Procedure 35 Lumbar Total Disk Arthroplasty
Indications
 Symptomatic, single-level degenerative disk disease in the lumbar spine (L3-S1) in skeletally mature patients with no more than grade I spondylolisthesis at the involved level and who have failed nonsurgical treatments for at least 6 months.
Symptomatic, single-level degenerative disk disease in the lumbar spine (L3-S1) in skeletally mature patients with no more than grade I spondylolisthesis at the involved level and who have failed nonsurgical treatments for at least 6 months.
Indications Pitfalls
• Active systemic infection or infection localized to the site of implantation
• Osteopenia or osteoporosis defined as dual energy x-ray absorptiometry (DEXA) bone density–measured T-score less than −1.0
• Allergy or sensitivity to implant materials (cobalt, chromium, molybdenum, polyethylene, titanium)
• Isolated radicular compression syndromes, especially resulting from herniation
• Involved vertebral end plate dimensionally smaller than 34.5 mm in the medial to lateral and/or 27 mm in the anterior to posterior directions
• Clinically compromised vertebral bodies at affected level because of current or past trauma
• Lytic spondylolisthesis or degenerative spondylolisthesis of grade greater than 1
• Scoliosis (lumbar curve greater than 11 degrees)
• Absolute contraindications for an anterior approach are significantly calcified aorta, and extensive abdominal wall reconstructions.
• Relative contraindications for the anterior approach are age, morbid obesity, previous intraabdominal or retroperitoneal surgery, history of severe pelvic inflammatory disease, and previous anterior spinal surgery.
Examination/Imaging
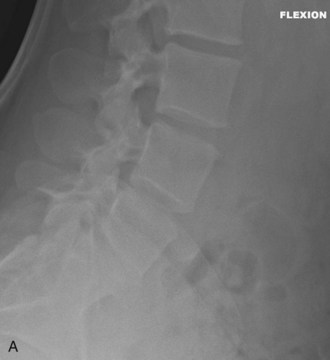
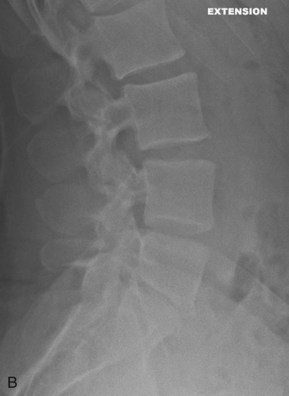
 Figure 35-1, A and B show flexion/extension lateral radiographs showing disk height loss and degeneration at L5-S1. Note the absence of instability.
Figure 35-1, A and B show flexion/extension lateral radiographs showing disk height loss and degeneration at L5-S1. Note the absence of instability.
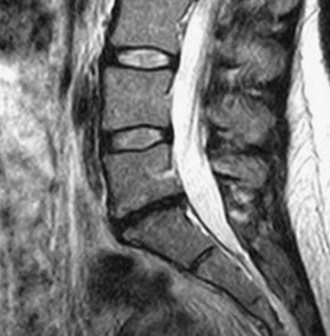
 Use T2 weighted-sagittal magnetic resonance imaging (MRI) to document disk degeneration (Figure 35-2).
Use T2 weighted-sagittal magnetic resonance imaging (MRI) to document disk degeneration (Figure 35-2).
 Use axial MRI images to assess significant facet joint degeneration, which would be a contraindication for arthroplasty.
Use axial MRI images to assess significant facet joint degeneration, which would be a contraindication for arthroplasty.
 Perform preoperative DEXA scan to verify adequate bone density (T-score greater than −1.0) before the procedure.
Perform preoperative DEXA scan to verify adequate bone density (T-score greater than −1.0) before the procedure.
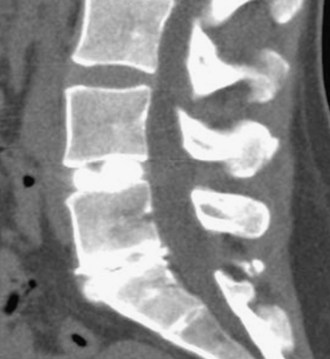
 Figure 35-3 is a computed tomography (CT) diskogram showing morphologic changes at L5-S1; the patient reported 10/10 concordant pain. The L4-5 level was normal with minimal discomfort.
Figure 35-3 is a computed tomography (CT) diskogram showing morphologic changes at L5-S1; the patient reported 10/10 concordant pain. The L4-5 level was normal with minimal discomfort.
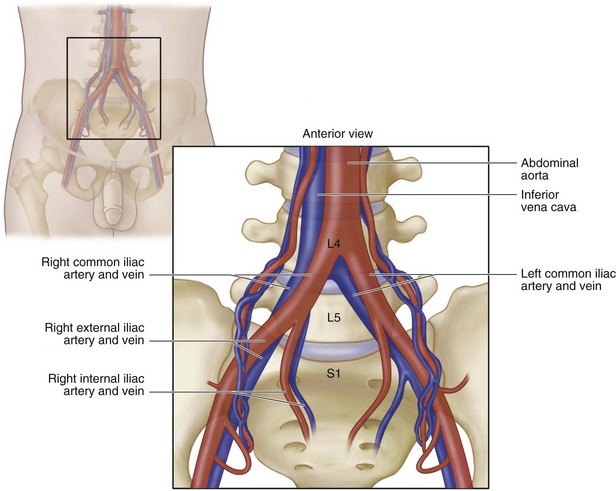
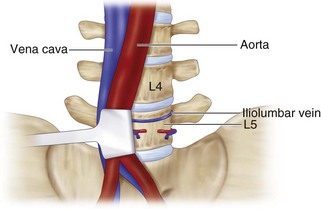
Surgical Anatomy
Positioning
 The patient is positioned supine on a regular operating table with arms padded at the elbow and taped across the chest.
The patient is positioned supine on a regular operating table with arms padded at the elbow and taped across the chest.
Portals/Exposures
 The anterior approach to the lumbar spine is used through either a transverse or horizontal incision.
The anterior approach to the lumbar spine is used through either a transverse or horizontal incision.
 The rectus fascia is incised in line with the skin incision, and the midline fascial raphe of the rectus is identified.
The rectus fascia is incised in line with the skin incision, and the midline fascial raphe of the rectus is identified.
 The retroperitoneal dissection starts on the medial border of the rectus and proceeds lateral and posterior to the muscle belly, having less potential chance of denervation of the rectus.
The retroperitoneal dissection starts on the medial border of the rectus and proceeds lateral and posterior to the muscle belly, having less potential chance of denervation of the rectus.
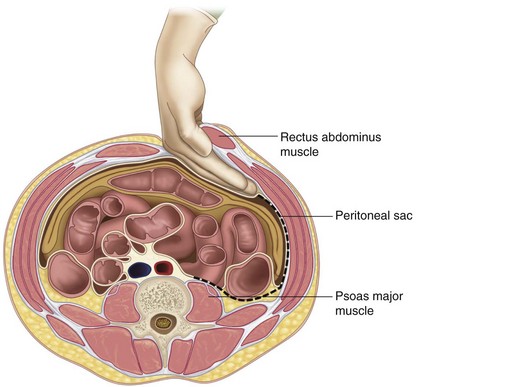
 The plane is bluntly dissected superficial to the abdominal contents along the left abdominal wall outside the peritoneum and taken posteriorly toward the psoas muscle (Figure 35-6).
The plane is bluntly dissected superficial to the abdominal contents along the left abdominal wall outside the peritoneum and taken posteriorly toward the psoas muscle (Figure 35-6).
 The entire peritoneal sac (with the ureter) can be bluntly dissected off the abdominal wall and retracted toward the midline with a handheld retractor.
The entire peritoneal sac (with the ureter) can be bluntly dissected off the abdominal wall and retracted toward the midline with a handheld retractor.
 Insertion of a screw or bent needle into the disk space should be done to verify the level and verify the midline of the disk space with fluoroscopic imaging (mark the position on the anterior spine with Bovie cautery before removing marker).
Insertion of a screw or bent needle into the disk space should be done to verify the level and verify the midline of the disk space with fluoroscopic imaging (mark the position on the anterior spine with Bovie cautery before removing marker).
Portals/Exposures Pearls
• Use of intraoperative fluoroscopy to show angle and level of disk space is helpful to plan an incision, especially when using a horizontal incision.
• For larger patients, a vertical incision is preferred.
• Avoid injury to the inferior epigastric vessels on the underbelly of the rectus muscle.
• The ureter should be identified and retracted along with the peritoneal sac and never dissected separately.
• For L5-S1 procedures, the middle sacral artery should be identified and ligated.
• For L3-4 and L4-5 procedures, segmental vessels may need to be identified and ligated.
• At L4-5, the ascending lumbar vein may limit vascular mobilization and require ligation.
Procedure
Step 1: Diskectomy
 Perform a complete diskectomy (leaving only the lateral annulus and posterior longitudinal ligament), with removal of cartilaginous end plate from both superior and inferior vertebral bodies (Figure 35-7).
Perform a complete diskectomy (leaving only the lateral annulus and posterior longitudinal ligament), with removal of cartilaginous end plate from both superior and inferior vertebral bodies (Figure 35-7).
Step 2: Remobilization
 Release of the posterior longitudinal ligament (PLL) off of the posterior vertebral bodies should be completed using a small curved curette (Figure 35-8).
Release of the posterior longitudinal ligament (PLL) off of the posterior vertebral bodies should be completed using a small curved curette (Figure 35-8).
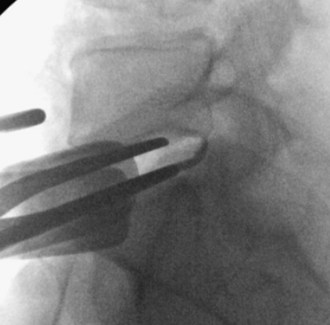
 Specialized distractors and paddles are inserted into the disk space to help with remobilization (Figure 35-9).
Specialized distractors and paddles are inserted into the disk space to help with remobilization (Figure 35-9).
Step 3: Trial Insertion
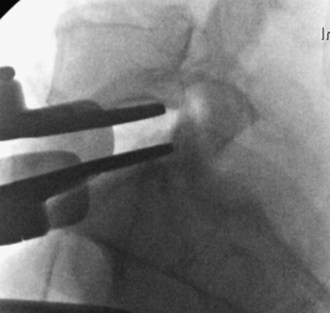
 Based on midline marking, insert appropriately sized trial into disk space under lateral fluoroscopy and visualize on anteroposterior (AP) fluoroscopy for verification (Figure 35-10).
Based on midline marking, insert appropriately sized trial into disk space under lateral fluoroscopy and visualize on anteroposterior (AP) fluoroscopy for verification (Figure 35-10).
 Start with a 10-mm trial, and increase in size depending on the resistance felt and amount of disk height restoration on the lateral fluoroscopic images.
Start with a 10-mm trial, and increase in size depending on the resistance felt and amount of disk height restoration on the lateral fluoroscopic images.
Step 4: Keel Preparation
Step 5: Device Insertion
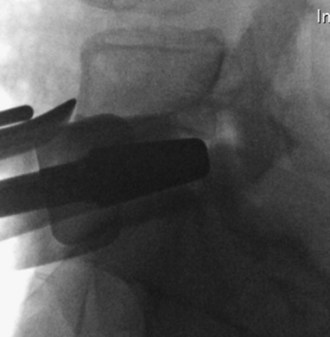
 The arthroplasty device should be inserted as far posterior as possible within the disk space (Figure 35-12).
The arthroplasty device should be inserted as far posterior as possible within the disk space (Figure 35-12).
 Lateral fluoroscopic images should be used frequently to verify the angle at which the device is being inserted and the depth.
Lateral fluoroscopic images should be used frequently to verify the angle at which the device is being inserted and the depth.
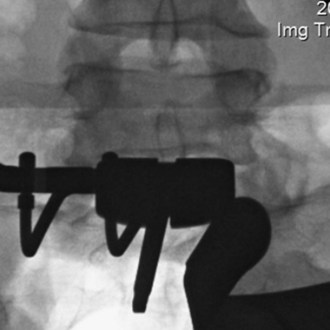
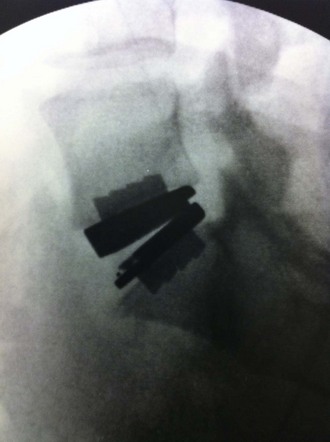
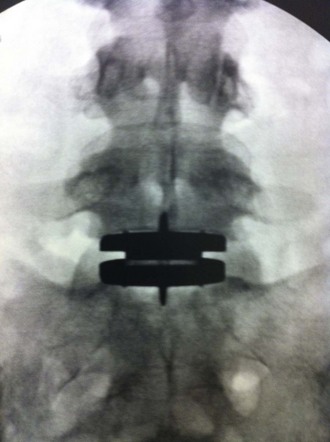
 A final AP image should be taken to verify that the device is positioned in the midline (Figure 35-13).
A final AP image should be taken to verify that the device is positioned in the midline (Figure 35-13).
Postoperative Care and Expected Outcomes
Bertagnoli R, Yue JJ, Shah RV, et al. The treatment of disabling single-level lumbar diskogenic low back pain with total disc arthroplasty utilizing the ProDisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30:2230-2236.
Blumenthal S, McAfee PC, Guyer RD, et al. A prospective, randomized, multicenter Food and Drug Administration Investigational Device Exemptions study of lumbar total disc replacement with the CHARITE Artificial Disc versus lumbar fusion: part I: evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
Brau SA, Delamarter RB, Schiffman ML, et al. Vascular injury during anterior lumbar surgery. Spine J. 2004;4:409-412.
David T. Long-term results of one-level lumbar arthroplasty: minimum 10-year follow-up of the CHARITE Artificial Disc in 106 patients. Spine. 2007;32:661-666.
Guyer RD, McAfee PC, Banco RJ, et al. Prospective, randomized, multicenter Food and Drug Administration Investigational Device Exemption study of lumbar total disc replacement with the CHARITE Artificial Disc versus lumbar fusion: five-year follow-up. Spine J. 2009;9:374-386.
Guyer RD, Tromanhauser SG, Regan JJ. An economic model of one-level lumbar arthroplasty versus fusion. Spine J. 2007;7:558-562.
Lemaire JP, Carrier H, Sariali el-H, et al. Clinical and radiological outcomes with the Charité Artificial Disc: a 10-year minimum follow-up. J Spinal Disord Tech. 2005;18:353-359.
Zigler J, Delamarter R, Spivak JM, et al. Results of the prospective, randomized, multicenter Food and Drug Administration Investigational Device Exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine. 2007;32:1155-1162.