History
Current Medications
Current Symptoms
Physical Examination
Comments
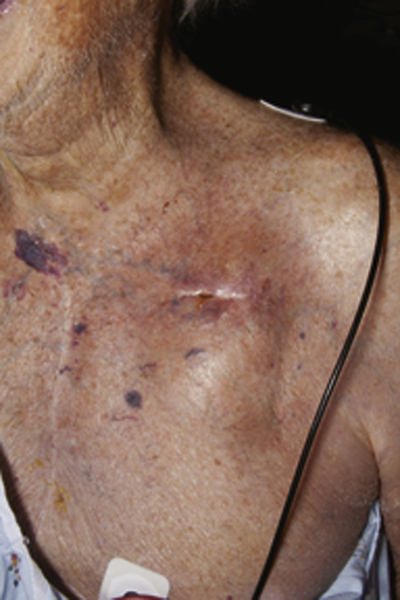
FIGURE 35-1 Pain, redness, and drainage from the device pocket in the left chest wall.
Laboratory Data
Comments
Electrocardiogram
Findings
Comments
Chest Radiograph
Findings
Comments
Echocardiogram
Findings
Comments
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Selected References
1. Baddour L.M., Epstein A.E., Erickson C.C. et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458–477.
2. Le K.Y., Sohail M.R., Friedman P.A. et al. Impact of timing of device removal on mortality in patients with cardiovascular implantable electronic device infections. Heart Rhythm. 2011;8:1678–1685.
3. Mela T., McGovern B.A., Garan H. et al. Long-term infection rates associated with the pectoral versus abdominal approach to cardioverter-defibrillator implants. Am J Cardiol. 2001;88:750–753.
4. Pichlmaier M., Knigina L., Kutschka I. et al. Complete removal as a routine treatment for any cardiovascular implantable electronic device-associated infection. J Thorac Cardiovasc Surg. 2011;142:1482–1490.
5. Romeyer-Bouchard C., Da Costa A., Dauphinot V. et al. Prevalence and risk factors related to infections of cardiac resynchronization therapy devices. Eur Heart J. 2010;31:203–210.
6. Sohail M.R., Hussain S., Le K.Y. et al. Risk factors associated with early- versus late-onset implantable cardioverter-defibrillator infections. J Interv Card Electrophysiol. 2011;31:171–183.
7. Sohail M.R., Uslan D.Z., Khan A.H. et al. Management and outcome of permanent pacemaker and implantable cardioverter-defibrillator infections. J Am Coll Cardiol. 2007;49:1851–1859.
8. Uslan D.Z., Gleva M.J., Warren D.K. et al. Cardiovascular implantable electronic device replacement infections and prevention: results from the REPLACE Registry. Pacing Clin Electrophysiol. 2012;35:81–87.
9. Uslan D.Z., Sohail M.R., St Sauver J.L. et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med. 2007;167:669–675.
10. Voigt A., Shalaby A., Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33:414–419.





