History
Comments
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
Electrocardiogram
Findings
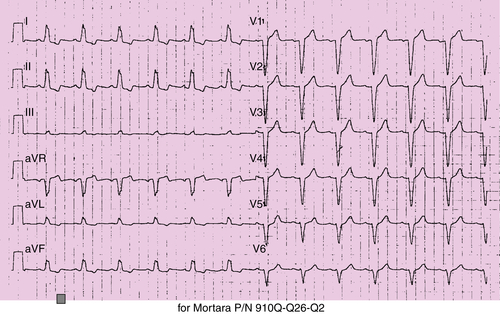
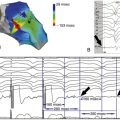
FIGURE 32-1
Echocardiography
Findings
Catheterization
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
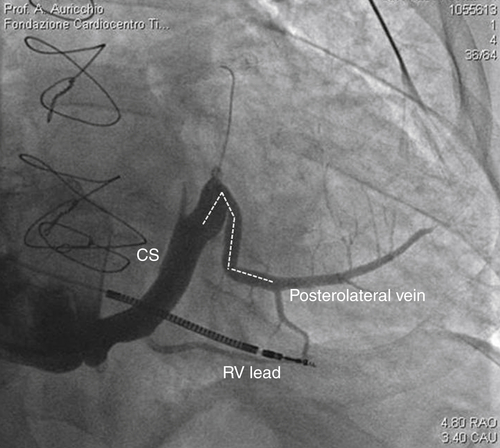
FIGURE 32-2
Intervention
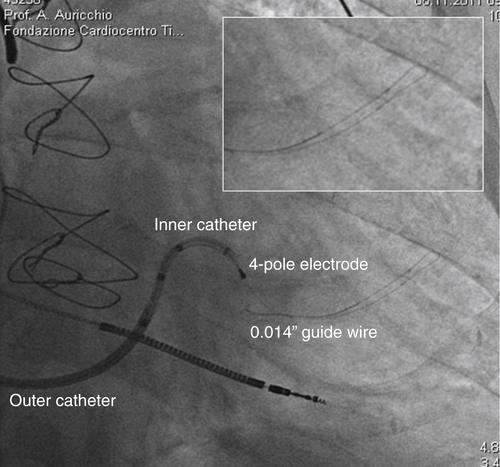
FIGURE 32-3
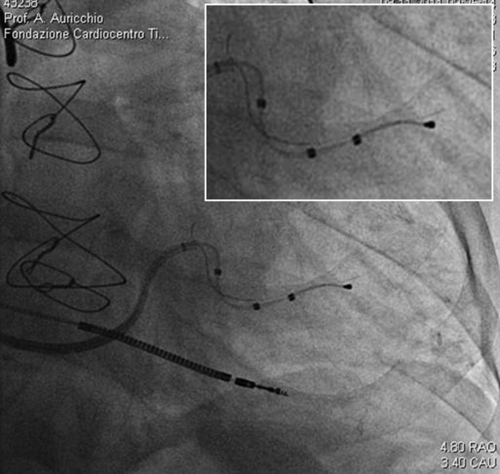
FIGURE 32-4
Question
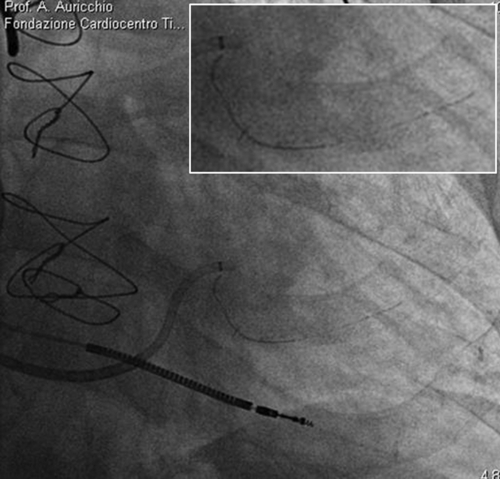
FIGURE 32-5
FIGURE 32-6
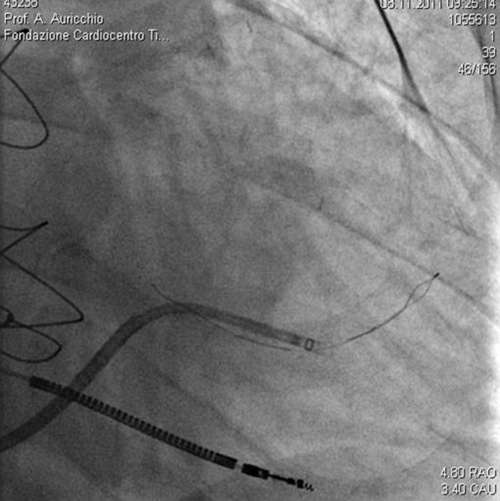
FIGURE 32-7
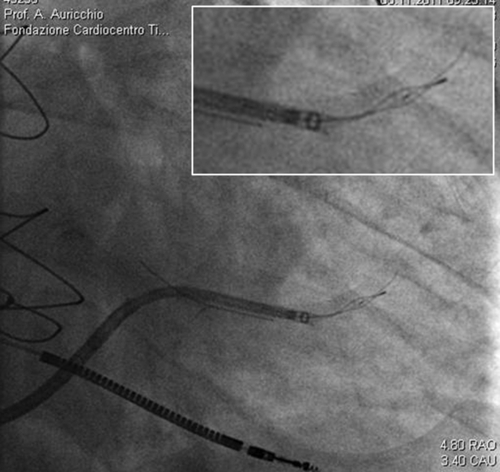
FIGURE 32-8
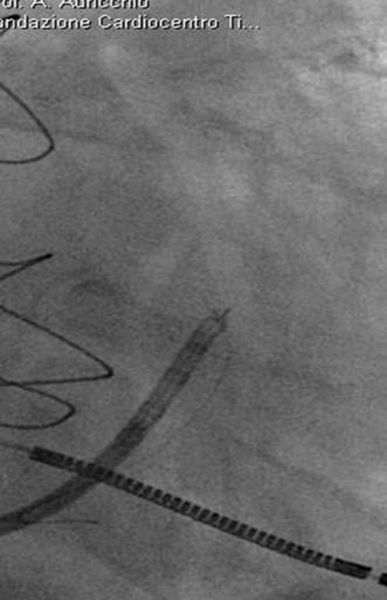
FIGURE 32-9
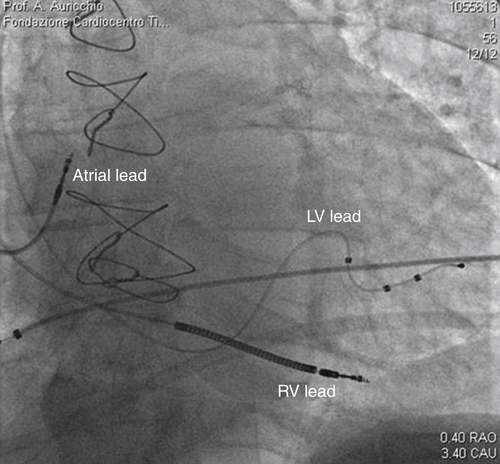
FIGURE 32-10
Outcome
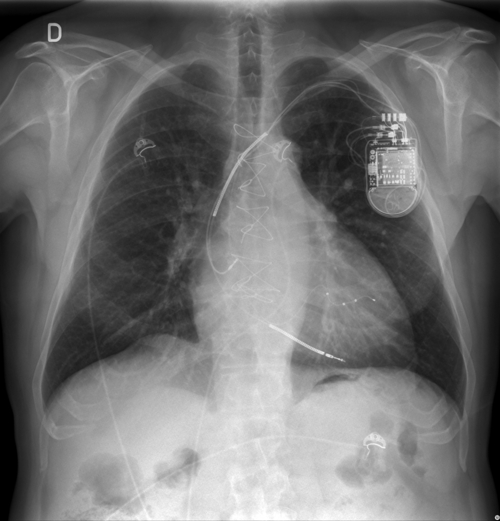
FIGURE 32-11
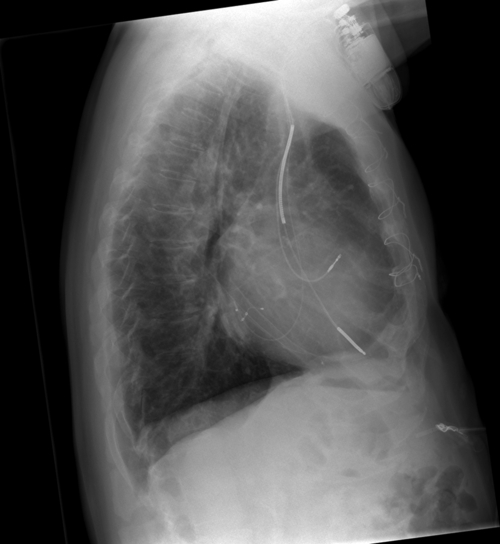
FIGURE 32-12
Selected References
1. Bristow M., Saxon L., Boehmer J. et al. for the Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION). Cardiac resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. New Engl J Med. 2004;350:2140–2150.
2. Cleland J., Daubert J., Erdmann E. et al. for the CARE-HF study investigators. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the Cardiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J. 2006;27:1928–1932.
3. Brignole M., Auricchio A., Baron-Esquivias G. et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace. 2013;15(8):1070–1118.
4. Moss A., Hall W., Cannom D. et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338.