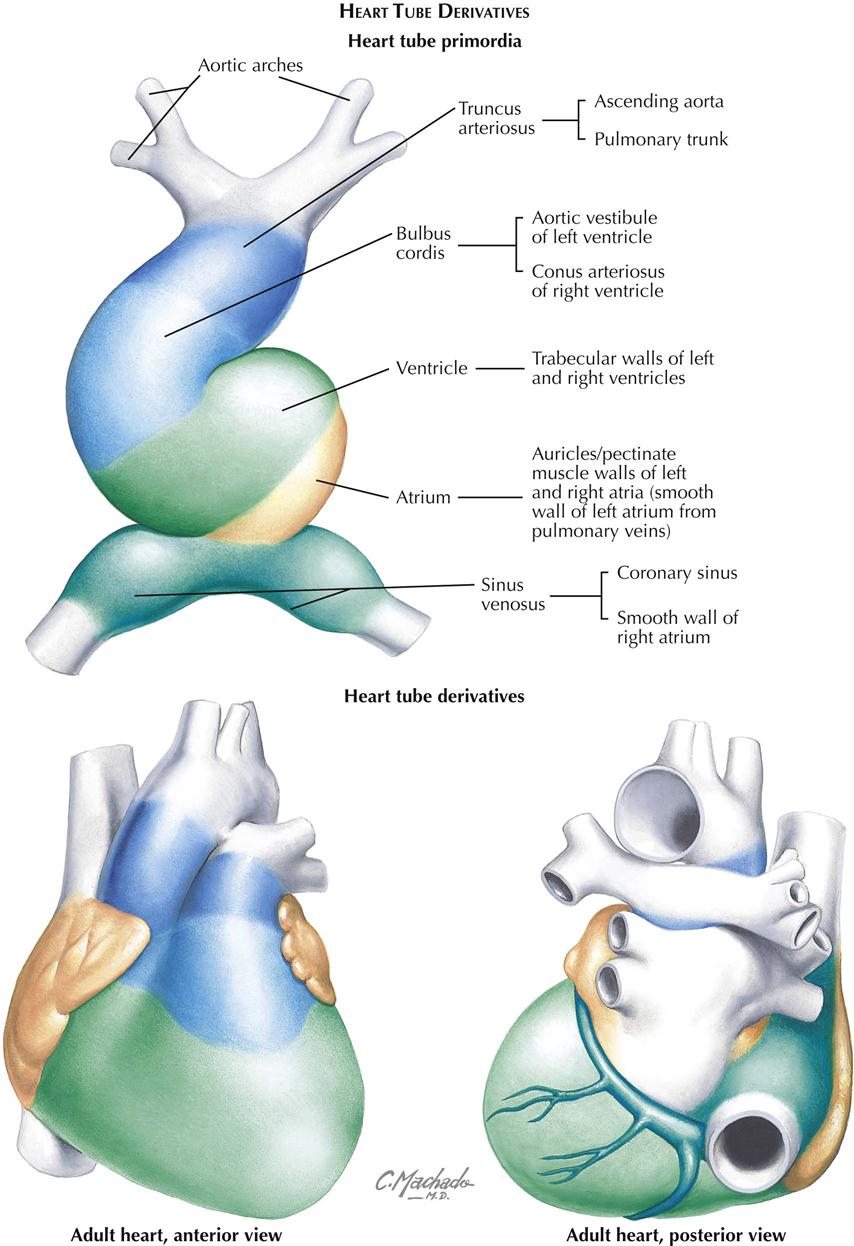
A series of chambers forms in sequence within the tube. At the venous end the sinus venosus develops left and right horns and receives blood from the common cardinal veins, vitelline veins, and umbilical veins (two initially, one later). These veins return blood from the embryo, umbilical vesicle (yolk sac), and placenta, respectively (see Plate 4-5). From the sinus venosus, blood flows into a primordial atrium, a primordial ventricle, a chamber called the bulbus cordis, and then out the arterial end of the heart tube through the truncus arteriosus. Blood from the truncus arteriosus flows into a dilated aortic sac ventral to the foregut. The first pair of pharyngeal (branchial) arch arteries (aortic arches) connect the aortic sac to the dorsal aortae. The ventricle and bulbus cordis grow faster than the other parts of the heart tube. Because its two ends are fixed, the heart tube is forced to bend in order to adapt to the available pericardial space. The bend is between the ventricle and bulbus cordis, forming a bulboventricular loop that extends ventrally and to the right. A result of the bend is the more cranial and dorsal position of the atrium and sinus venosus behind the truncus arteriosus outflow tract of the heart tube. At the same time, perforations appear in the dorsal mesocardium, leading to its disappearance as the openings increase in size. The result is the formation of the transverse pericardial sinus, the space between the arteries and veins at the top of the adult heart that connects the left and right aspects of the pericardial cavity.
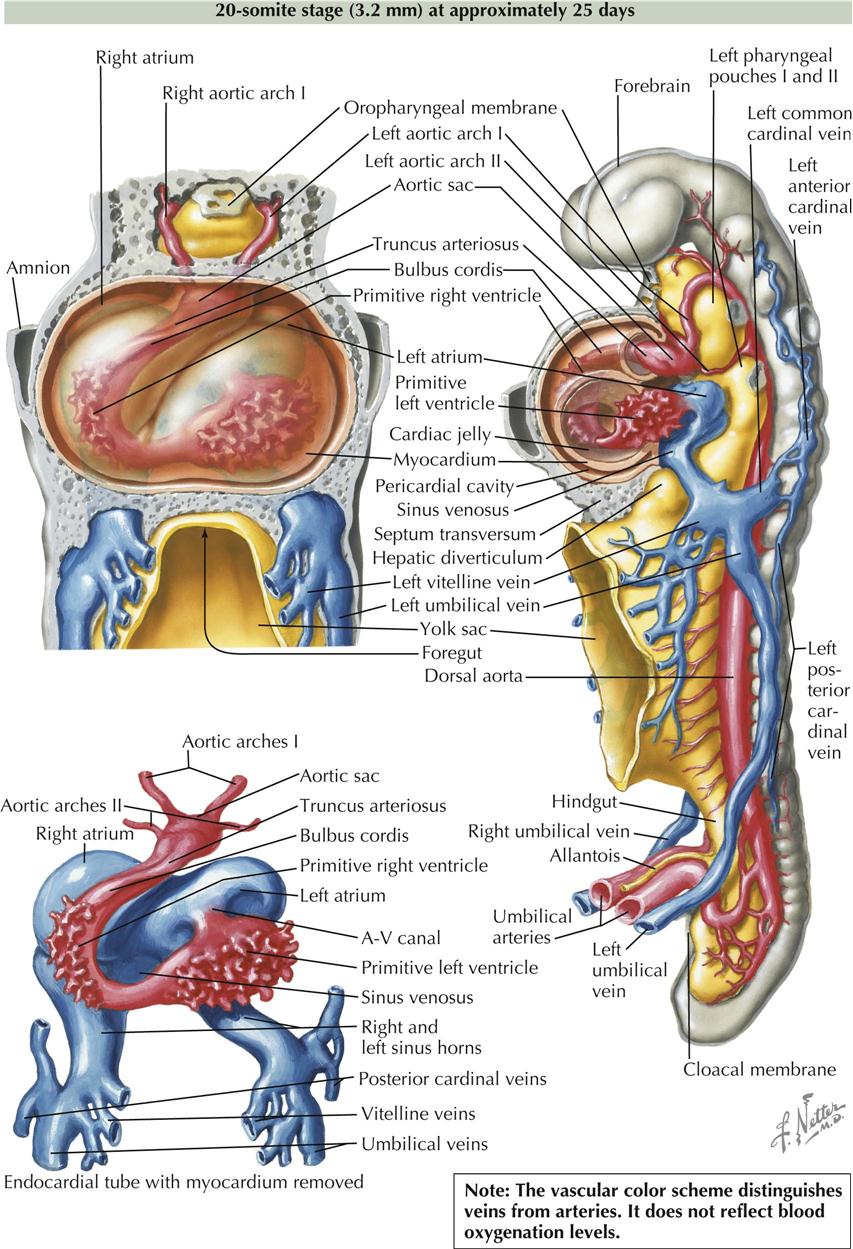
20-Somite Stage
At the close of this phase of development, numerous diverticula appear in the ventral aspect of the early ventricle and in the proximal third of the bulbus cordis. These diverticula project initially into the cardiac jelly and later into the myocardium, expanding the capacity of the heart sections involved and becoming the mechanism of formation of the trabecular muscle of the ventricular walls. At this point, although bending and local elaborations have changed its appearance, the heart still consists of essentially a single tube. A small, interventricular septum appears marking the start of left and right ventricles and an interventricular canal, but these are still in sequence with regard to blood flow. Although still a tube, the heart’s external appearance already strongly suggests its future four-chambered structure. The elaboration of septa will further define the atria and ventricles. The embryo is now about 3.2 mm long and approximately 25 days old, and it possesses 20 somites (see Plate 4-6).
Formation of Cardiac Septa
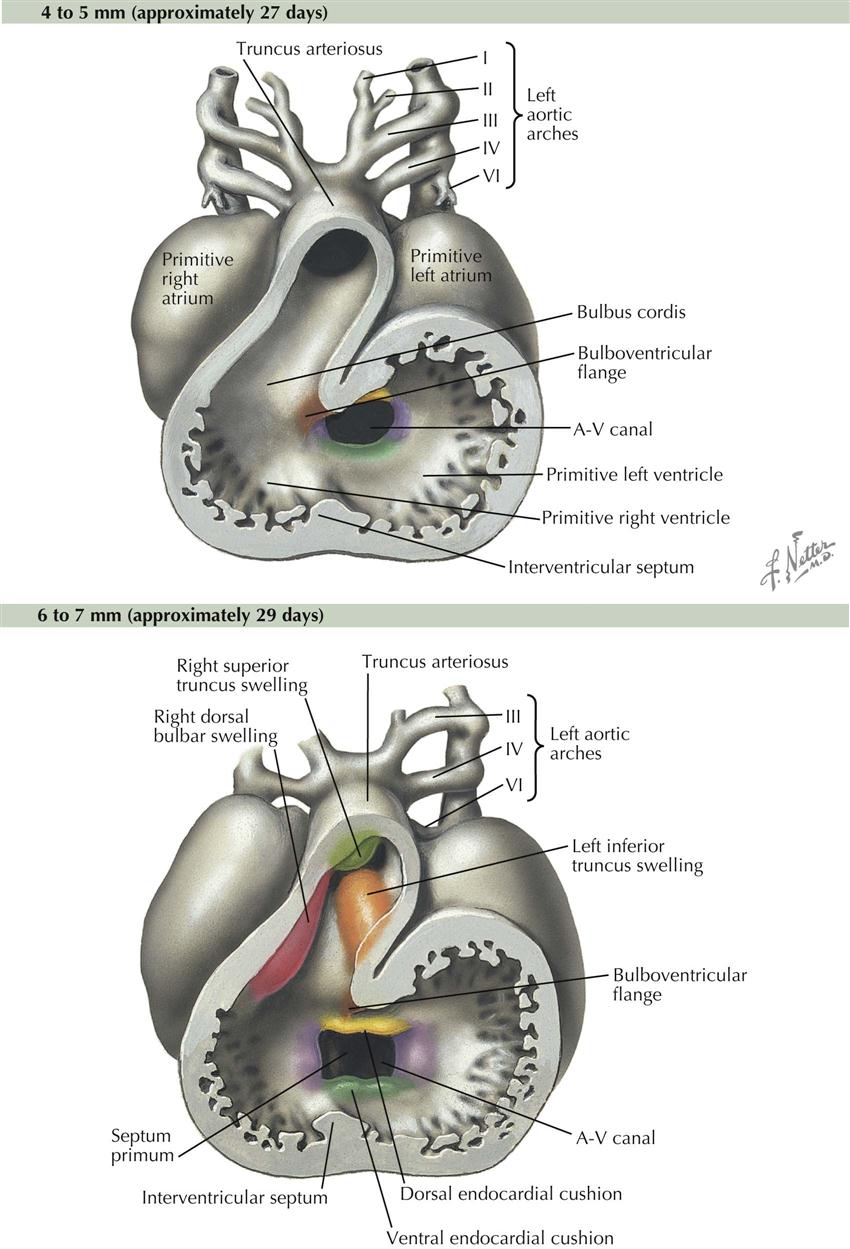
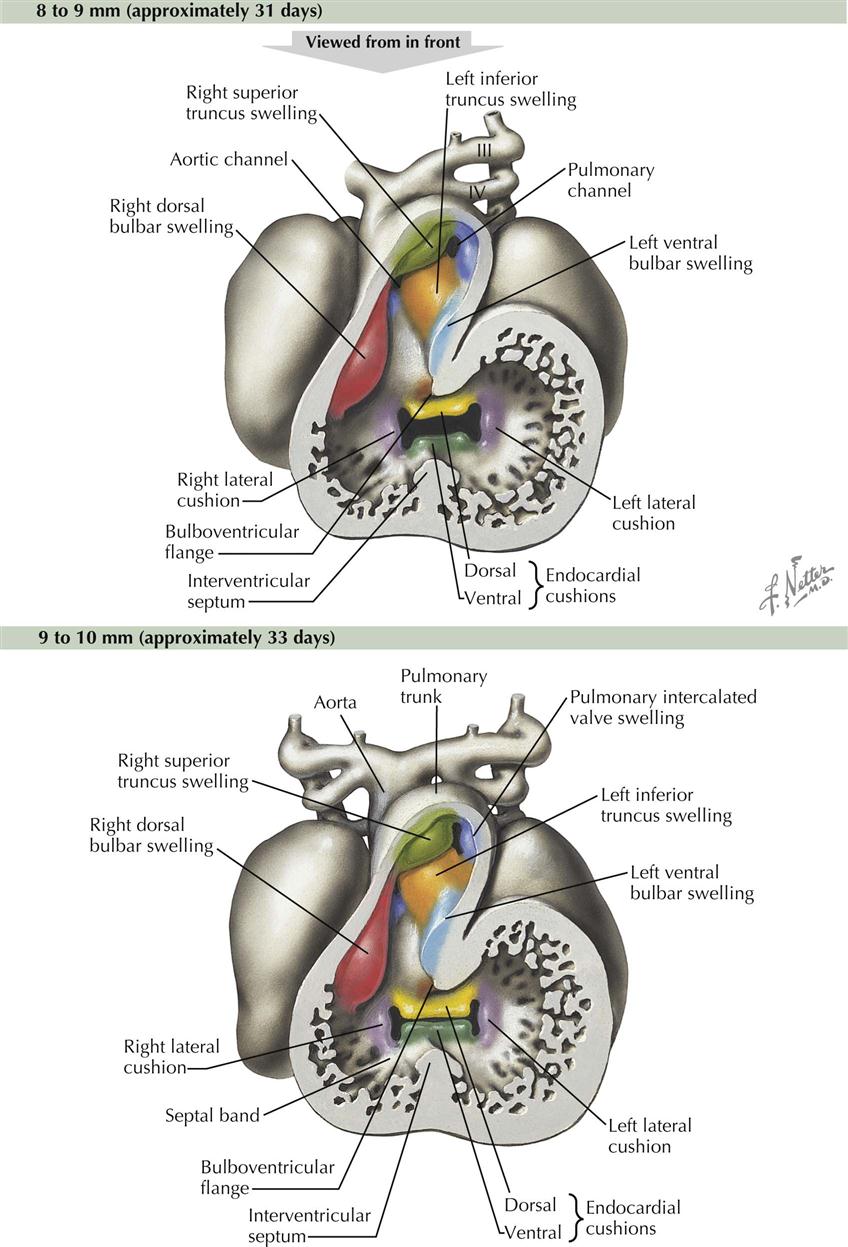
At the close of the preceding phase of development, the heart completely occupies the pericardial cavity. Blood flows in a single path through the sinus venosus and atrium, through an atrioventricular canal into the left ventricle, though an interventricular canal above the free edge of the primordial interventricular septum into the right ventricle, then out the bulbus cordis and truncus arteriosus. The stage is now set for the septation of the heart, which lasts about 10 days. No major changes occur in the external appearance of the heart. The formation of the various cardiac septa occurs more or less simultaneously; for descriptive purposes, however, it is necessary to consider their development separately.
Atrioventricular Canal
Blood flow through the heart is first separated into left and right sides by dorsal and ventral endocardial cushions that appear about day 24. Endocardial cushions are swellings of mesenchymal tissue that grow toward each other, then fuse to divide the atrioventricular (A-V) canal into left and right A-V canals (see Plate 4-7). The primordial atrium begins to shape into a left atrium and a right atrium, although at this early stage the atria are in wide communication with each other. With the appearance of the endocardial cushions and primordial interventricular septum, the four chambers can be identified, but blood from the sinus venosus still enters the heart in one location, the right atrium, and it exits the heart in one location, the right ventricle. Half the blood in the right atrium passes through the right A-V canal into the right ventricle, then out through the bulbus cordis and truncus arteriosus. The other half of the blood in the right atrium passes into the left atrium, through the left A-V canal into the left ventricle, then to the right ventricle and the same exit path through bulbus cordis and truncus arteriosus.
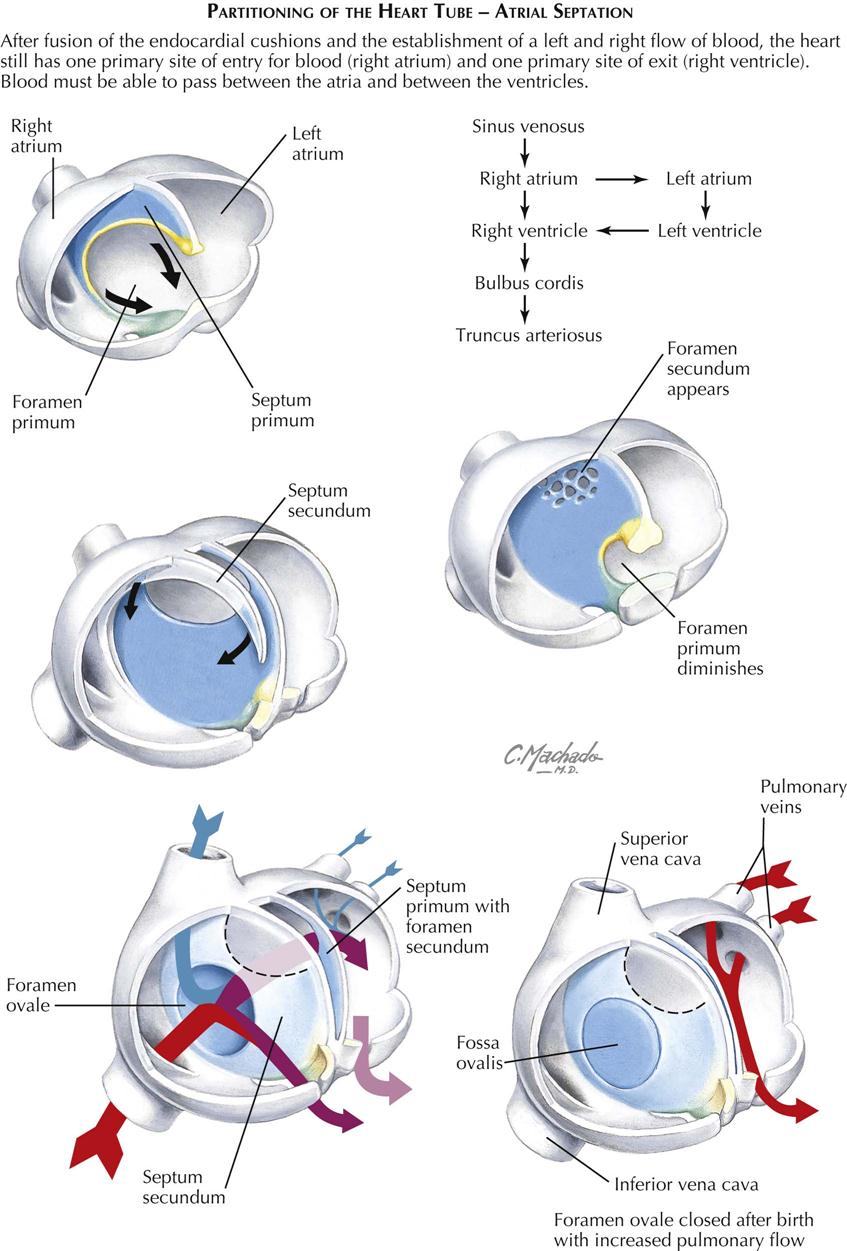
Atria, Atrial Septum, and Pulmonary Veins
The atria are divided by two adjacent septa that function as a valve permitting blood to flow from primitive right atrium to left atrium, but not the other direction. This plays a crucial role in having blood bypass the nonfunctioning prenatal lungs, and in converting the prenatal circulatory pattern to the postnatal configuration soon after the first breath of the newborn.
The truncus arteriosus forms a depression on the external surface of the common atrium that corresponds on the inside to a crescent-shaped ridge, the septum primum (see Plate 4-7). It grows inferiorly toward the fused endocardial cushions. The transient interatrial passage inferior to it is the foramen primum, which disappears as the septum primum fuses with the endocardial cushions. Before this happens, holes appear high up on the septum primum and coalesce to form a foramen secundum in the septum primum. On the right atrium side of the septum primum, a thick, muscular, septum secundum grows inferiorly toward the endocardial cushions. It also has a crescent shape that, with continued growth, will circumscribe an oval foramen in the septum secundum, the foramen ovale. The foramen secundum is at a higher level than the foramen ovale. Oxygen-rich blood in the fetal inferior vena cava is directed at the foramen ovale. It pushes the septum primum away from the septum secundum to allow blood to pass through the foramen secundum into the left atrium. With increased blood pressure in the left atrium at birth from increased pulmonary blood flow, however, the septum primum is pressed against the relatively stiff septum secundum, effectively closing the foramen ovale. After fusion of septum primum with septum secundum, the foramen ovale becomes the fossa ovalis of the right atrium. Growth of the septum secundum occurs during the fifth and sixth weeks.
A single embryonic pulmonary vein, present in a 5- to 6-mm embryo, develops as an outgrowth of the posterior left atrial wall. It connects with the splanchnic plexus of veins in the region of the developing lung buds. Later in development, the vein itself and parts of its first four branches (two from the left lung and two from the right) expand tremendously and become incorporated into the left atrium to form the larger, smooth, posterior part of the adult atrium. In the fully developed heart, the original embryonic left atrium is represented by little more than the trabeculated atrial appendage (auricle). The intrapulmonary part of the splanchnic venous plexus ultimately loses its connections with the systemic veins and drains exclusively by way of the pulmonary veins.
On the right side, the right sinus horn is similarly incorporated into the right atrium; it enlarges mainly in its vertical diameter, and the relative distance between the common cardinal vein (proximal superior vena cava) and the inferior vena cava (from the right vitelline vein) increases. The original embryonic right atrium becomes the right atrial appendage (auricle), containing the earliest-appearing pectinate muscles. A lateral wall with pectinate muscle will grow to become the largest component of the right atrial wall. Thus the primitive right atrium becomes the right atrial appendage with its pectinate muscle; the right horn of the sinus venosus becomes the smooth back wall of the right atrium; and new pectinate muscle develops into the lateral wall.
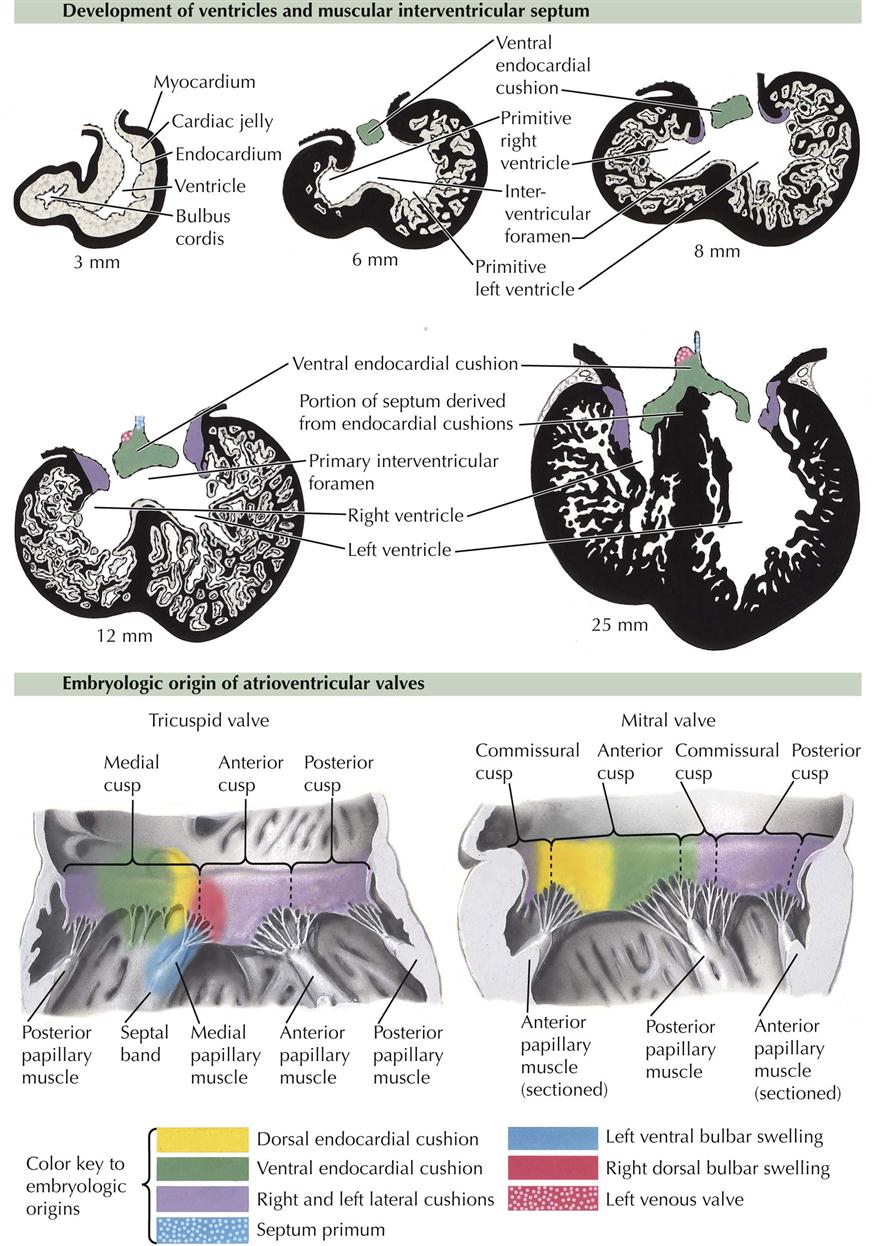
Development of the Ventricles
The primordial right and left ventricles are little more than sequential local widening of the original cardiac tube, and they are connected to each other by a smooth-walled, relatively narrow channel, the primary interventricular foramen. As the heart tube folds, the interventricular foramen is bounded inferiorly by the developing interventricular septum (see Plate 4-8). Completion of the ventricular separation is intimately related to division of the outflow tract of the primitive heart tube: the bulbus cordis and truncus arteriosus.
In an embryo of about 4 to 5 mm, the A-V canal still leads into the primitive left ventricle, and blood can reach the primitive right ventricle only by way of the primary interventricular foramen. After division of the A-V canal by the endocardial cushions into left and right A-V canals, blood still must pass from the left ventricle to the right ventricle before exiting the heart. If the interventricular septum simply grew to fuse with the endocardial cushions, there would be no exit of blood from the left ventricle.
Enlargement of the ventricles is accomplished by centrifugal growth of the myocardium, always closely followed by increasing diverticulation and formation of trabeculae internally; this prevents the compact outer layer of the myocardium from becoming too thick and solid. Typically, the ventricles of the embryonic heart consist of a tremendous mass of trabeculae enclosed by a rather thin outer layer of compact myocardium. Most of the trabeculae eventually disappear. Of the remaining trabeculae, some coalesce to form larger structures such as papillary muscles and the moderator band; others are reduced to thin, fibrous strands (e.g., chordae tendineae) that connect the papillary muscles to the atrioventricular valve cusps (see Plate 4-7).
The primary interventricular septum is thick and gives rise to the inferior, muscular part of the adult septum. Again, it does not continue to grow to fuse with the endocardial cushions. Instead, it will fuse with a septum that divides the bulbus cordis and truncus arteriosus. The primary interventricular foramen never closes, but actually enlarges and, in the fully developed heart, gives access to the aortic vestibule, the smooth upper part of the left ventricle that leads to the aortic valve.
Truncus Arteriosus and Bulbus Cordis
Distal to the ventricles in the outflow part of the heart tube is a dilatation, the bulbus cordis, followed by a tapering truncus arteriosus (see Plate 4-8). Potentially confusing terms have been used to describe these structures; some consider these two outflow chambers to be a single structure, sometimes referred to as the bulbus cordis, sometimes as the truncus arteriosus. Another term used for the interface between the two is the “conus cordis.” Word combinations are also used, such as “truncoconal” to describe septal swellings (see Plate 4-9). This section uses more recent and common terms, the bulbus cordis leading to the truncus arteriosus.
The bulbus cordis and truncus arteriosus are divided lengthwise by a spiral septum, also called the aorticopulmonary septum, named after the ascending aorta and pulmonary trunk that are derived from the truncus arteriosus (“arterial trunk”). Two streams of blood spiral through this part of the heart tube, and longitudinal septa form in the path of least resistance between the two streams (see Plate 4-10).
The process begins in the 6-mm embryo at the end of the fourth week and is completed near the end of the sixth week (14- to 15-mm embryo). It proceeds in a distal to proximal direction; the truncus arteriosus is divided first, followed by the bulbus cordis. The two opposing ridges dividing the bulbus cordis are called left and right bulbar ridges, which are the proximal parts of the developing spiral septum. The ridges are continuous with the attached edges of the muscular interventricular septum. The fusion of these ridges with each other, with the interventricular septum, and with the endocardial cushions completes division of the ventricles and creates an outflow path for each chamber. The thin, upper, membranous interventricular septum derives from an extension of tissue on the right side of the endocardial cushions. It fuses with the muscular interventricular septum and bulbar ridges of the spiral septum. Membranous septal defects are the most common heart defect (25% of all congenital heart defects), partly because three basic primordia (interventricular septum, spiral septum, endocardial cushions) are required to fuse in an area of very dynamic blood flow. There is considerable opportunity for a failure of fusion of these elements at the location of the membranous interventricular septum.
The spiral septum is a suitable synonym for the aorticopulmonary septum because of its orientation in the outflow part of the embryonic heart tube and the resulting relationship of the adult arterial derivatives of the chambers it divides. The ascending aorta arises posterior to the pulmonary trunk in the adult heart, spirals up to the right of the pulmonary trunk, and continues anteriorly to form the aortic arch that passes over the bifurcation of the trunk into left and right pulmonary arteries.
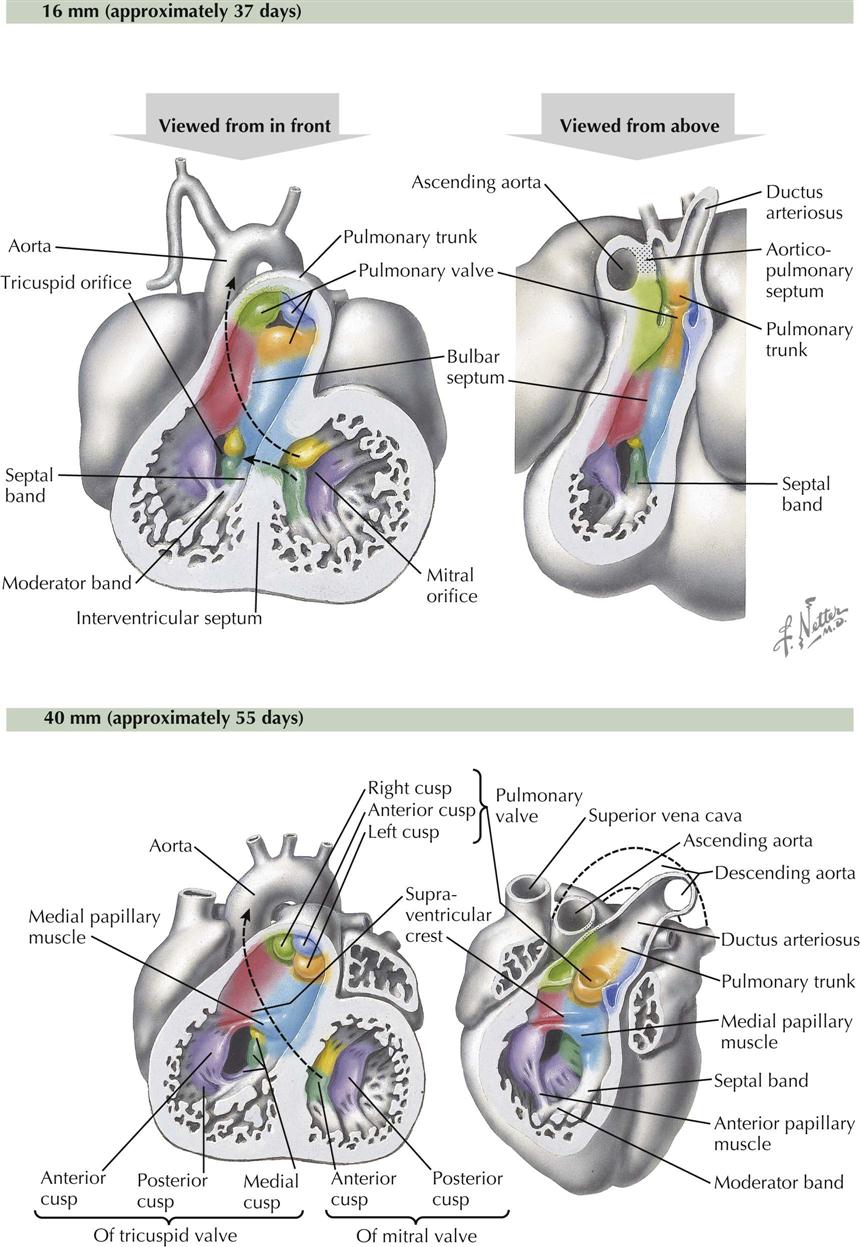
The bulbus cordis is incorporated into the ventricles, forming the upper, smooth-walled, outflow part of each ventricle: the conus arteriosus in the right ventricle, just below the pulmonic semilunar valve, and the aortic vestibule of the left ventricle, leading to the aortic semilunar valve (see Plate 4-11). The inferior, trabeculated part of each ventricle derives from the primitive ventricle. Because of the oblique orientation of the bulbar (spiral septum) ridges, part of the primitive right ventricle is captured by the left ventricle when the ridges fuse to the interventricular septum. As a result, the embryonic interventricular foramen above the primary interventricular septum is retained as the interface between aortic vestibule and trabecular part of left ventricle.
Sinus Venosus
The cardiovascular system in the early embryo is paired and symmetric. At about day 23, the four-somite stage, the paired endothelial heart tubes fuse, beginning in the bulboventricular region and progressing toward the venous pole of the heart. The sinus venosus maintains its paired condition. Early in the fourth week, a central unpaired part of the sinus venosus opens into the primitive atrium and right and left sinus horns.
At this stage the sinus venosus receives three pairs of veins. Most medially, at the junction of the sinus horns and the central portion, the vitelline veins enter the floor of the sinus. Lateral to the vitelline veins, the umbilical veins enter the sinus horns from below, with the common cardinal veins coming from above. The proximal parts of the umbilical veins soon disappear (and the distal segment of the left umbilical vein connects with the developing inferior vena cava). Because anastomotic channels develop between the right and left systemic veins (e.g., future left brachiocephalic vein), and blood flow is preferential to the right side of the embryo, the right horn of the sinus venosus and the right proximal cardinal and vitelline veins become more important, whereas their left counterparts are greatly reduced in size. Thus the right sinus horn becomes larger, more vertical, and incorporated into the part of the primitive atrium that will become the right atrium. The right horn will form the smooth posterior wall of the right atrium, the sinus venarum, named after its sinus venosus origin. The communication between the sinus venosus and the developing right atrium is now limited to the right sinus horn. The left horn of the sinus venosus becomes the coronary sinus. The left common cardinal vein usually disappears.
The sinoatrial orifice is tall and narrow, and the folds on either side constitute the valve of the sinus venosus, with the right fold larger than the left fold. The vertical dimension increases until a constriction in the middle creates separate openings for the developing superior and inferior venae cavae. The left fold of the valve fuses with the septum secundum to become part of the interatrial septum. The cranial part of the right valve fold becomes a thick, vertical ridge of muscle, the crista terminalis, that marks the boundary between the two primordia (sinus venosus and primitive atrium) that contribute to the right atrial wall. Posterior to the crista terminalis is the smooth-walled sinus venarum; anterior to the crista terminalis is the wall of the right atrium lined with pectinate muscle, including the right atrial appendage (auricle). The inferior part of the right fold of the valve of the sinus venosus becomes the valve of the inferior vena cava and the smaller valve of the coronary sinus.
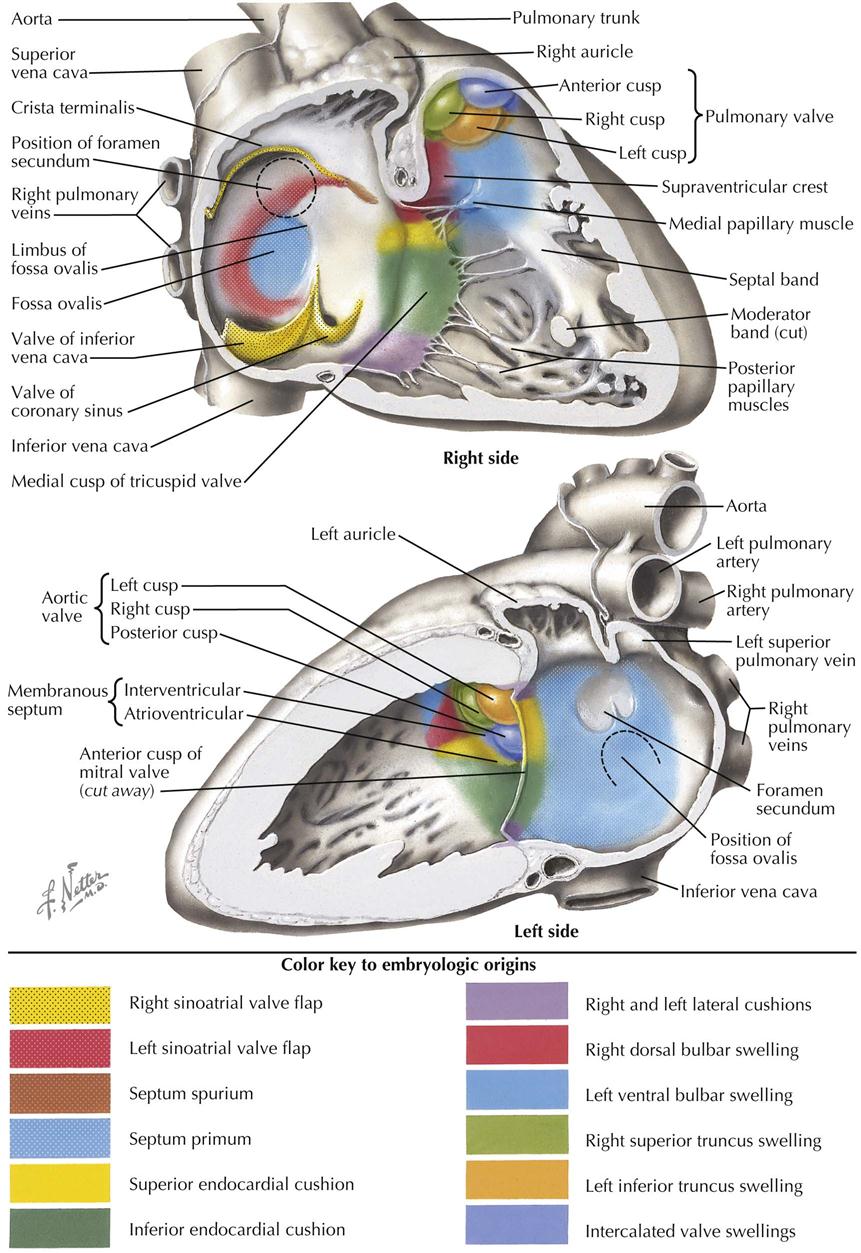
Plate 4-11 summarizes the primitive heart tube chambers and their adult derivatives.
Atrioventricular and Semilunar Valves
The atrioventricular valve cusps form from extensions of mesenchyme and ventricular muscle surrounding the A-V canals (see Plate 4-7). The cusps are initially thick and fleshly, becoming thin and fibrous later. The left, bicuspid mitral valve initially has four cusps of equal size. The left and right cusps diminish in size and are usually identifiable in the adult valve as very small left and right commissural cusps. The remaining two cusps are the anterior (aortic) cusp and posterior cusp. The right tricuspid valve develops similarly, except three cusps develop instead of the original four (becoming two) in the mitral valve. The lateral cusp and part of the anterior cusp develop first. The medial cusp overlying the membranous interventricular septum develops later. The papillary muscles and their chordae tendineae develop from trabecular muscle. Initially thick and fleshy, the chordae tendineae become thin and fibrous as their muscular component disappears. Development of the basic structure of the mitral valve is completed by the end of the sixth week; the tricuspid valve is completed soon after (see Plates 4-12 and 4-13).
The primordia of the aortic and pulmonary semilunar valves appear near the end of partitioning of the truncus arteriosus by the truncal component of the spiral septum. Four swellings of mesenchyme surround the lumen of the truncus arteriosus (see Plate 4-9). Left and right swellings are divided by the aorticopulmonary septum to form left and right valve cusps in both the ascending aorta and pulmonary trunk. The anterior swelling forms the anterior cusp in the pulmonary valve, and the posterior swelling in the truncus arteriosus forms the posterior cusp of the aortic valve. Excavation of the superior surfaces of the swellings and later thinning result in the semilunar shape of each cusp. Dilatation of the proximal origins of the ascending aorta and pulmonary trunk gives rise to the pulmonary and aortic sinuses, the expanded space between each cusp and the walls of the arteries. The left and right coronary arteries arise from the left and right aortic sinuses (of Valsalva), respectively.
Development of Major Blood Vessels
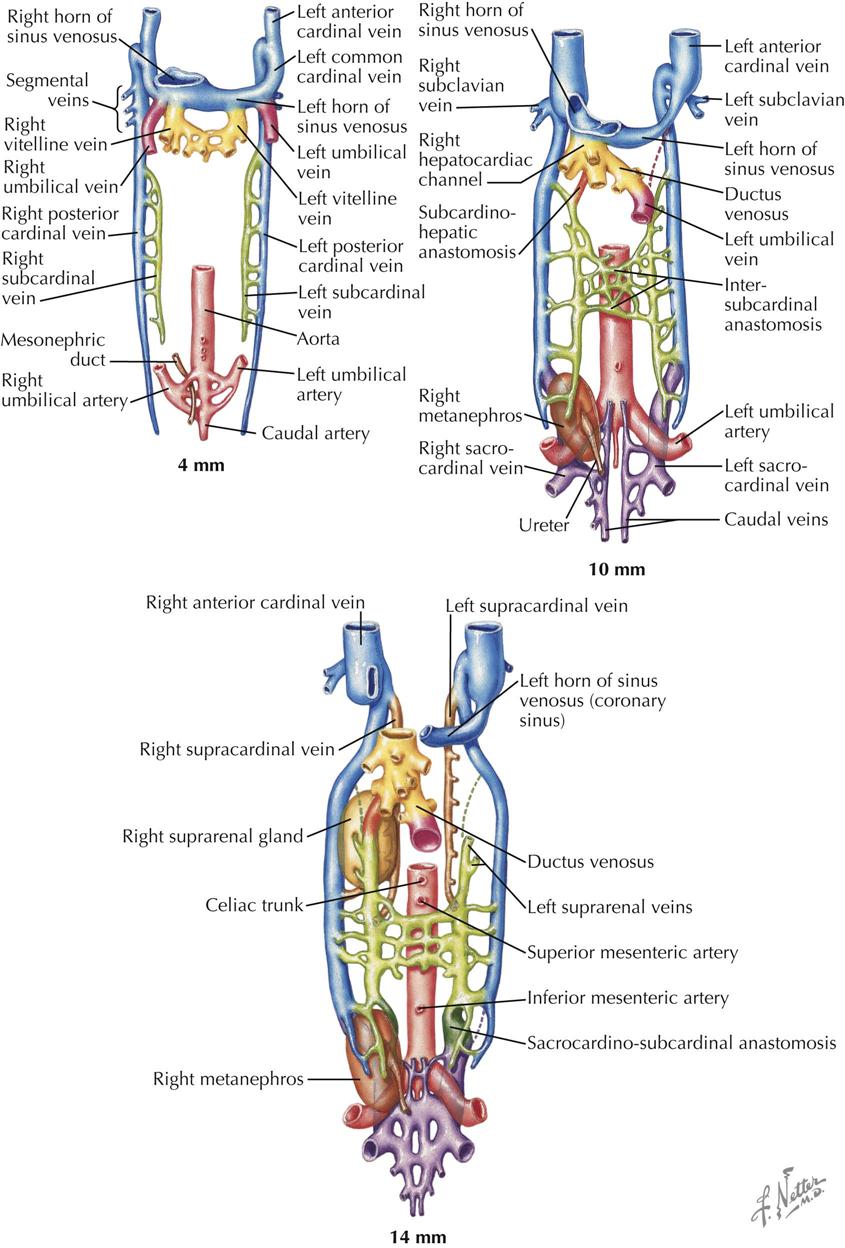
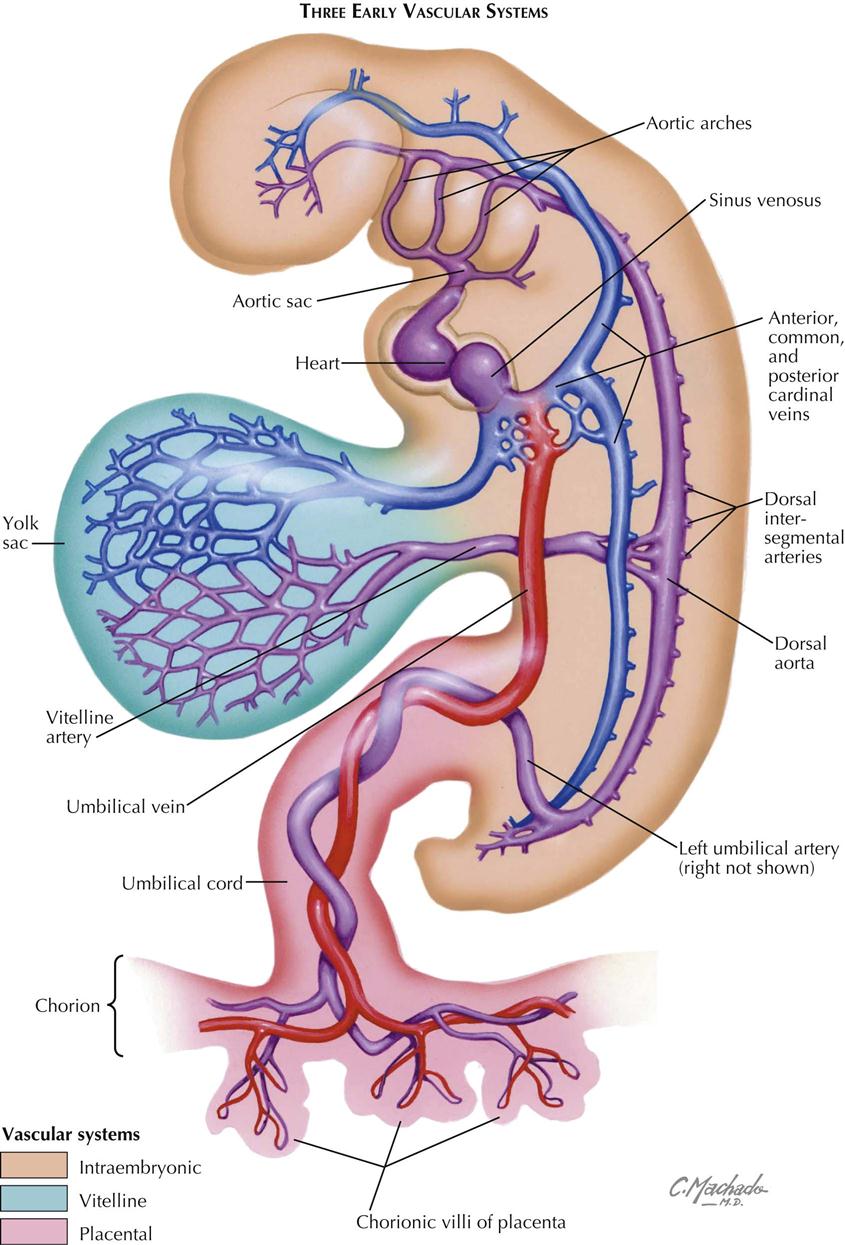
The early embryonic vascular system is plexiform (intercalating). Preferential flow related to the development of organ systems, however, leads to enlargement of certain channels in the plexus. This expansion is brought about in part by the fusion and confluence of adjacent smaller vessels and by the enlargement of individual capillaries. Thus a number of vascular systems develop. As the embryo grows, new organs appear; others are transient and disappear. The various vascular systems are also continuously modified to satisfy changing needs.
Initially, the arteries and veins consist simply of endothelial tubes and cannot be distinguished from each other histologically. In later development, typical vessel walls are differentiated from the surrounding mesenchyme. The final pattern of the vascular system is genetically determined and varies with the animal species. Variations are, however, extremely common in both arterial and venous patterns, and local modifications occur in cases of abnormal development of organs.
Aortic Arch System
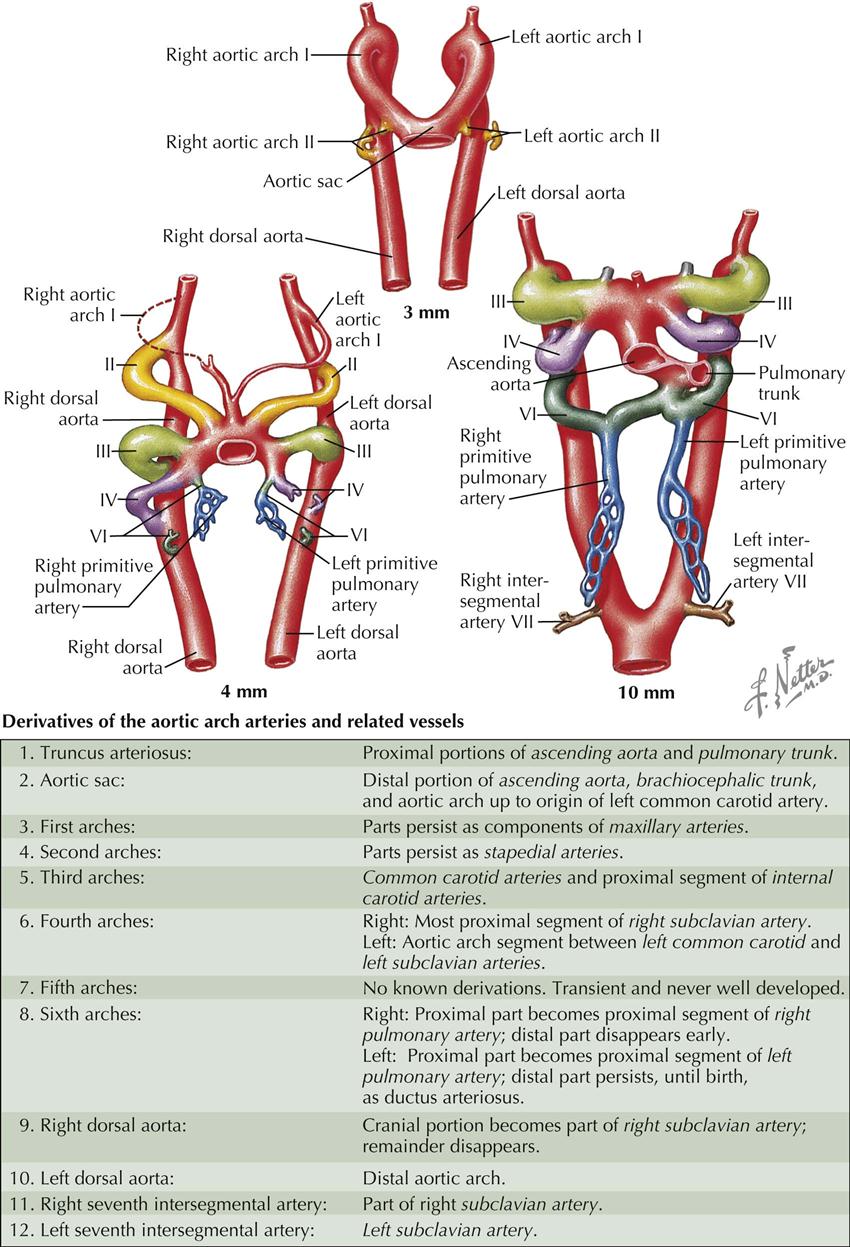
The major arteries in an early embryo are represented by a pair of vessels, the dorsal aortae, which run with the long axis of the embryo and form the continuation of the endocardial heart tubes. Because of the changing position of the cardiogenic mesoderm containing the heart tubes, the cranial portion of each dorsal aorta comes to describe an arc on both sides of the foregut, thus establishing the first pair of aortic arch arteries, termed aortic arches (see Plate 4-14).
In primitive vertebrates, six pairs of aortic arches appear in conjunction with the development of the corresponding pharyngeal (“branchial”) arches, which are transverse swellings of mesenchyme flanking the foregut ventrally and laterally. The pharyngeal arches and their blood supply initially evolved in part to form the gills (branchiae) of aquatic vertebrates, thus their original designation as “branchial arches.” In humans and other lung-breathing vertebrates, five of the six pharyngeal arches are present (the fifth pair of arches and arteries are rudimentary) only in early embryonic life; they become greatly modified in development as their mesenchyme differentiates into the facial skeleton and many other structures and tissues of the head and neck. As part of this process, certain aortic arches (pharyngeal arch arteries) are retained and modified to form the large arteries of the neck and thorax.
Near the end of the third week (3-mm embryo), the first pair of arches is large; the second pair is just forming. The junction of the truncus arteriosus and the first pair of arches is somewhat dilated and is called the aortic sac. From this aortic sac, subsequent aortic arches originate, and new arches are added as the heart and aortic sac undergo relatively caudal displacement. Distally, the dorsal aortae fuse to form a single artery; this fusion progresses in a cranial direction, in both an absolute and a relative sense.
Two days later (4-mm embryo), the first arch has largely disappeared, but part of it persists as a portion of the maxillary artery. The second arch also regresses; all that remains of it is the tiny stapedial artery in the middle ear. The third arch is well developed and large. The fourth and sixth arches form as ventral and dorsal sprouts from the aortic sac and dorsal aortae, respectively, fuse with each other. The ventral portion of the sixth arches already have their major branches, the proximal portions of the left and right primitive pulmonary arteries, even though the arch itself has not yet been completed (see Plate 4-14).
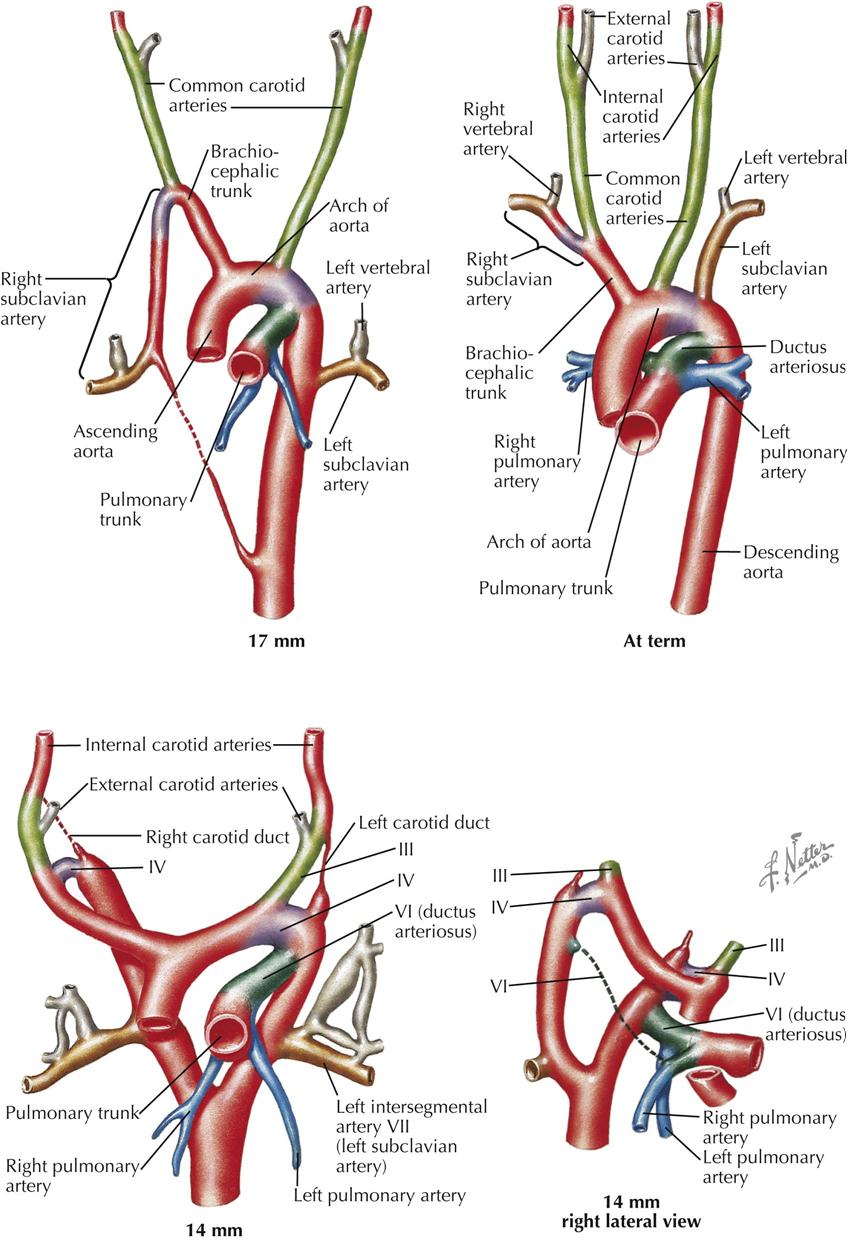
Soon after the end of the fifth week (10-mm embryo), the first two aortic arches have disappeared as such; the third, fourth, and sixth arches are large. The truncus arteriosus and proximal aortic sac have divided into the ascending aorta and pulmonary trunk. The trunk is aligned with the left side of the sixth arch, which becomes the ductus arteriosus, a shunt from the pulmonary trunk into the aorta. The sixth aortic arch on the right disappears, and the left and right pulmonary arteries remain connected to the pulmonary trunk. Intersegmental arteries form between the somites; the seventh cervical pair will play an important role in the formation of the subclavian arteries, and are located at about the level where the dorsal aortae join each other.
By now, the aortic arch system has largely lost its original symmetric pattern (see Plate 4-15). The dorsal aortae between the third and fourth arches have disappeared on each side, and the third arches begin to elongate as the heart descends farther. The aortic sac becomes the arch of the aorta and brachiocephalic trunk, and the third arches extending from these become the left and right common carotid arteries, respectively. The fourth arch on the left becomes the short, descending part of the aortic arch between the left common carotid artery and the slightly more distal connection with the ductus arteriosus. On the right, the fourth arch becomes the proximal portion of the right subclavian artery. The rest of this artery derives from a segment of the right dorsal aorta and right, seventh intersegmental artery. The right dorsal aorta disappears between the developing right subclavian artery and the site where the right dorsal aorta joins the left. If this segment persists as an abnormal origin of the right subclavian artery, it will form a “vascular sling” behind the foregut.
In summary, it may be helpful to think of the aortic arch derivatives from a purely topographic perspective. The pharyngeal (and aortic) arch territories in the embryo extend from the jaws to the thorax. Aortic arches I and II largely disappear, but arch I contributes to the maxillary arteries in the head; and arch VI gives rise to the pulmonary arteries and ductus arteriosus in the mediastinum. The common carotid arteries span the halfway point of the territory of the arches, so it makes sense that these arteries come from arch III in the middle of the sequence. Arch V is not functional, leaving arch IV to contribute to the arteries between the common carotid and pulmonary vessels: the arch of the aorta and the proximal part of the right subclavian artery (see Plates 4-14 and 4-15).
Major Systemic Veins
The development of the great systemic veins is a complex process of clinical importance. Few organ systems in the body are so subject to variations and anomalies in their final, fully developed state. Although generally of little functional significance to the individual, the many venous variations and anomalies can cause confusion in diagnostic angiocardiographic studies and potentially disastrous accidents when surgical correction of cardiac anomalies is attempted.
In the early embryo the major veins develop from an initially plexiform network, and a number of channels run mainly in a longitudinal direction. Three main pairs of veins connect to the sinus venosus. The vitelline veins carry the blood from the umbilical vesicle (yolk sac), the first site of the production of embryonic blood cells. The umbilical veins, initially paired, bring blood from the chorionic villi (developing placenta) into the embryo, entering the sinus venosus lateral to the vitelline veins. The cardinal venous system is entirely intraembryonic. The paired anterior cardinal veins drain the cranial region of the embryo. The posterior cardinal veins arise somewhat later; they drain the body of the embryo, including the large mesonephric kidneys, the first functioning embryonic/fetal kidneys. The anterior and posterior cardinal veins join to form the short common cardinal veins that enter the right and left horns of the sinus venosus just lateral to the umbilical veins.
Soon after the posterior cardinal veins have been established, a new venous system develops as a pair of veins, the subcardinal veins, appear medially to the posterior cardinal veins (see Plate 4-16). Their main function is to drain the urogenital system of the developing embryo: first the mesonephric kidneys and gonads, then the metanephric kidneys (future adult kidneys), gonads, and suprarenal glands. Cranially, the subcardinal veins empty into the posterior cardinal veins.
In the 5-week embryo (8 to 10 mm), the cardinal venous system is symmetric and equally developed bilaterally, but this will rapidly change. The vitelline veins—in the region of the septum transversum, the developing liver, and around the duodenum—have broken up into an anastomosing plexus that will give rise to hepatic veins and the proximal portion of the hepatic portal system of veins. The original left and right vitelline veins connecting this plexus with the sinus venosus are now called hepatocardiac channels. The left vitelline disappears, but the right vein becomes greatly enlarged and persists as the terminal, posthepatic part of the inferior vena cava (IVC). The superior vena cava derives from the right common cardinal vein. The right umbilical vein disappears, and the left umbilical vein connects with the vitelline venous plexus, after which its proximal portion connecting to the sinus venosus also disappears. All the umbilical venous blood now enters the vitelline venous (liver) plexus. A direct route, the ductus venosus, is created between the left umbilical vein and the right hepatocardiac channel (future IVC), allowing most of the umbilical venous blood to enter the right atrium through the IVC.
The subcardinal veins have gained importance, and numerous anastomoses with the posterior cardinal veins have been established. The growing mesonephric kidneys have brought the left and right subcardinal veins closer together, and an anastomosing plexus of veins has developed between them, the intersubcardinal anastomosis. The right subcardinal vein connects to the right hepatocardiac channel to form the hepatic segment of the IVC.
A new venous system appears bilaterally in the caudal region of the embryo, although some view this system as originating from the posterior cardinal veins. It consists of sacrocardinal veins that empty into the posterior cardinal veins. Two smaller caudal veins, the sacrocardinal vein and caudal vein, form the iliac system of veins (common, internal, external branches) and veins of the pelvis.
As the subcardinal veins enlarge, the left posterior cardinal vein decreases in size, and the left horn of the sinus venosus, the future coronary sinus, becomes attenuated. Venous return is shifting to the right side of the embryo. Most the left and right posterior cardinal veins soon disappear as the subcardinal system continues to develop.
The right subcardinal vein and its anastomosis with the right hepatocardiac channel (from the right vitelline vein) rapidly become the principal venous channel to the heart. The anastomosis becomes the intrahepatic component of the IVC, and the right subcardinal vein becomes an infrahepatic segment of the IVC. The subcardinal veins lose their cranial connections with the posterior cardinal veins; remaining branches become the renal and suprarenal veins as well as the gonadal veins.
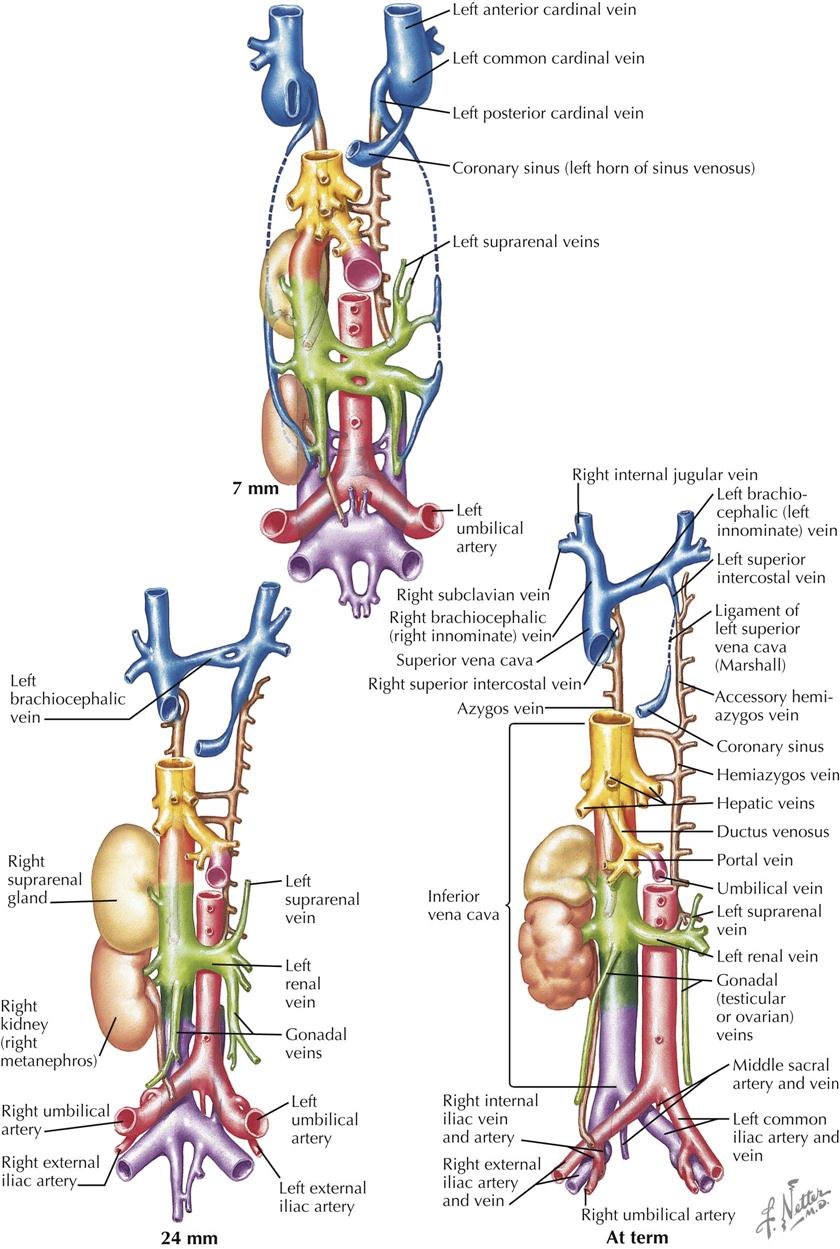
Yet another new venous system appears in the form of two longitudinal channels, the supracardinal veins (see Plate 4-16). Cranially, the veins empty into the terminal part of the posterior cardinal veins, and caudally, anastomose with the subcardinal veins. The supracardinal veins form the azygos system of veins that drain the thoracic body wall by way of the intercostal veins, taking over this function from the posterior cardinal veins. The cranial part of the left supracardinal vein degenerates; the caudal thoracic portion forms the hemiazygos vein that connects over the midline with the right supracardinal vein (developing azygos vein). The azygos vein forms an arch that drains the azygos system into the superior vena cava (SVC). The azygos develops from the terminal portion of the right supracardinal vein and right posterior cardinal vein. Recall that the right common cardinal vein forms the SVC. The right supracardinal vein below its connection to the subcardinal vein enlarges to become the inferior segment of the IVC. The left supracardinal disappears. If the terminal part of the IVC fails to develop from the right vitelline vein, blood from the lower part of the body will enter the right atrium through a greatly enlarged azygos vein.
The upper limbs and head and neck are drained by veins that empty into the left and right anterior cardinal veins (see Plate 4-17). An anastomosis between them forms the left brachiocephalic vein, which brings superior venous return to the right side of the embryo, the same shift that occurs in venous return below the heart. The right anterior cardinal vein becomes the right brachiocephalic vein that continues into the SVC (from right common cardinal vein). The left horn of the sinus venosus has attenuated further. The left anterior and common cardinal veins become the ligament of the left SVC (ligament of Marshall), which is continuous with the coronary sinus (from left horn of sinus venosus). The most common anomaly of the anterior cardinal veins is a persistent left SVC. An oblique vein of the left atrium may also persist. The only functioning remnant of the left anterior cardinal vein is the small, left superior intercostal vein.
The inferior vena cava derives from more primordia than any other vessel and thus merits a summary. The contributions are as follows.
| 1. Terminal part, right vitelline vein: | Posthepatic segment |
| 2. Subcardinohepatic anastomosis: | Hepatic segment |
| 3. Part of right subcardinal vein: | Renal segment |
| 4. Right supracardinal vein: | Prerenal segment |
Of the original six connections to the sinus venosus—paired vitelline, umbilical, and common cardinal veins—only two remain: derivatives of the right common cardinal vein (superior vena cava) and right vitelline vein (IVC). Terminal part of the right vitelline vein is also the hepatocardiac channel.
With three vascular systems in the early embryo and three sequential systems of cardinal veins, it is not surprising that variations and anomalies are extremely common.
Fetal Circulation and Changes at Birth
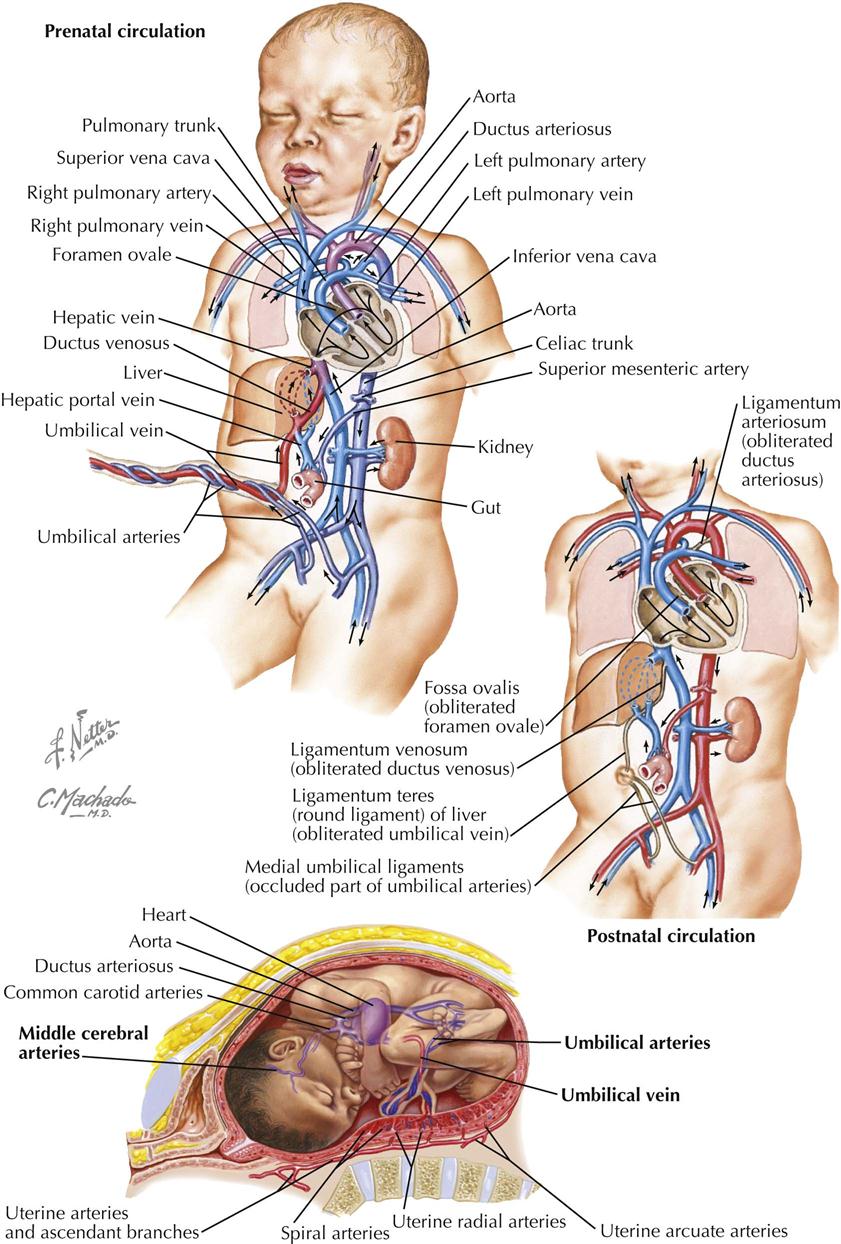
The primary vascular concept of prenatal circulation is the requirement that the intraembryonic circulation of blood bypass the nonfunctioning lungs and liver (see Plate 4-18). The placenta (villous chorion) serves the role of these organs with gas and metabolic exchange between maternal and fetal blood. The airway in the lungs is filled with amniotic fluid, and pulmonary vascular resistance is high. The lungs only receive enough blood to nourish the tissues, and pulmonary venous blood flow into the left atrium is minimal. The plan for the prenatal circulation also requires that it convert the postnatal pattern soon after the first breath of the newborn. Two lung shunts (and a liver shunt) and the design of the interatrial septum serve these needs.
The original placental circulation consists of paired umbilical arteries that pass from the internal iliac arteries to the placenta through the umbilical cord and paired umbilical veins that bring highly oxygenated blood into the embryo to connect with the sinus venosus of the developing heart tube (see Plate 4-19). The right umbilical vein disappears, and the left proximal umbilical vein also disappears as the remaining intraembryonic segment connects with the developing IVC under the liver. As with the lungs, blood flow in the liver is also minimal. Most of the blood in the umbilical vein bypasses the liver through the ductus venosus, a straight continuation of the umbilical vein into the IVC.
After partitioning of the heart tube, the IVC is in line with the foramen ovale in the right atrium. Its stream of blood pushes the septum primum away from the septum secundum, and much of this oxygenated blood passes from the right atrium to the left. This is the first lung shunt. From the left atrium, the blood will pass into the left ventricle and out the ascending aorta.
Venous blood from the SVC is directed at the right atrioventricular valve. Although some mixing of blood occurs from superior and inferior venae cavae, the streams of the blood pass each other to a degree, and the most highly oxygenated blood in the fetal heart ends up on the left side. The blood in the right ventricle passes into the pulmonary trunk and continues into the arch of the aorta via the ductus arteriosus (from sixth aortic arch). This is the second lung shunt based on simple pressure differences between the pulmonary and systemic circulations. Again, vascular resistance in the lungs is high. Pressure is much lower in the aorta, and most of the right ventricle blood flows to the descending aorta. The ductus arteriosus connects with the aortic arch just past the origin of the great arteries. The result is that the brain (and head in general) and upper extremities receive the most highly oxygenated blood in the fetus. There is a mixing of blood from the ductus arteriosus and ascending aorta, but its oxygen content is still higher than typical venous blood, and it is obviously sufficient to address the metabolic needs of the growing lower half of the fetus.
The conversion to the postnatal/adult configuration is triggered by the first breath. Amniotic fluid comes out of the airway, vascular beds open in the lungs, and blood now rushes from the ductus arteriosus into the lungs rather than the aortic arch. Blood flow from the lungs to the left atrium increases rapidly, and the septum primum is pressed against the septum secundum to effectively close the foramen ovale. Over the next few weeks the ductus arteriosus will begin to form a fibrous cord, the ligamentum arteriosum, and the two interatrial septa will permanently fuse.
Blood in the umbilical arteries now has a higher oxygen content, which causes the arteries to spasm. Eventually the umbilical arteries will form fibrous cords, the medial umbilical ligaments, converging on the umbilicus on the internal surface of the abdominal wall. The umbilical vein collapses from lack of blood and eventually becomes the fibrous round ligament of the liver (ligamentum teres). The ductus venosus becomes the ligamentum venosum.