History
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
Electrocardiogram
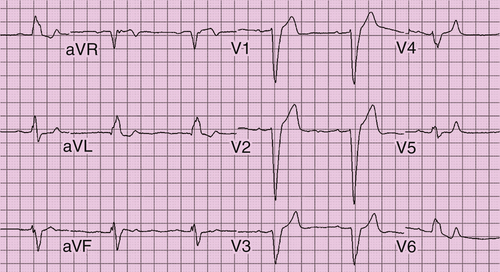
FIGURE 38-1 Case 1. Baseline electrocardiogram showing sinus bradycardia, left bundle branch block, and first-degree atrioventricular block.
Chest Radiograph
Comments
Echocardiogram
Comments
Exercise Testing
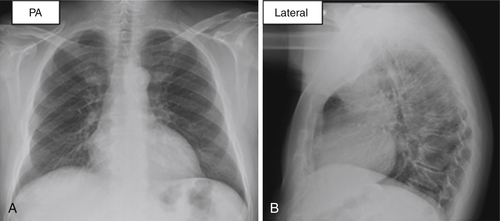
FIGURE 38-2 Case 1. Posteroanterior and lateral views of chest radiograph showing no congestive changes, infiltrate, or effusion.
Cardiac Catheterization
Cardiac Magnetic Resonance Imaging
Final Diagnosis
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
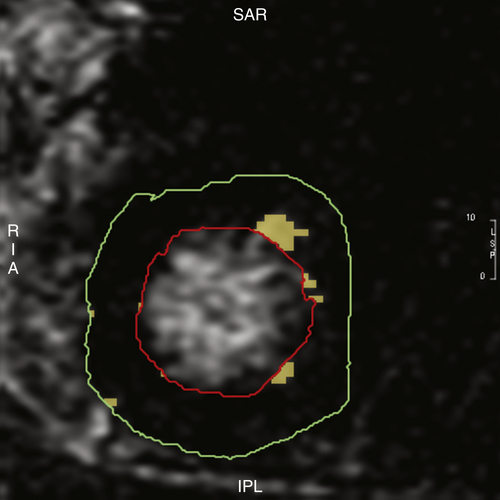
FIGURE 38-3 Case 1. Cardiac magnetic resonance imaging with late gadolinium enhancement suggestive of scar in the anterior and anterolateral segments of left ventricle. Scar burden was estimated to be 2% of the total left ventricular mass.
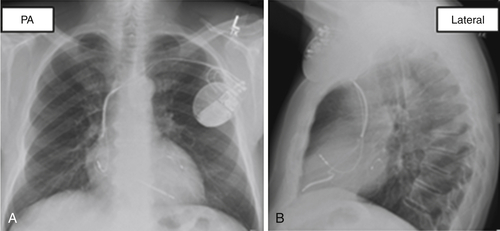
FIGURE 38-4 Case 1. Left ventricular lead location in the basal lateral segment on a postimplant chest radiograph.
Question
Discussion
Plan of Action
Intervention
Outcome
Case 2
| Age | Gender | Occupation | Working Diagnosis |
| 64 Years | Male | Teacher | Acute Coronary Syndrome |

History
Current Medications
Physical Examination
Laboratory Data
Electrocardiogram
Chest Radiograph
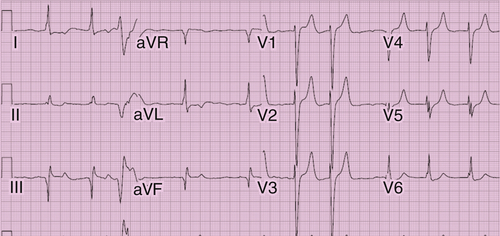
FIGURE 38-5 Case 2. Baseline ECG demonstrating atrial fibrillation and nonspecific interventricular conduction delay.
Echocardiogram
Comments
Exercise Testing
Cardiac Magnetic Resonance Imaging
Question
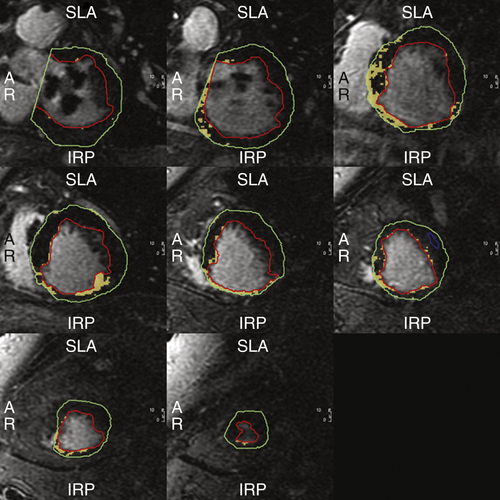
FIGURE 38-6 Case 2. Cardiac magnetic resonance imaging demonstrating extensive inferior wall scar. Extent of myocardial scar is 12.3% of the total left ventricular mass.
Discussion
Question
Discussion
TABLE 38-1
Selected Studies Investigating the Impact of Myocardial Scar on Cardiac Resynchronization Therapy Response
| Study | Patient Characteristics | Scar Assessment | Conclusions |
| Mele et al12 2009 |
71 patients with ICM | Echocardiography | Poor CRT response with a higher number of scar segments and closer location to pacing lead |
| Adelstein et al1 2007 |
50 patients with ICM | Myocardial perfusion Imaging | Higher nonresponse to CRT with higher SPS score, scar density, and greater scar density near the left ventricular lead |
| Ypenburg et al15 2007 | 34 patients with ICM | DE-CMR | Total scar burden was inversely related to CRT response |
| Delgado et al6 2011 |
397 patients with ICM | Speckle-tracking radial strain analysis and DE-CMR | Left ventricular lead location on scar was a predictor of worse outcome |
| Adelstein et al2 2011 | 190 patients with ICM | Thallium-201 SPECT MPI | Higher scar burden (SRS >27) was associated with poor survival |
| Chalil et al5 2007 | 62 patients with ICM | DE-CMR | Presence of posterolateral scar and pacing on scar were independent predictor of response |
| Jansen et al9 2008 |
57 patients with ICM + NICM | CMR | Left ventricular dyssynchrony is more important than scar |
| Ypenburg et al15 2007 |
51 patients with ICM | Technitium-99m SPECT | Both the extent of scar tissue and its location near the left ventricle lead prohibits CRT response |
| Birnie et al4 2009 | 49 patients with ICM and NICM | Rubidium and fluorine-18-fluorodeoxyglucose PET | Responders had less lateral wall scar than nonresponders but a similar extent of global and septal scar |
| Bleeker et al3 2006 |
40 patients with ICM | CMR | Posterolateral wall scar was associated with poor response to CRT |
| Riedlbauchova et al13 2009 | 66 patients with ICM | PET scan | Response to CRT was observed regardless of the presence of total scar and left ventricular lead location in the region of scar or ischemia or hibernation |
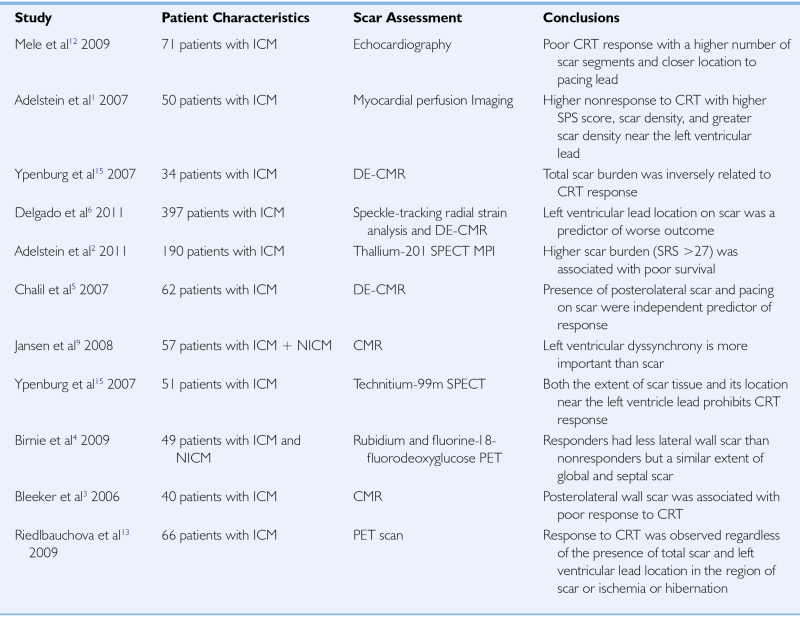
CMR, Cardiac magnetic resonance; CRT, cardiac resynchronization therapy; DE-CMR, delayed-enhancement CMR; ICM, ischemic cardiomyopathy; MPI, myocardial perfusion imaging; NICM, nonischemic cardiomyopathy; PET, positron emission tomography; SPECT, single-photon emission computed tomography; SRS, summed rest score; SPS, summed perfusion score.
Final Diagnosis
Comments
Intervention
Outcome
Selected References
1. Adelstein E.C., Saba S. Scar bruden by myocardial perfusion imaging predicts response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105–112.
2. Adelstein E.C., Tanaka H., Soman P. et al. Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J. 2011;32:93–103.
3. Bleeker G.B., Kaandorp T.A., Lamb H.J. et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976.
4. Birnie D., DeKemp R.A., Ruddy T.D. et al. Effect of lateral wall scar on reverse remodeling with cardiac resynchronization therapy. Heart Rhythm. 2009;6:1721–1726.
5. Chalil S., Foley P.W., Muyhaldeen S.A. et al. Late gadolinium enhancement-cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace. 2007;9:1031–1037.
6. Delgado V., van Bommel R.J., Bertini M. et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to protect long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–78.
7. Epstein A.E., Dimarco J.P., Ellenbogen K.A. et al. ACC/AHA/HRS 2008 Guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:e1–62.
8. Goldenberg I., Moss A.J., Hall W.J. et al. Predictors of response to cardiac resynchronization therapy in the Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011;124:1527–1536.
9. Jansen A.H., Bracke F., van Dantzig J.M. et al. The influence of myocardial scar and dyssynchrony on reverse remodeling in cardiac resynchronization therapy. Eur J Echocardiogr. 2008;9:483–488.
10. Khan F.Z., Virdee M.S., Palmer C.R. et al. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509–1518.
11. Klem I., Weinsaft J.W., Bahnson T.D. et al. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408–420.
12. Mele D., Agricola E., Galderisi M. et al. Echocardiographic myocardial scar burden predicts response to cardiac resynchronization therapy in ischemic heart failure. J Am Soc Echocardiogr. 2009;22:702–708.
13. Riedlbauchova L., Brunken R., Jaber W.A. et al. The impact of myocardial viability on the clinical outcome of cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2009;20:50–57.
14. Xu Y.Z., Cha Y.M., Feng D. et al. Impact of myocardial scarring on outcomes of cardiac resynchronization therapy: extent or location? J Nucl Med. 2012;53:47–54.
15. Ypenburg C., Schalij M.J., Bleeker G.B. et al. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J. 2007;28:33–41.





