History
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
Electrocardiogram
Findings
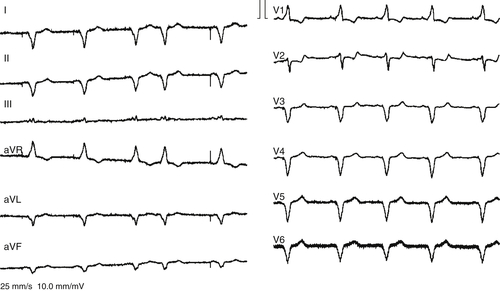
FIGURE 30-1 12-Lead electrocardiogram with cardiac resynchronization therapy “on.”
Echocardiogram
Findings
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Selected References
1. Fox K., Garcia M.A., Ardissino D. et al. Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology; ESC Committee for Practice Guidelines (CPG).Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–1381.
2. Fox K.M. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362:782–788.
3. Schaer B.A., Osswald S., Di Valentino M. et al. Close connection between improvement in left ventricular function by cardiac resynchronization therapy and the incidence of arrhythmias in cardiac resynchronization therapy-defibrillator patients. Eur J Heart Fail. 2010;12:1325–1332.
4. Schaer B., Sticherling C., Szili-Torok T. et al. Impact of left ventricular ejection fraction for occurrence of ventricular events in defibrillator patients with coronary artery disease. Europace. 2011;13:1562–1567.