History
Comments
Current Medications
Comments
Current Symptoms
Comments
Physical Examination
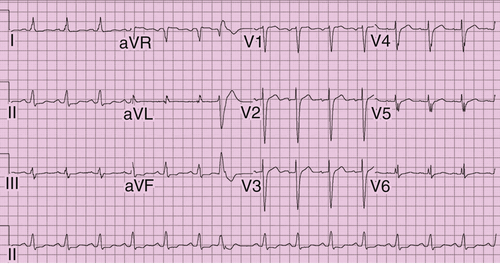
FIGURE 36-1 Electrocardiogram before implant.
Comments
Laboratory Data
Comments
Electrocardiogram
Findings
Comments
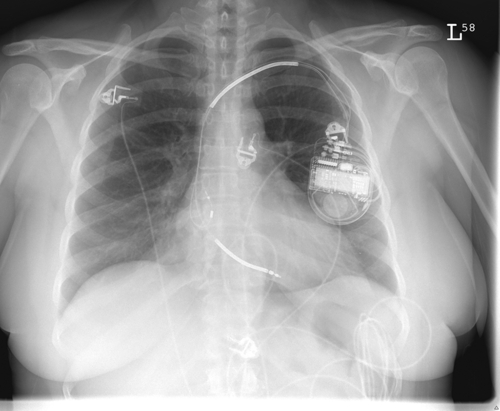
FIGURE 36-2 Chest radiograph.
Chest Radiograph
Findings
Comments
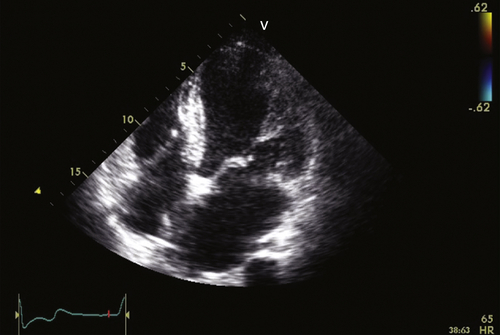
 Echocardiogram
Echocardiogram
Findings
Comments
Findings
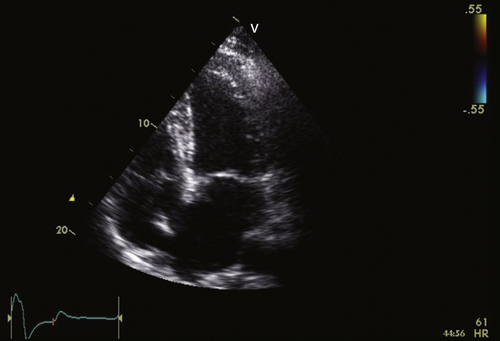
FIGURE 36-3 Apical four-chamber view. See expertconsult.com for video. ![]()
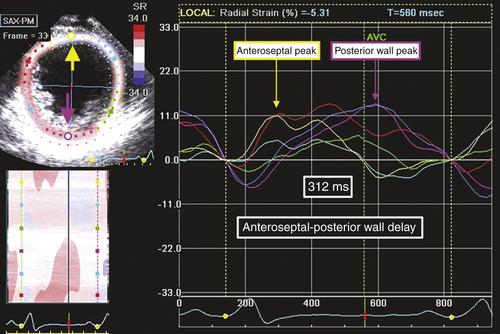
FIGURE 36-4 Speckle tracking radial strain from midventricular short-axis view.
Comments
Findings
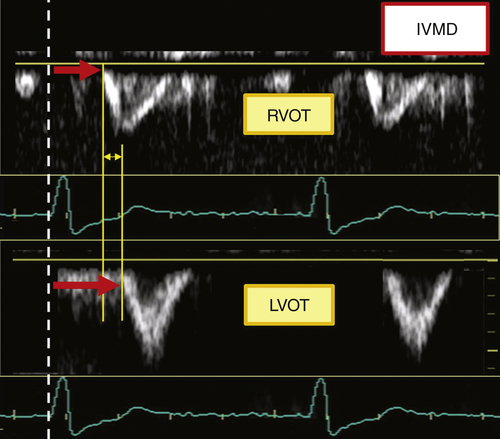
FIGURE 36-5 Pulsed Doppler interventricular mechanical delay (IVMD). LVOT, Left ventricular outflow tract; RVOT, right ventricular outflow tract.
Comments
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Findings
Comments
Selected References
1. Delgado V., van Bommel R.J., Bertini M. et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–78.
2. Gorcsan 3rd. J., Abraham T., Agler D.A. et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191–213.
3. Gorcsan 3rd. J., Oyenuga O., Habib P.J. et al. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation. 2010;122:1910–1918.
4. Hara H., Oyenuga O.A., Tanaka H. et al. The relationship of QRS morphology and mechanical dyssynchrony to long-term outcome following cardiac resynchronization therapy. Eur Heart J. 2012;33:2680–2691.
5. Tracy C.M., Epstein A.E., Darbar D. et al. 2012 ACC/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2012;126:1784–1800.