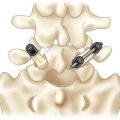
Procedure 31 Interspinous Process Motion-Sparing Implant
Indications
 Lumbar stenosis at one or two levels
Lumbar stenosis at one or two levels
 Patient must report a resolution of symptoms when seated or flexed forward (i.e., “shopping cart” sign) when upright.
Patient must report a resolution of symptoms when seated or flexed forward (i.e., “shopping cart” sign) when upright.
Indications Pitfalls
• Symptoms unresolved with lumbar flexion
• Axial back pain as the main complaint (versus buttock and/or leg pain)
• Osteoporosis with recent history of fragility fracture (severe osteoporosis)
• Spondylolisthesis greater than 25% on static or dynamic radiographs
• Significant scoliosis (greater than 25 degrees at the level of stenosis)
Indications Controversies
• “Mild to moderate” stenosis indication for ISP implantation refers to patients’ symptoms (able to walk at least 50 feet), not the MRI findings.
• Three-level implantation of the device is not currently approved. Some surgeons have had success with treatment of three-level stenosis with ISP implantation.
Figures 31-3, 31-5, 31-6, and 31-8 © 2011 Medtronic Spine, LLC. Used with permission.
Examination/Imaging
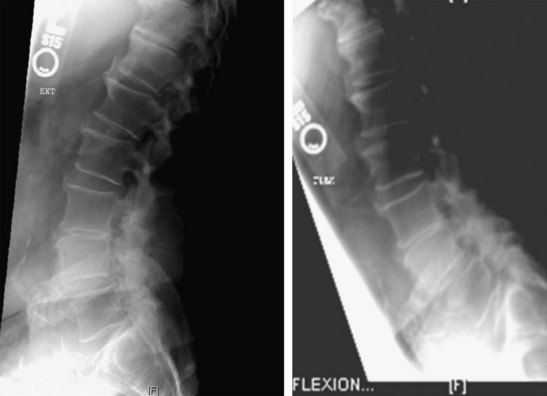
 Standing anteroposterior (AP), lateral (neutral, extension [left] and flexion [right]) views (Figure 31-1)
Standing anteroposterior (AP), lateral (neutral, extension [left] and flexion [right]) views (Figure 31-1)
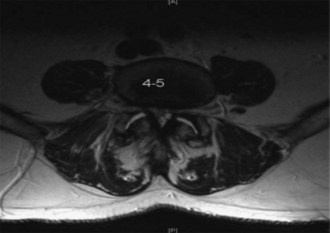
 MRI with axial, sagittal, and coronal imaging to assess disk herniation, canal stenosis, and Modic changes (Figure 31-2)
MRI with axial, sagittal, and coronal imaging to assess disk herniation, canal stenosis, and Modic changes (Figure 31-2)
Surgical Anatomy
Positioning
 Lateral positioning on radiolucent table (Figure 31-4)
Lateral positioning on radiolucent table (Figure 31-4)
 Prone positioning on a Jackson table with a Wilson frame for maximal flexion at the index level
Prone positioning on a Jackson table with a Wilson frame for maximal flexion at the index level
Portals/Exposures
 Perform blunt subperiosteal dissection with Cobb elevator, avoiding excessive muscle injury.
Perform blunt subperiosteal dissection with Cobb elevator, avoiding excessive muscle injury.
 Avoid injury to the supraspinous ligament as well as the facet anatomy.
Avoid injury to the supraspinous ligament as well as the facet anatomy.
Procedure
Step 1: Positioning
Step 2: Incision
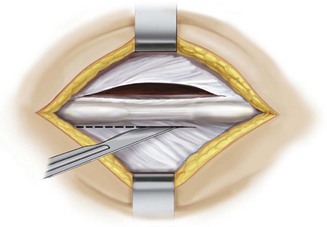
 Make a midline skin incision with bilateral fascial incision exposing the inferior and superior aspects of the spinous processes at the selected interspinous segment (Figure 31-5).
Make a midline skin incision with bilateral fascial incision exposing the inferior and superior aspects of the spinous processes at the selected interspinous segment (Figure 31-5).
Step 3: Preparing Interspace
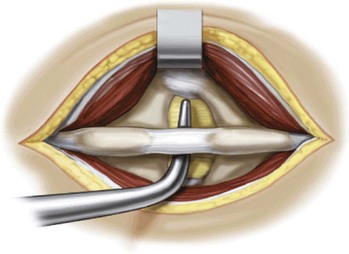
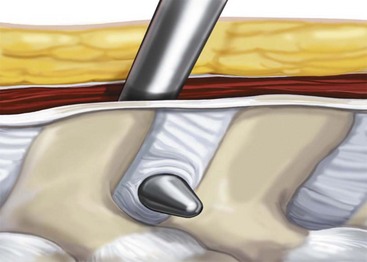
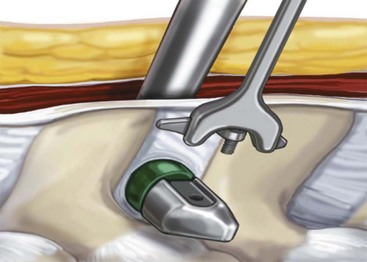
 Develop the interspinous space with the small and large dilators, and confirm the position on a radiograph (Figure 31-6).
Develop the interspinous space with the small and large dilators, and confirm the position on a radiograph (Figure 31-6).
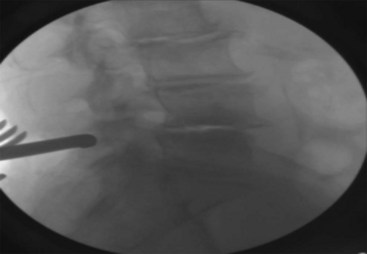
 Gradually increase the space by dilation using the sizer until it is noted, clinically, that the interspinous ligament is taut (sometimes a “vacuum disk” is noted radiographically at the posterior aspect of the disk space, confirming a disk space distraction) (Figure 31-7).
Gradually increase the space by dilation using the sizer until it is noted, clinically, that the interspinous ligament is taut (sometimes a “vacuum disk” is noted radiographically at the posterior aspect of the disk space, confirming a disk space distraction) (Figure 31-7).
Step 3 Pitfalls
• Ensure, radiographically, the correct level, as well as that the dilators and sizers for the interspace are positioned sufficiently anteriorly, just posterior to the spinolaminar junction.
• Avoid overdilating, because this may lead to fracture of the spinous processes or rupture of the interspinous/supraspinous ligaments.
Postoperative Care and Expected Outcomes
 The patient can mobilize postoperatively with adequate analgesia.
The patient can mobilize postoperatively with adequate analgesia.
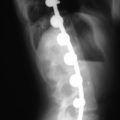
 A 2-week follow-up should be done for wound evaluation as well as standing AP and lateral radiographs to ensure maintained implant positioning.
A 2-week follow-up should be done for wound evaluation as well as standing AP and lateral radiographs to ensure maintained implant positioning.
Postoperative Pearls
• Most patients state that their preoperative claudication pain is relieved immediately after surgery.
• Inadequate preoperative symptom relief should be evaluated with further imaging and may potentially require additional therapy, including physical therapy and at-time epidural steroid injections.
• If patient continues complaining of axial low back pain, they should be reminded that the surgery was to address their buttock and leg pain; once the leg and buttock pain is relieved, the patient may start complaining of the remaining low back pain.
Christie S, Song J, Fessler R. Dynamic interspinous process technology. Spine. 2005;30(Suppl 16):S73-S78.
This article is an overview of interspinous technology.
Kabir SM, Gupta SR, Casey AT. Lumbar interspinous spacers: a systematic review of clinical and biomechanical evidence. Spine. 2010;35:E1499-E1506.
Kim D, Albert T. Interspinous process spacers. J Am Acad Orthop Surg. 2007;15:200-207.
This article is an overview of interspinous technology.
Siddiqui M, Nicol M, Karadimas E, et al. The positional magnetic resonance imaging changes in the lumbar spine following insertion of a novel interspinous process distraction device. Spine. 2005;30:2677-2682.
This article reports the MRI changes before and after interspinous process implantation.
Weiner BK. Interspinous process decompression system device affords superior outcomes and equal safety to non-operative therapy. Spine. 2005;30:2846-2847.
The article highlights the clinical advantages of the interspinous spacer over nonoperative therapy.
Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: two-year follow up results. Spine. 2005;30:1351-1358.