CHAPTER 34. Endocrine Care
Laura Currie
OBJECTIVES
At the conclusion of this chapter, the reader will be able to:
1. Describe the basic function of the endocrine system including the hormones produced by the thyroid, parathyroid, pituitary, and adrenal glands.
2. Identify the signs, symptoms, and diagnostic testing used to assess endocrine gland function.
3. Identify the surgical procedure and perioperative considerations for the patient with hyperthyroidism, hypothyroidism, pheochromocytoma, hypersecretion and hyposecretion of the pituitary and adrenal glands.
4. Identify the postanesthesia plan of care for the patient having subtotal thyroidectomy, bilateral adrenalectomy, hypophysectomy, and parathyroidectomy.
5. Discuss the postanesthesia considerations of the patient with endocrine dysfunctions: thyrotoxicosis, hypercalcemia, Cushing’s syndrome, Addison’s disease, diabetes insipidus, syndrome of inappropriate antidiuretic hormone.
6. Discuss the postanesthesia care of the diabetic patient and diabetic emergencies: hypoglycemia, diabetic ketoacidosis, and hyperglycemic hyperosmolar syndrome.
I. THYROID GLAND
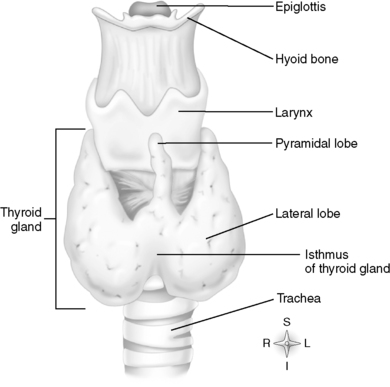
A. Anatomy and physiology (Figure 34-1)
1. Location
a. Sits in anterior portion of the neck
b. Right lobe below the larynx
c. Left lobe beside the trachea
d. Middle portion called the isthmus lies at the base of the neck between second and fourth tracheal rings.
2. Blood supply from external carotid arteries
3. Nerve supply from cervical sympathetic trunk
4. Functions of thyroid gland
a. Regulates energy, metabolism and growth, and development
(1) Hormone production from the hypothalamic-pituitary-thyroid axis
(a) Hypothalamus secretes thyrotropin-releasing hormone (TRH)→stimulates the anterior pituitary to secrete thyroid-stimulating hormone (TSH)→increases the production of the thyroid hormones (THs) thyroxine (T 4) and triiodothyronine (T 3) and the uptake of iodide
(2) Negative feedback loop (Figure 34-2)
(a) Hypothalamus secretes TRH to regulate the synthesis and release of TSH.
(b) When TH levels decrease, TSH and TRH levels increase.
(c) Conversely, if TH levels increase, TSH and TRH levels decrease.
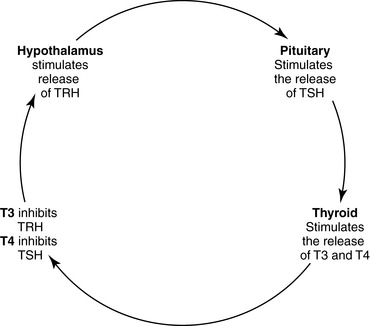 |
| FIGURE 34-2 ▪
Hypothalamus-pituitary-thyroid axis.
|
b. T 3 has a short half-life, and T 4 has a half-life of 5 to 7 days.
c. Peripheral tissue converts T 4 to T 3.
d. T 3 considered the true tissue TH
e. T 4 considered a plasma prohormone
B. Comparison of hyperthyroid and hypothyroid conditions (Table 34-1)
| T3 , Triiodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone. | ||
| Hyperthyroid | Hypothyroid | |
|---|---|---|
| Description | Excessive secretion of thyroid hormones | Insufficient secretion of thyroid hormones |
| Causes |
Multinodular, toxic, diffuse enlargement (goiter)—Graves’ disease
Malignancy
Thyroiditis
Viral, autoimmune
Excessive iodine intake
Amiodarone toxicity secondary to high concentrations of iodine; inhibits the conversion of T 4 to T 3
|
Chronic thyroiditis—progressively destroys thyroid function (Hashimoto’s thyroiditis)
Autoimmune diseases
Iodine deficiency
Surgical removal of thyroid
Secondary dysfunction related to pituitary problems
Tertiary dysfunction related to hypothalamus problems
|
| Signs and symptoms |
Cardiopulmonary
Hypertension
Tachycardia or new atrial fibrillation
Low blood pressure—fluid loss
Potential heart failure
Tachypnea
Eyes/Ears/Nose/Throat
Exophthalmos
Enlarged thyroid /goiter
Hoarseness/difficulty swallowing
Gastrointestinal
Weight loss
Increased peristalsis
Diarrhea and abdominal pain
Musculoskeletal
Body thinness
Muscle atrophy and weakness
Skin
Diaphoresis
Fine, silky, thin hair
Hyperpigmentation
Nervous System
Hyperactive emotional state
Heat intolerance
Insomnia
Genitourinary
Menstrual cycle changes
Infertility
|
Cardiopulmonary
Bradycardia
Decreased cardiac output
High blood pressure—fluid retention
Peripheral vasoconstriction
Increased cholesterol levels
Eyes/Ears/Nose/Throat
Puffy eyes, enlarged tongue
Goiter
Hoarseness/difficulty swallowing
Gastrointestinal
Weight gain
Constipation
Musculoskeletal
Muscle weakness
Joint pain
Skin
Dry
Alopecia
Myxedema (late)
Nervous System
Fatigue, inability to concentrate
Miscellaneous
Cold intolerance
Genitourinary
Heavy menstrual bleeding
Infertility
|
| Diagnostic tests |
TSH decreased
T 3 increased
Free T 4 increased
Thyroid scan: radioactive iodine uptake
Ultrasonography: identification of tumor type
Fine-needle aspiration
|
TSH increased
T 3 decreased
Free T 4 decreased
|
| Operative procedures to correct condition |
Purpose: remove tracheal/esophageal obstructions or malignancy
Subtotal thyroid lobectomy (partial lobe)
Thyroid lobectomy (total lobe)
Total thyroidectomy (removal of entire gland)
|
No specific surgery
Comorbid condition
|
| Preoperative objectives |
Promote a euthyroid state by:
Regulating antithyroid drugs
Controlling hyperdynamic cardiac status
Educate patient and family related to type of surgery/procedure, incision site, drains, and pain.
Thyroid surgery–specific head and neck support when turning
|
Promote a euthyroid state by:
Regulating thyroid replacement
Educate patient and family related to type of surgery/procedure, incision site, drains, and pain.
|
| Anesthesia concerns |
Corneal drying or abrasions
Considerations of agents based on euthyroid state
Stability of cardiac status
Airway status
Oxygen requirements increased with hypermetabolic state and increased temperature
Vocal cord visualization for injury to recurrent laryngeal nerves
|
Predisposition to hypothermia, cardiac failure, and delayed gastric emptying
Metabolism of medications may be delayed.
Adrenal insufficiency: may consider glucocorticoids to correct insufficiency
Neuromuscular weakness may affect weaning.
Potential difficult intubation secondary to predisposition for an enlarged tongue
|
C. Medical therapy: goal is to promote a euthyroid state.
1. Hyperthyroid conditions
a. Inhibition of TH synthesis
(1) Propylthiouracil: 600- to 1000-mg loading dose followed by 200 to 250 mg every 4 hours
(a) Blocks conversion of T 4 and T 3
(b) Administered at least 6 to 12 weeks preoperatively to achieve euthyroid state
(c) Avoid acetylsalicylic acid because it displaces T 3 from protein binding.
(2) Methimazole: 60 to 120 mg/day in divided doses
(a) Blocks uptake of iodine
(b) Administered 6 to 12 weeks preoperatively to achieve euthyroid state
b. Inhibition of TH release
(1) Saturated solution of potassium iodide
(a) 50 mg iodine per drop: 1 to 2 drops three times per day
(b) Iodide blocks T 4 release from the thyroid gland.
(c) Acute management
(2) Lugol’s solution (5% iodine, 10% potassium solution)
(a) 8 mg iodine per drop
(b) Acute management: 4 to 8 drops Lugol’s solution or saturated solution of sodium iodide every 6 to 8 hours; administer at least 2 to 3 hours after initial dosing of inhibitors of TH synthesis.
(3) Lithium carbonate: 300 mg every 6 hours
c. Inhibition of sympathetic nervous system innervation
(1) Beta-blockers first choice
(a) Propanolol: 0.5 to 1 mg intravenously (IV) every 15 minutes as needed, as loading dose until onset of action of oral propranolol (60-80 mg every 4 hours)
(b) Esmolol: loading dose of 250 to 500 mcg/kg followed by infusion of 50 to 100 mcg/kg per minute
(2) Calcium channel blockers if unable to tolerate beta-blockers
d. Prevent peripheral conversion of T 4 to T 3 during acute thyrotoxic storm.
(1) Hydrocortisone: 300 mg initially, followed by 100 mg every 8 hours IV
(2) Dexamethasone: 2 mg IV every 6 hours
(3) Prednisone: 40 mg/day—amiodarone-induced thyrotoxicosis
2. Hypothyroid conditions
a. Replace hormone
(1) Chronic—levothyroxine: 1 to 1.5 mcg/kg per day orally initially; adjust as needed every 6 weeks until TSH in normal range; average dosage 1.6 to 1.8 mcg/kg per day (1.3 mcg/kg per day in the elderly)
(2) Acute—myxedema coma: initial dosage 200 to 500 mcg IV daily; reduce dosage to 50 to 100 mcg IV daily until patient is able to take medication orally.
D. Postanesthesia nursing plan of care (Boxes 34-1 and 34-2)
BOX 34-1
DIFFERENCES BETWEEN THYROTOXIC CRISIS AND MALIGNANT HYPERTHERMIA
| Thyrotoxic Crisis | Malignant Hyperthermia | |
|---|---|---|
| Trigger | Increase in circulating thyroid hormones due to physiological stress | Exposure to anesthetic agents such as succinylcholine and/or volatile inhalation agents |
| Acute signs and symptoms |
Hyperthermia
Tachycardia
Hypercarbia
No muscle rigidity
|
Hyperthermia
Tachycardia
Hypercarbia
Muscle rigidity
|
| Treatment | Beta Blockers Steroids | Dantrolene sodium |
BOX 34-2
POSTANESTHESIA NURSING PLAN OF CARE: THYROID SURGERY/CONDITIONS
Managing Hyperthyroid Conditions After Thyroid Surgery
Nursing Diagnosis
▪ Ineffective airway clearance related to edema of surgical area
▪ Impaired gas exchange related to increased metabolic demands
▪ Alteration in tissue perfusion related to hyperdynamic metabolic state
▪ Ineffective thermoregulation related to hyperdynamic metabolic state
Interventions
Airway Management
▪ Assess for signs of distress resulting from edema of the glottis or hematoma formation: dyspnea, cyanosis, stridor, retraction of neck muscles, tracheal deviation.
▪ Manage secretions to decrease strain on incision line caused by coughing.
▪ Manage oxygenation secondary to increased metabolic demands with supplemental humidified oxygen.
▪ Manage ventilation by monitoring rate, depth, and acid-base balance (arterial blood gases).
Cardiac Status
▪ Assess cardiac status secondary to hypermetabolic state, activation of the sympathetic nervous systems from the stress of surgery.
Wound Management
▪ Assess incision line for wound hemorrhaging (early complication) and report immediately.
▪ Monitor drainage devices if used.
▪ Assess laryngeal nerve damage by quality of vocalization and ability to swallow.
Positioning
▪ Maintain proper positioning after surgery.
30 ° or higher head positioning
Proper neck support by avoiding extreme head flexion or extension
General
▪ Monitor for tetany and hypocalcemia if combined with removal of parathyroid glands.
Laryngeal spasm, tingling in toes, fingers, mouth
Positive Chvostek’s sign: twitching of facial muscles if cheek is tapped over facial nerve
Positive Trousseau’s sign: carpopedal spasm if circulation in arm is impeded with blood pressure cuff
▪ Monitor for thyrotoxic crisis (storm) versus malignant hyperthermia (see Box 34-1).
Managing Hypothyroid Conditions After Surgery
Nursing Diagnosis
▪ Impaired gas exchange related to decreased metabolism of medications
▪ Ineffective airway clearance related to neurological weakness
▪ Ineffective thermoregulation related to decreased metabolic state
▪ Alteration in tissue perfusion related to decreased metabolic state
Interventions
Airway Management
▪ Assess for signs of distress related to neurological weakness, sensitivity to medications, and predisposition for an enlarged tongue.
▪ Manage oxygenation secondary to decreased metabolism of medications.
▪ Manage ventilation by monitoring rate, depth, and acid-base balance (arterial blood gases).
Cardiac Status
▪ Assess for signs and symptoms of low cardiac output/heart failure.
▪ Assess for bradycardia.
Thermoregulation
▪ Monitor temperature secondary to predisposition to hypothermia.
II. PARATHYROID GLANDS (see Figure 34-1)
A. Anatomy and physiology
1. Consists of four small ovoid masses of tissue lying behind the thyroid gland
2. Parathyroid hormone (PTH) secreted from parathyroid glands
a. PTH and vitamin D responsible for the regulation of calcium and phosphorous
b. Serum calcium maintained by:
(1) Regulating bone turnover
(2) Absorption of calcium from the gut (with vitamin D)
(3) Release of calcium in the urine
c. PTH release inhibited by rising serum calcium level
d. PTH release dependent on normal serum magnesium levels
B. Hyperparathyroid disease (Table 34-2)
 |
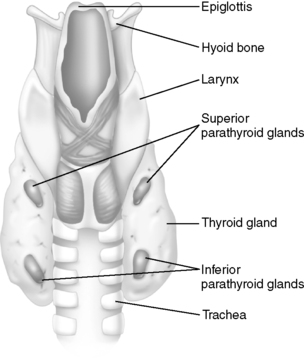 |
| FIGURE 34-1 ▪
Anatomy of thyroid and parathyroid gland.
(From Thibodeau GA, Patton KT: Anatomy & physiology, ed 6, St Louis, 2007, Mosby.)
|
| H2, Histamine type 2; PTH, parathyroid hormone. | ||
| *Normal values vary with laboratories. |
||
| Primary Hyperparathyroidism | Secondary Hyperparathyroidism | |
|---|---|---|
| Description | Excessive secretion of PTH, resulting in hypercalcemia | Hyperplasia of the parathyroid secondary to the dysfunction of another organ or secondary to another condition |
| Causes |
Adenomas (single or multiple gland) most common
Hyperplasia of one or more glands
Malignancies (rare)
Previous head or neck radiation
|
Vitamin D conditions (deficiency, malabsorption, metabolism, osteomalacia [i.e., rickets])
Calcium disorders
Phosphate disorders
Chronic renal failure
|
| Signs and symptoms | Result from hypercalcemia:
Cardiopulmonary
Hypertension
Dysrhythmias
Nervous System
Irritability
Somnolence
Lethargy
Genitourinary
Renal calculi
Polyuria
Musculoskeletal
Osteopenia and osteoporosis
Muscle weakness
Joint or back pain
Gastrointestinal
Abdominal pain
Constipation
Nausea
Risk for gastric ulcers and pancreatitis
|
|
| LABORATORY TESTS* | ||
| PTH |
Normal: 10–65 mg/mL
Hyperparathyroid conditions: Elevated
|
|
| Ionized calcium |
Normal: 4.5–5.6 mg/dL
Hyperparathyroid conditions: Elevated
|
|
| Calcium |
Normal: 9.0–10.5 mg/dL
Hyperparathyroid conditions: Elevated
|
|
| Phosphorus |
Normal: 2.5–4.5 mg/dL
Hyperparathyroid conditions: Decreased
|
|
| Operative procedures to correct condition |
Surgical removal of parathyroid
Total parathyroidectomy: removal of all glands
Partial parathyroidectomy: removal of up to 3.5 of 4 glands
Minimally invasive parathyroidectomy
|
|
| Preoperative objectives |
Treat hypercalcemia and correct associated conditions.
Saline hydration and furosemide administration for rapid correction
Calcitonin
Mithramycin for thrombocytopenia and renal problems
Prednisone
Dysrhythmia management
Educate patient and family related to type of surgery/procedure, incision site, drains, and pain.
Parathyroid surgery–specific head and neck support when turning
|
|
| Anesthesia concerns |
Intravascular volume changes
Postoperative airway obstruction related to recurrent laryngeal nerve injury or bleeding
Renal, cardiac, and nervous system abnormalities
Consider prophylaxis with H 2 receptor blockers.
|
|
C. Postanesthesia nursing plan of care (Box 34-3)
BOX 34-3
POSTANESTHESIA NURSING PLAN OF CARE: PARATHYROID SURGERY
Nursing Diagnosis
▪ Ineffective airway clearance related to edema of surgical area
▪ Impaired gas exchange related to postoperative bleeding or swelling or inability to move secretions
▪ Alteration in fluid and electrolyte balance secondary to total or partial removal of parathyroid gland(s)
▪ Alteration in tissue perfusion related to cardiac dysrhythmias
▪ Altered sensory perception related to postoperative hypocalcemia
Interventions
Airway Management
▪ Assess for signs of distress resulting from edema of the glottis or hematoma formation: dyspnea, cyanosis, stridor, retraction of neck muscles, tracheal deviation.
▪ Manage secretions to decrease strain on incision line caused by coughing.
▪ Manage oxygenation secondary to increased metabolic demands with supplemental humidified oxygen.
▪ Manage ventilation by monitoring rate, depth, and acid-base balance (arterial blood gases).
Cardiac Status
▪ Assess cardiac status secondary to hypocalcemia.
Wound Management
▪ Assess incision line for wound hemorrhaging or hematoma and report immediately.
▪ Assess laryngeal nerve damage by quality of vocalization and ability to swallow.
▪ Maintain proper positioning after surgery.
30 ° or higher head positioning
Proper neck support by avoiding extreme head flexion or extension
General
▪ Monitor for tetany and hypocalcemia with removal of parathyroid glands (immediate to 72 hours postoperatively).
Laryngeal spasm, tingling in toes, fingers, mouth
Positive Chvostek’s sign: twitching of facial muscles if cheek is tapped over facial nerve
Positive Trousseau’s sign: carpopedal spasm if circulation in arm is impeded with blood pressure cuff
▪ Consider treatments for hypocalcemia.
Calcium chloride IV administration
Vitamin D to replace PTH to increase serum calcium level
IV, Intravenous; PTH, parathyroid hormone.
III. PITUITARY GLAND
A. Anatomy and physiology
1. Location: pituitary gland located at the base of the skull in the sphenoid bone
a. Lies within the sella turcica, near the hypothalamus and the optic chiasm
b. Connected to the hypothalamus by the pituitary stalk, which links the endocrine and nervous systems
c. Composed of anterior (80% of gland) and posterior lobes (20% of gland)
B. Pathophysiology
1. Causes of glandular dysfunction
a. Adenomas
b. Malignancies
c. Congenital abnormalities
d. Hypothalamic dysfunction
2. Hormones of the anterior and posterior pituitary gland (Table 34-3)
| Hormone | Normal Physiology | Hypersecretion Conditions | Hyposecretion Conditions |
|---|---|---|---|
| ANTERIOR PITUITARY | |||
| Growth hormone | Stimulates protein synthesis and lipolysis and promotes growth by working with other hormones | Acromegaly | Dwarfism |
| Adrenocorticotropic hormone (ACTH; corticotropin) | Stimulates adrenal glands to produce and release corticosteroids: cortisol (glucocorticoid) and aldosterone (mineralocorticoid) | Cushing’s syndrome | Addison’s disease |
| Thyroid-stimulating hormone (TSH) | Stimulates thyroid gland to produce thyroid hormones |
Hyperthyroid conditions
Graves’ disease
|
Hypothyroid conditions
Myxedema
|
|
Luteinizing hormone (LH)
Follicle-stimulating hormone (FSH) (gonadotropins)
|
Stimulates ovaries and testes to produce estrogen and testosterone
Responsible for ovulation and spermatogenesis
|
Polycystic ovary conditions |
Infertility
Amenorrhea
Decreased sperm production
|
| Prolactin (PRL) | Stimulates mammary glands to produce milk |
Galactorrhea: an increase in milk production in men and non–breast-feeding women
Suppresses production of LH and FSH
|
Reduction in milk production |
| POSTERIOR PITUITARY | |||
| Antidiuretic hormone (ADH) Vasopressin |
Regulation of water by increasing water permeability in renal collecting duct, controlling extracellular fluid osmolality
Regulation of blood pressure by constricting arterioles
|
Syndrome of inappropriate antidiuretic hormone (SIADH) | Diabetes insipidus |
C. Clinical considerations of the anterior and posterior pituitary gland (Tables 34-4 and 34-5)
| ACTH, Adrenocorticotropic hormone (corticotropin); CT, computed tomography; MRI, magnetic resonance imaging; TSH, thyroid-stimulating hormone. | ||
| Hypersecretion | Hyposecretion | |
|---|---|---|
| Clinical signs and symptoms |
Acromegaly
Bone overgrowth or malformations usually of the mandible causing the jaw to protrude
Larynx cartilage may thicken, causing a deep voice
Tongue enlargement
Barrel chest
Joint pain
Coarse body hair
Enlarged sweat glands causing excessive perspiration
Enlarged heart
Headaches
Nerve disturbances
Menstrual changes
|
Dwarfism
Hypothyroidism
Obesity
Headaches
Decreased secondary sexual characteristics
Lethargy
|
| Diagnostic evaluation |
CT/MRI scan of pituitary gland
Increase in hormonal levels of human growth hormone/ACTH levels
|
CT/MRI scan of pituitary gland
Decrease in ACTH or human growth hormone levels
|
| Treatments |
Hypophysectomy—removal of pituitary gland
Craniotomy
Transsphenoidal through the nasal floor
|
Management of target organ disease state
Surgical resection of adenoma
|
| Anesthesia /operative concerns |
Airway management secondary to soft tissue/bone overgrowth
Management of blood pressure secondary to increases in ACTH and TSH
Management of hyperglycemia secondary to increases in ACTH (glucocorticoid release)
Management of dysrhythmias secondary to increases in ACTH and TSH
Management of fluids and electrolytes related to stimulation of ACTH (aldosterone release)
Risk for infection secondary to surgical procedure
|
Airway management secondary to obesity
Management of bradydysrhythmias secondary to decreases in ACTH and TSH
Management of hypoglycemia secondary to decreases in ACTH
Management of core temperature secondary to hypometabolism
Metabolism of medications may be delayed.
Neuromuscular weakness may effect weaning.
|
| ADH, Antidiuretic hormone; BP, blood pressure; CT, computed tomography; CVP, central venous pressure; DI, diabetes insipidus; HR, heart rate; IM, intramuscular; LOC, level of consciousness; MRI, magnetic resonance imaging; SIADH, syndrome of inappropriate antidiuretic hormone (secretion). | ||
| Hypersecretion | Hyposecretion | |
|---|---|---|
| Clinical signs and symptoms |
SIADH
Water intoxication/fluid overload
Headache
Decreased LOC
Decreased urine output
Seizures secondary to hyponatremia
Elevated BP, HR, CVP
Heart failure
Nausea, vomiting, and diarrhea
|
DI
Neurogenic: insufficient synthesis of ADH
Nephrogenic: inability to respond to ADH
Dehydration
Headache, lethargy
Visual disturbances
Increased HR
Decreased BP, CVP, and cardiac output
Polydipsia
Polyuria
|
| Diagnostic evaluation |
CT/MRI scan of pituitary gland for tumors
Increase in plasma levels of ADH
Increased urine osmolality greater than serum osmolality
Decreased urine aldosterone
Dilutional serum hyponatremia
|
CT/MRI scan of pituitary gland
Decrease in ACTH or human growth hormone levels
Hemoconcentrated hypernatremia
Decreased serum ADH with neurogenic DI
Increased serum ADH with nephrogenic DI
Decreased urine osmolality
|
| Treatments |
Hypophysectomy—removal of pituitary gland
Surgical resection of tumors
Restrict fluids.
Sodium level >125 mEq/L: fluid restriction of 800–1000 mL/day. Demeclocycline (Declomycin) can be administered: allows excretion of water because it inhibits the effect of ADH on renal tubules.
Sodium level <105 mEq/L: administer hypertonic (3% saline) infusion over 2–3 hours
Furosemide to increase urinary water excretion
|
Management of target organ disease state
Surgical resection of adenoma
Treat with exogenous ADH (vasopressin/Pitressin).
5–10 units Pitressin subcutaneously or IM
Thiazide diuretics
Replace volume lost by titrating hypotonic or dextrose fluids to urine output.
|
| Anesthesia /operative concerns |
Management of volume secondary to systemic fluid retention
Manage or prevent seizure activity secondary to hyponatremia.
Limit the use of drugs that may increase ADH release (morphine, barbiturates, beta-adrenergics).
|
Management of volume secondary to signs of intravascular dehydration
Management of BP secondary to dehydration
Management of cardiac status secondary to vasopressin administration (potent vasoconstrictor)
|
D. Postanesthesia plan of care: pituitary surgery/conditions (Box 34-4)
BOX 34-4
POSTANESTHESIA PLAN OF CARE: PITUITARY SURGERY/CONDITIONS
Nursing Diagnosis
▪ Potential impaired gas exchange secondary to difficult intubation
▪ Ineffective thermoregulation related to changes in metabolic demands
▪ Potential for infection related to impaired glucocorticoid levels and surgery
▪ Impaired fluid and electrolyte balance related to fluid volume excess or deficit
▪ Potential alteration in neurological status
Interventions
Airway
▪ Assess for signs of distress: dyspnea, cyanosis, stridor, retraction of neck muscles, tracheal deviation.
▪ Manage secretions.
▪ Manage oxygenation secondary to increased metabolic demands with supplemental humidified oxygen.
▪ Manage ventilation by monitoring rate, depth, and acid-base balance (arterial blood gases).
Thermoregulation
▪ Monitor for hyperthermia (hypothalamic influences)
▪ Monitor for hypothermia (from decreased thyroid-stimulating hormone levels).
Infection
▪ Monitor blood glucose levels to range between 80 and 120 mg/dL.
▪ Monitor incisions for signs and symptoms of infection.
Fluid and Electrolytes
▪ Fluid restriction as indicated
▪ Monitor intake, output, and weight.
▪ Monitor electrolytes.
▪ Manage dysrhythmias.
▪ Mouth care to protect mucous membranes
▪ Monitor for signs and symptoms of fluid overload or deficit.
▪ Monitor mental status as a result of fluid status and electrolyte (sodium) imbalance.
▪ Monitor urine specific gravity.
Neurological Status
▪ Monitor for signs of changes in level of consciousness.
▪ Monitor for signs of seizure activity.
▪ Monitor for cerebrospinal fluid leakage at incision site/transsphenoidal approach.
IV. ADRENAL GLANDS
A. Anatomy and physiology
1. Location
a. Lie retroperitoneal beneath the diaphragm capping the medial aspect of the superior pole of each kidney
b. Right adrenal is triangular and adjacent to the inferior vena cava.
c. Left adrenal is round or crescent shaped and sits posterior to the stomach and the pancreas.
2. Adrenal medulla
a. Ten percent of the gland secretes catecholamines.
3. Adrenal cortex
a. Ninety percent of the gland secretes steroids and hormones.
B. Pathophysiology
1. Normal regulation of adrenal hormones
a. Regulated by the release of corticotropin from the hypothalamus
b. Functions of glucocorticoids (cortisol)
(1) Carbohydrate metabolism
(2) Protein metabolism
(3) Promotes lipolysis
(4) Increases tissue responsiveness to other hormones
(5) Anti-inflammatory effects
c. Functions of mineralocorticoids (aldosterone)
(1) Control blood pressure by regulating sodium and water reabsorption
(2) Increases potassium secretion
2. Medullary hormones
a. Catecholamines (epinephrine, norepinephrine, dopamine)
(1) Control blood pressure and heart rate by regulation of sympathetic nervous system
(2) Regulation of gluconeogenesis and lipolysis
C. Adrenal gland conditions (Table 34-6)
| ACTH, Adrenocorticotropic hormone (corticotropin); CT, computed tomography; ECG, electrocardiogram; MRI, magnetic resonance imaging. | ||||
| Hyperaldosteronism | Addison’s Disease | Cushing’s Syndrome | Pheochromocytoma | |
|---|---|---|---|---|
| Definition |
Primary
Overproduction of aldosterone
Secondary
High renin activity from other pathological conditions and hypertension
|
Hyposecretion of cortisol and aldosterone | Hypersecretion of corticosteroids | Overproduction of catecholamines |
| Physiology | Adrenal cortex | Adrenal cortex | Adrenal cortex | Adrenal medulla |
| Etiology |
Primary
Adenomas
Adrenocortical malignancies
Adrenocortical hyperplasia
Secondary
Ascites
Hypertension
Heart failure
Obstructed renal artery disease
|
Autoimmune reaction
Infection
Secondary effect from other glandular conditions
Secondary from steroid therapy for other conditions
Congenital disorders
|
Adenomas
Carcinomas
Overstimulation of adrenal cortex by ACTH release from pituitary gland (negative feedback loop)
Prolonged use of glucocorticoids
Congenital disorders
|
Benign tumor of adrenal medulla
Tumors that secrete high levels of catecholamines (epinephrine and norepinephrine)
|
| Effects of conditions |
Primary
Hypernatremia
Hypervolemia
Hypertension
Hypokalemia
Weakness
Paresthesias
Tetany
Hyperglycemia
Secondary
Hypovolemia
Hyponatremia
Hypokalemia
|
From Aldosterone Deficiency
Hypernatremia
Hypokalemia
Weakness
Dizziness
Polyuria
From Corticosteroid Deficiency
Hypoglycemia
Hyperpigmentation from excessive corticotropin stimulation as a result of pituitary stimulation (negative feedback loop)
Addisonian crisis
Prolonged hypotension and cardiac dysrhythmias
Shock
Lack of response to vasopressors
|
From Corticosteroid Hypersecretion
Altered distribution of body fat primarily to the back of the neck (“buffalo hump”) and the trunk of the body (centripetal)
“Moon face”
Ecchymosis
Osteoporosis
Poor wound healing
|
Severe hypertension
Hyperglycemia
Hypermetabolism
Increased levels of norepinephrine and epinephrine
Tachycardia
Palpitations
Nausea
Weight loss
Abdominal pain
Irritability
Diaphoresis
Headaches
Visual disturbances
|
| Diagnostic evaluation |
Elevated sodium
Decreased potassium
Increased urinary excretion of aldosterone
Hyperglycemia and glycosuria
ECG changes secondary to electrolyte imbalance
CT/MRI scan of adrenal gland
Hyperglycemia clinical signs
|
Elevated potassium
Decreased sodium
CT/MRI scan of adrenal gland
ACTH stimulation test: failure to stimulate ACTH helps with confirming diagnosis of Addison’s disease
Hypoglycemia
|
Elevated cortisol levels
Decreased potassium levels
Increase plasma ACTH levels (if pituitary cause)
Decrease in eosinophils
CT/MRI scan of adrenal gland
Dexamethasone suppression test: failure to suppress cortisol helps to confirm a diagnosis of Cushing’s syndrome
|
Increase in serum catecholamines (epinephrine and norepinephrine)
Increase in urine catecholamines
CT/MRI scan of adrenal gland
Clonidine administration: decreases plasma norepinephrine
|
| Treatments | Adrenalectomy (unilateral or bilateral) | Corticosteroid administration | Adrenalectomy (unilateral or bilateral) | Adrenalectomy |
| Anesthesia/operative/postanesthesia concerns |
Management of blood pressure
Assess lung expansion postoperatively
Hypertension
Antihypertensive agents
Vasodilators
Angiotensin-converting enzyme inhibitors
Hypotension
Volume expanders (blood, albumin)
Vasopressors
Dysrhythmia management secondary to potassium changes and catecholamine releases
|
Management of corticosteroid administration
Preoperative usage from preexisting conditions such as arthritis, colitis, asthma
Inadequate corticosteroid replacement during and after surgical procedures
|
Airway management
Compromised lung expansion secondary to truncal obesity or moon face
Positioning for exposure and preventing stress fractures or skin trauma
Managing blood pressure
Managing hyperglycemia
|
Avoidance of medications causing histamine release of sympathetic stimulation
Management of blood pressure before and after excision of tumor
Before: hypertensive management with vasodilators
After: rebound hypotension with vasopressors, blood expanders, cortisol replacement
|
D. Postanesthesia nursing plan of care: adrenal gland surgery/conditions (Box 34-5)
BOX 34-5
POSTANESTHESIA NURSING PLAN OF CARE: ADRENAL GLAND SURGERY/CONDITIONS
Nursing Diagnosis
▪ Potential for alterations in neurological status secondary to hypertension, increased circulating catecholamines, or rapid removal/change in circulating catecholamines after removal of a pheochromocytoma
▪ Potential for impaired gas exchange related to postoperative atelectasis/pneumothorax secondary to 12th rib resection during adrenalectomy
▪ Altered cardiac output secondary to activation or inactivation of the sympathetic nervous system
▪ Alteration in tissue perfusion secondary to cardiac dysrhythmias related to electrolyte disturbances
▪ Alteration in fluid and electrolyte balance secondary to adrenalectomy/excision of pheochromocytoma
▪ Potential for infection secondary to hyperglycemia
Interventions
Neurological Status
▪ Monitor for signs/sudden changes in level of consciousness.
▪ Assess pupils for reactivity/light accommodation.
Airway Management
▪ Assess for signs of distress secondary to risk of atelectasis/pneumothorax: dyspnea, cyanosis, stridor, retraction of neck muscles, tracheal deviation.
▪ Manage secretions to minimize risk of hypoxemia.
▪ Evaluate lung expansion by chest x-ray verification.
▪ Manage oxygenation with supplemental humidified oxygen.
▪ Manage ventilation by monitoring rate, depth, and acid-base balance (arterial blood gases).
Cardiac Management
▪ Assess and treat hyper/hypotension
▪ Administer vasoactives as needed to maintain hemodynamics secondary to decreased circulating catecholamines.
▪ Administer vasodilators as needed secondary to hypertension caused by pheochromocytoma.
▪ Monitor for bleeding.
▪ Monitor for rebound epinephrine shock secondary to insensitive receptors and impaired vascular reflexes.
▪ Maintain hemodynamics as indicated.
▪ Monitor laboratory values: changes in serum sodium, potassium, and glucose.
▪ Administer IV fluids (hypertonic saline for low serum sodium levels), blood products, albumin as indicated.
Wound Management
▪ Assess incision line for wound hemorrhaging or hematoma and report immediately.
▪ Monitor for signs and symptoms of infection: redness, swelling, increased tenderness.
▪ Monitor for trends in WBC counts
IV, Intravenous; WBC, white blood cell.
V. DIABETES MELLITUS IN THE SURGICAL PATIENT
A. Pathophysiology
1. Etiology
a. Deficits in insulin secretion, action, or both
b. Chronic hyperglycemia can lead to dysfunction and failure of various organs, especially the eyes, kidneys, nerves, heart, and blood vessels.
c. Diabetic patient at higher risk for surgery and anesthetic complications than nondiabetic patient
2. Types of diabetes mellitus
a. Type 1 (ketosis prone)
(1) Characteristics
(a) Insulin deficient
(b) Ketotic
(c) Children and young adults
(d) Rarely obese
(e) Prone to other autoimmune disorders
(f) Accounts for 5% to 10% of the population with diabetes
(2) Causes
(a) Genetic
(b) Autoimmune destruction of pancreatic beta cells (insulin-producing cells)
(c) Environmental
b. Type 2
(1) Characteristics
(a) Insulin resistance and insulin deficiency
(b) Nonketotic
(c) Overweight/obese
(d) Accounts for 90% to 95% of the population with diabetes
(2) Causes
(a) Resistance to insulin action
(b) Inadequate compensatory insulin secretory response
(c) Risk factors:
(i) Age
(ii) Obesity
(iii) Inactivity
(iv) Hypertension
(v) Dyslipidemia
3. Other types of diabetes mellitus
a. Gestational
b. Drug or chemical induced
c. Genetic defects in beta-cell function
d. Genetic defects in insulin action
e. Diseases of the exocrine pancreas
f. Infections
g. Endocrinopathies
B. Perioperative considerations
1. Preoperative evaluation
a. Assess for macrovascular complications secondary to diabetes.
(1) Coronary artery disease (CAD) (see Chapter 32)
(a) Common cause of mortality in diabetic patients
(b) Assess for pain/electrocardiogram changes, serum troponin values secondary to incidence of painless angina.
(c) Lipid profile
(2) Peripheral vascular system
(a) Shiny taut skin
(b) Diminished or absent pulses
(c) Loss of hair on lower extremity
(d) Cool extremities
(e) Leg pain at rest/night
(f) Intermittent claudication
(g) Color changes in legs with positioning (red with legs dependent; white with legs elevated)
(3) Cerebral circulation
(a) History of transient ischemic attacks/stroke
(b) Confusion/disorientation
(c) Chronic hypertension
b. Assess for microvascular complications.
(1) Diabetic nephropathy
(a) Serum creatinine
(b) Blood urea nitrogen
(c) Albumin levels in urine
(d) Urinary output
(2) Diabetic retinopathy
(a) Presence of cataracts
(3) Diabetic neuropathy
(a) Postural hypotension
(b) Sensory motor impairment
(c) Genitourinary impairment
(d) Delayed gastric emptying
c. Assess laboratory values.
(1) Glucose levels before surgery
(2) Glycosylated hemoglobin (hemoglobin A1C) to determine long-term (3 months) control of diabetes
(3) Electrolytes
(a) Potassium
(b) Sodium
(c) Chloride
(d) Bicarbonate
(4) Creatine Kinase
(5) Troponin
d. Assess for medications associated with altering glucose levels.
(1) Medications associated with contributing to hyperglycemia
(a) Thiazides and loop diuretics
(b) Glucocorticoids
(c) Dilantin
(d) Calcium channel blockers
(e) H 2 receptor blockers
(f) Beta-adrenergic receptor agonists
(g) Morphine sulfate
(2) Medications associated with contributing to hypoglycemia
(a) Insulin
(b) Sulfonylureas
(c) Beta-adrenergic receptor antagonists
(d) Angiotensin-converting enzyme inhibitors
(e) Alcohol
2. Intraoperative and anesthesia considerations
a. Glycemic control to target range during surgery
(1) Stress of surgery contributes to insulin resistance in all patients.
(2) Prevent diabetic emergencies such as diabetic ketoacidosis and hyperglycemic hyperosmolar syndrome.
(a) Frequent blood glucose monitoring intraoperatively
(b) Maintain patient in well-hydrated anabolic state.
b. Anesthetic agents
(1) No specific anesthetic for diabetic patients
(2) Inhalation agents may cause less pronounced changes in blood glucose.
(3) Regional blocks may be considered because they cause fewer metabolic disturbances.
c. Avoidance of hypoglycemia and serious cerebral dysfunction
(1) Maintain blood glucose levels for diabetic patients undergoing surgery to target ranges (American Diabetes Association recommends 80–110 mg/dL; Box 34-6).
BOX 34-6
EVIDENCED-BASED PRACTICE CONSIDERATIONS FOR PATIENTS WITH DIABETES MELLITUS
According to the American Diabetic Association (2008) position statement titled Standards of Medical Care in Diabetes Mellitus, targeting glucose control in the hospital can potentially improve mortality, morbidity, and health economic outcomes. The patient can manifest hyperglycemia from the stress of the hospitalization or the procedure, the withholding of antihyperglycemic medications or the administration of hyperglycemia-provoking agents such as glucocorticoids or vasopressors, or the decompensation of type 1, type 2 diabetes. Many studies have looked at varying populations of hospitalized patients and the effects of targeted glucose on morbidity and mortality. It is recommended that the target glucose for critically ill patients should be 80-110 mg/dL. The incidence of hypoglycemia was found to be higher in medical critically patients compared with surgical critically ill patients.
Specific to perianesthesia care, most of the studies on glycemic control have been done on the cardiac surgical population. These patients experienced the lowest mortality rates and risks of sternal wound infections with a target blood glucose <150 mg/dL. This can translate to perioperative hyperglycemia as a predictor of infection in patients with diabetes.
From this review, many variations of insulin protocols and regimens have been created.
Each patient’s clinical situation should be evaluated and goals for glycemic control targeted and achieved. It is recommended that hospital facilities develop standards for glycemic control and provide support to achieve the goals. In addition, quality improvement initiatives should be developed to evaluate progress and facilitate improvement.
(2) Monitor for signs of hypoglycemia during surgery.
(a) Elevated heart rate
(b) Decrease in urinary output
(c) Seizure activity
d. Avoidance of hyperglycemia
(1) Assess for increase in urine output.
(2) Assess for risk of intravascular dehydration secondary to osmotic diuresis.
(3) Assess for hyperglycemia with administration of vasoactive agents such as epinephrine.
e. Avoidance of cardiopulmonary complications
(1) Prevent myocardial infarction.
(a) Consider perioperative beta-blockers.
(b) Monitor electrolytes: potassium, magnesium.
(c) Maintain hemodynamics.
(d) Monitor and treat dysrhythmias.
(2) Prevent hypotension caused by increased urinary output by administering IV fluid.
(3) Prevent hypoxemia.
(a) Assess glycosylated hemoglobin: increased levels influence tissue oxygenation.
(b) Assess oxygen saturations: oxygen consumption increased secondary to increased shunting as a result of general anesthesia.
f. Avoidance of injury
(1) Maintain proper positioning during surgery secondary to peripheral neuropathy.
(2) Monitor for risk of aspiration secondary to impaired gastric emptying (gastroparesis).
(a) Consider rapid sequence induction.
(b) Elevate the head to decrease the risk when possible.
3. Diabetic emergencies (Table 34-7)
| DKA, Diabetic ketoacidosis; ECG, electrocardiogram; HCO3, bicarbonate; HHS, hyperglycemic hyperosmolar syndrome; IV, intravenous. | |||
| Hypoglycemia | DIABETIC KETOACIDOSIS | HYPERGLYCEMIC HYPEROSMOLAR SYNDROME | |
|---|---|---|---|
|
Characteristics/
clinical signs
|
Shakiness/tremors
Diaphoresis
Tachycardia
Irritability
Decreased level of consciousness
Confusion
Slurred speech
Seizures
Coma
|
Type 1 diabetics
Polydipsia
Polyuria
Polyphagia
Decreased level of consciousness
Warm and dry
Decreased blood pressure
Elevated heart rate
ECG changes: tall, peaked T waves
Abdominal pain
Nausea and vomiting
Kussmaul’s respirations
Fruity acetone breath
|
Type 2 diabetics
Polydipsia
Polyuria
Polyphagia
Decreased level of consciousness
Warm and dry or cool and moist
Normal or decreased blood pressure
Normal heart rate
Abdominal pain
Nausea and vomiting
|
| Possible causes |
Interactions with other drugs
Alcohol
Insulin overdose or incorrect dosages
Inadequate food intake
Hormonal deficiencies
Renal diseases
Neoplasms
|
Infection
Poorly controlled type 1 diabetes
Insulin omission
Surgical stress
Medications that interfere with insulin
|
Precipitated by an acute illness
Poorly controlled type 2 diabetes
Infection: pneumonia and urinary tract infections most common
Surgical stress
Medications that interfere with insulin
|
| Laboratory values | Glucose <60 mg/dL |
Glucose 250–800 mg/dL
pH <7.3
HCO 3 <15 mEq/L
Serum and urine ketones >2+
Elevated potassium
|
Glucose >800 mg/dL
pH >7.3
HCO 3 >15 mEq/L
Serum and urine ketones <2+
|
| Treatments |
Mild Reactions
Administer 10–15 g carbohydrate (i.e., 4 oz of orange juice) or inject 1 mg glucagon or via feeding tube, administer a liquid source of glucose (soda).
Moderate and Severe Reactions
Administer 50% dextrose equivalent to 25 g glucose and follow with continuous IV infusion.
Monitor glucose levels frequently for several hours.
|
Treatment goals are similar for both DKA and HHS.
Administer fluids.
0.9% normal saline at rapid rates (adjusted for other comorbidities and corrected sodium levels)
Titrate with consideration to urine output, blood pressure, and central venous pressures.
Administer 5% dextrose in 0.45% normal saline once the blood glucose level reaches 250 mg/dL.
Treat hyperglycemia (protocols may vary).
Initiate IV bolus of regular insulin recommended at 0.15 units/kg as IV bolus.
Continuous IV insulin infusion recommended at a rate of 0.1 units/kg per hour. Titrate to target ranges as indicated.
Decrease insulin infusion at a glucose level of 250 mg/dL to decrease the risk of hypoglycemia and to protect against cerebral edema.
Replace electrolytes IV as needed.
Potassium
Magnesium
Calcium
Replace lost bicarbonate as needed.
|
|
4. Postanesthesia nursing plan of care: diabetes mellitus (Box 34-7)
BOX 34-7
POSTANESTHESIA NURSING PLAN OF CARE: DIABETES MELLITUS
Nursing Diagnosis (Actual or Potential)
▪ Alteration in cerebral circulation secondary to hypoglycemia or hyperglycemia
▪ Impaired gas exchange secondary to hypoxemia
▪ Decreased cardiac output secondary to cardiovascular complications from diabetes
▪ Fluid volume deficit secondary to hyperglycemia
▪ Infection secondary to diabetes
Interventions
▪ Monitor and manage serum glucose to target range.
▪ Assess for and treat diabetic emergencies as indicated.
Neurological Management
▪ Assess level of consciousness.
▪ Assess for changes in cerebral function.
Slurred speech
Seizure activity
Irritability
Airway Management
▪ Assess for signs of distress secondary to hypoxemia: dyspnea, cyanosis, stridor, retraction of neck muscles, tracheal deviation.
▪ Manage secretions to increase oxygenation.
▪ Manage oxygenation secondary to hypoxemia with supplemental humidified oxygen.
▪ Manage ventilation by monitoring rate, depth, and acid-base balance (arterial blood gases).
Cardiovascular Management
▪ Assess for dysrhythmias secondary to electrolyte imbalance.
▪ Assess extremities for color, sensation, and motor function.
▪ Maintain hemodynamics.
Fluid Volume Management
▪ Monitor intake and output.
▪ Continue hydration as indicated.
▪ Monitor electrolytes.
Infection
▪ Monitor for signs and symptoms of infection.
▪ Assess incision site and/or invasive line site for erythema, pain, purulent drainage.
▪ Assess WBC counts for trends.
WBC, White blood cell.
VI. PANCREAS TRANSPLANTATION
A. Overview
1. Select criteria/considerations for patient selection for pancreas transplantation
a. Frequent episodes of hypoglycemia, marked hyperglycemia, ketoacidosis requiring medical attention
b. Clinical and emotional problems with exogenous insulin therapy or administration
c. Consistent failure of insulin-based management to prevent acute complications
d. Diabetes for at least 20 years
e. Risks of secondary complications of diabetes mellitus are greater than those of the surgical procedure and the posttransplant immunosuppression.
2. Goals of pancreas transplantation
a. Improve the quality of life of people with diabetes, by eliminating the acute complications commonly experienced by patients with type 1 diabetes (hypoglycemia, marked hyperglycemia, and ketoacidosis).
b. Eliminate the need for exogenous insulin, frequent daily blood glucose measurements, and many of the dietary restrictions.
c. Reverse or stabilize the long-term renal and neural complications of diabetes.
d. Frequently combined with kidney transplant
B. Preoperative assessment
1. Health history
a. Absence of infection
(1) Screened for remote infection (i.e., urinary tract infection, respiratory, dental)
(2) Preoperative antibiotics
b. Central nervous system
(1) Evaluate mental/emotional illness secondary to postoperative compliance.
(2) Evaluate for autonomic neuropathy.
(a) Gastroparesis
(b) Cystopathy
(c) Orthostatic hypotension
c. Coronary artery disease
d. Renal disease
e. Peripheral vascular disease
f. Sensory neuropathies
2. Preoperative preparation
a. Patient/family education to long-term management
b. Invasive lines
C. Anesthesia/operative concerns
1. Rapid induction may be considered secondary to gastroparesis.
2. Control serum glucose levels during surgery to range of 80 to 110 mg/dL.
3. Manage electrolyte levels, with primary emphasis on maintaining potassium at levels of 3.5 to 5.3 mEq/L.
4. Administration of immunosuppressive agents before graft reperfusion
a. High-dose immunosuppressants administered
(1) Antilymphocyte antibody induction therapeutic agents intraoperatively and early postoperatively
(2) Steroid agents/immunosuppressants for maintenance
5. Positioning secondary to long surgical time
6. Operative techniques
a. Provide adequate arterial blood flow to the pancreas and duodenal segment during transplantation.
b. Provide adequate venous outflow of the pancreas via the portal vein.
c. Pancreas graft arterial revascularization using the recipient right common or external iliac artery
d. The Y-graft portal vein anastomosed to iliac vein
D. Postanesthesia concerns
1. Control of glucose level to 80 to 110 mg/dL
a. Insulin infusion indicated versus bolusing to maintain a steady euglycemic state
b. Decrease in insulin infusion after surgery common secondary to euglycemic condition after transplantation
2. Manage volume as indicated by patient condition.
a. Blood products may be indicated.
b. IV hydration as appropriate to clinical condition
c. Monitor urine output.
3. Wound management
4. Pain management
5. Skin and sensory perception management secondary to prolonged positioning
6. Postoperative complications
a. Thrombosis secondary to low-flow states of the graft: first 24 to 48 hours after surgery
b. Pancreatitis: frequent and temporary; seen 48 to 96 hours postoperatively; evidenced by elevated serum amylase level
BIBLIOGRAPHY
1. American Diabetes Association, Diagnosis and classification of diabetes mellitus, Diabetes Care 31 (Suppl 1) ( 2008) S55–S60.
2. American Diabetes Association, Position statement standards of medical care in diabetes mellitus, http://care.diabetesjournals.org/cgi/content/full/31/Supplement_1/S12#SEC8; Available at: Accessed January 31, 2008.
3. Brunton, L., Goodman & Gilman’s the pharmacological basis of therapeutics. ed 11 ( 2006)McGraw-Hill, New York.
4. Chulay, M.; Burns, S., AACN essentials of critical care nursing: Endocrine system. ( 2006)McGraw Hill, New York.
5. In: (Editors: Flomenbaum, N.; Goldfrank, L.; Hoffman, R.; et al.) Goldfrank’s toxicological emergenciesed 8 ( 2006)McGraw-Hill, New York.
6. Kaufman, D.B., Pancreas transplantation, www.emedicine.com/med/topic2605; Available at: Accessed January 12, 2008..
7. Klein, I.; Danzi, S., Thyroid disease and the heart, Circulation 116 (15) ( 2007) 1725–1735.
8. Noble, K., Thyroid storm, J Perianesth Nurs 21 (2) ( 2006) 119–122.
9. In: (Editors: Porter, R.; Kaplan, J.; Homeier, B.; et al.) Merck manual online: Endocrine and metabolic disorders ( 2006)Merck, Whitehouse Station, NJ.
10. Rothrock, J., Alexander’s care of the patient in surgery. ed 13 ( 2007)Mosby, St. Louis.
11. Townsend, C.; Beauchamp, R.D.; Evers, B.M.; et al., Sabiston’s textbook of surgery. ed 18 ( 2007)Saunders, Philadelphia.



