History
Comments
Current Medications
Current Symptoms
Comments
Physical Examination
Laboratory Data
Comments
Electrocardiogram
Findings
Comments
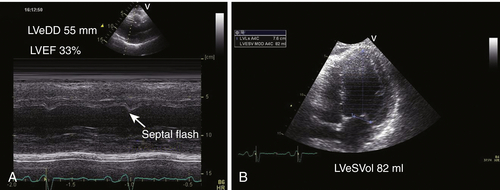
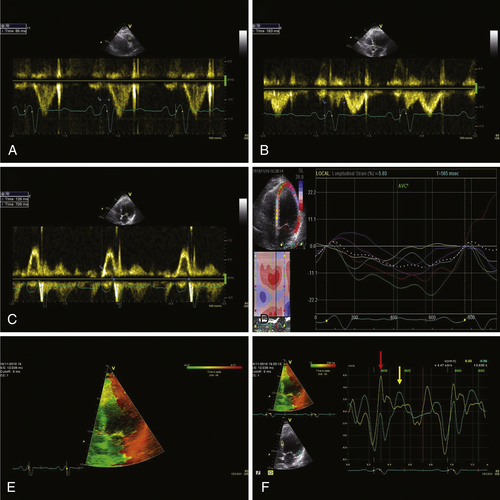
Echocardiogram
Findings
Comments
Findings
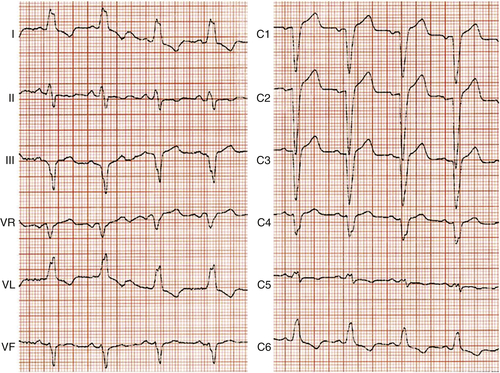
FIGURE 4-1
Catheterization
Coronary Angiography
Findings
Comments
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Findings
Comments
Selected References
1. Bristow M.R., Saxon L.A., Boehmer J. et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Eng J Med. 2004;350:2140–2150.
2. Cleland J.G., Daubert J.C., Erdmann E. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549.
3. Gold M.R., Thébault C., Linde C. et al. The effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) Study. Circulation. 2012;126:822–829.
4. Hsu J.C., Solomon S.D., Bourgoun M. et al. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: The MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with the Cardiac Resynchronization Therapy) Study. J Am Coll Cardiol. 2012;59:2366–2373.
5. Linde C., Abraham W.T., Gold M.R. et al. Randomized trial of cardiac resynchronization therapy in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1823–1843.
6. McMurray J.J., Adamopoulos S., Anker S.D. et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;14:803–869.
7. Moss A.J., Hall W.J., Cannom D.S. et al. Cardiac resynchronization therapy for the prevention of heart failure events. N Eng J Med. 2009;361:1329–1338.
8. Sipahi I., Carrigan T.P., Rowland D.Y. et al. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy. Arch Intern Med. 2011;171:1454–1462.
9. Tang A.S., Wells G.A., Talajic M. et al. Cardiac resynchronization therapy for mild to moderate heart failure. N Engl J Med. 2010;363:2385–2395.
10. Vaillant C., Martins R.P., Donal E. et al. J Am Coll Cardiol. 2013;61:1089–1095.
11. Zareba W., Klein H., Cygankiewicz I. et al. Effectiveness of cardiac resynchronization therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial–Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011;123:1061–1072.