Toxinology Emergencies
Edited by Mark Little
30.1 Snakebite
Geoffrey Isbister
Introduction
Australia has a number of venomous snakes with some of the most potent venoms in the world. All the medically important snakes are elapids (front-fanged), although bites rarely occur from colubrids and non-venomous snakes. New Zealand has no snakes of medical importance. The risk of significant coagulopathy and uncommonly death, even after apparently trivial contact with Australian snakes, remains and must be appreciated by healthcare workers [1].
Epidemiology
It is thought that approximately 3000 suspected snakebites occur annually in Australia, but this figure is difficult to estimate and depends on how many suspected bites, non-venomous bites and non-envenomed cases are included. The number of envenomed cases is far less and probably in the order of 100–200 each year; the majority of which occur in rural and regional areas. Snakebite deaths continue to occur (about 1–5 per year) and are usually a result of early cardiac arrest in brown snakebites or major haemorrhage in coagulopathic patients [1].
The commonest clinical manifestation is coagulopathy which occurs in about three-quarters of envenomed cases, the majority in brown snake bites [2]. Neurotoxicity and myotoxicity are now uncommon and mechanical ventilation is rarely required for treatment [2]. The types of snakes causing major envenoming differ across Australia. Bites in snake handlers remain an important problem with about 10% of all bites being in snake handlers. However, they are almost all bites from Australian snakes, albeit the more uncommon and interesting snakes and exotic snakebite is very rare [3]. Although snake handlers often want to avoid antivenom, they should be treated like anyone else because there is little evidence to support they are at higher risk of antivenom reactions. Snake handlers and people working with snake venoms can develop systemic hypersensitivity reactions to venom itself, so venom anaphylaxis must be a differential diagnosis in these patients [3].
Prevention
Most snakebites are preventable and result from snake handling or interference with snakes in the wild, sometimes in the setting of alcohol consumption. Ideally, snakes should be left alone and those working with or keeping snakes should have appropriate training and licences. Simple precautions, such as wearing thick long pants and boots when walking in the bush or when working with snakes, can prevent most bites due to the short length of Australian elapid fangs. Snake handlers should carry and maintain first-aid kits that include at least four broad elastic bandages (15 cm; e.g. Ace) and have practised applying the bandage. If exotic snakes are being held, including Australasian snakes out of their geographical distribution, appropriate antivenoms should be available.
Clinical features
Systemic envenoming results when venom is injected subcutaneously and reaches the systemic circulation. Whether or not a snakebite results in systemic envenoming depends on a number of factors including fang length, average venom yield of the snake, effectiveness of the bite and bite site. Recent studies have suggested that only a small amount of the injected venom actually reaches the systemic circulation [1,4]. Most snakebites do not result in envenoming because either insufficient venom reaches the systemic circulation or the snake is non-venomous.
Envenoming is characterized by local and systemic effects, although Australasian elapids rarely cause major local effects, such as necrosis and local haemorrhage. The clinical features of envenoming depend on the particular toxins present in each snake’s venom but non-specific systemic symptoms (nausea, vomiting, headache, abdominal pain, diarrhoea and diaphoresis) occur in many cases. The major clinical syndromes are coagulopathy, neurotoxicity, myotoxicity and acute kidney injury [2]. Severe envenoming can result in early collapse associated with dizziness, loss of consciousness, apnoea and hypotension [1]. In the majority of cases, there is spontaneous recovery over 5–15 min but, in some case, this does not occur and multiorgan failure and death ensue if resuscitation is delayed [1].
The medically important Australian snakes and their associated clinical effects are listed in Table 30.1.1.
Table 30.1.1
| Snake | Coagulopathy | Neurotoxicity | Myotoxicity | Systemic symptoms | Thrombotic microangiopathy | Cardiovascular effects | Antivenom |
| Brown snake | VICC1 | Rare and mild | – | <50% | 10% | Collapse (25%) Cardiac arrest (5%) |
Brown snake |
| Tiger snake group | |||||||
| Tiger snake | VICC | Uncommon | Uncommon | Common | 5% | Rare | Tiger snake |
| Rough-scale snake | VICC | Uncommon | Uncommon | Common | <5% | Rare | Tiger snake |
| Hoplocephalus spp.2 | VICC | – | – | <50% | – | – | Tiger or brown snake |
| Black snakes | |||||||
| Mulga snake | Anticoagulant | – | Common | Common | – | – | Black snake3 |
| Red-bellied black snake | Anticoagulant | – | Uncommon | Common | – | – | Tiger snake4 |
| Death adder | – | Common | – | Common | – | – | Death Adder3 |
| Taipan | VICC | Common | Rare | Common | 5% | Uncommon | Taipan3 |
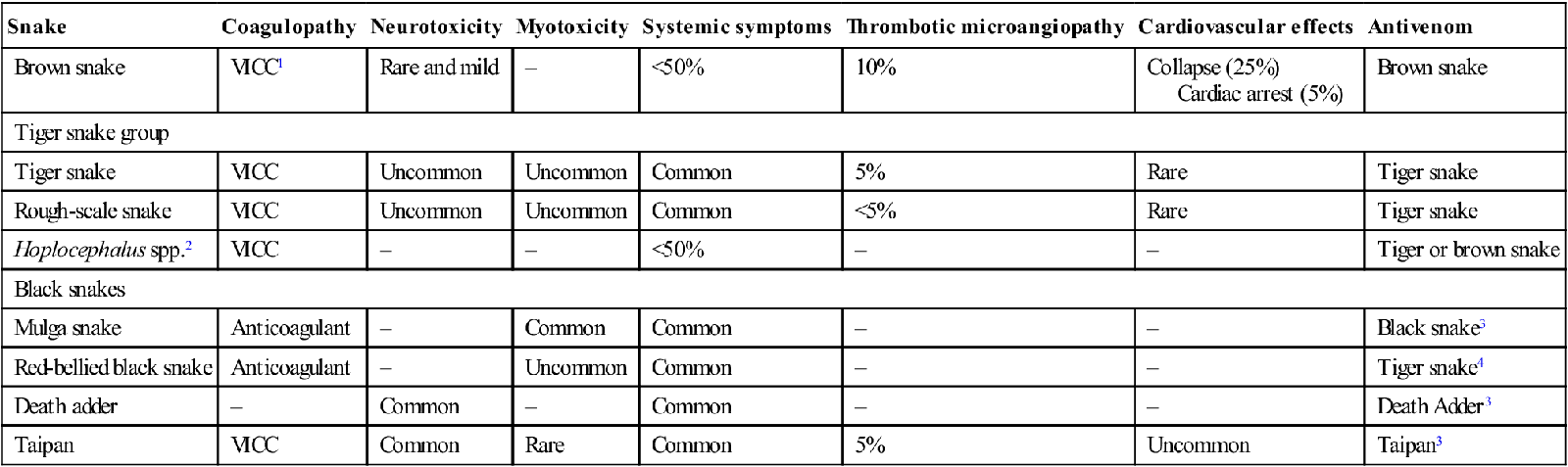
1VICC: venom-induced consumption coagulopathy;
4Polyvalent or tiger snake antivenom cannot be used for sea snake envenoming.
Coagulopathy
Venom-induced consumption coagulopathy (VICC)
This is the commonest and most important clinical effect in Australian snake envenoming. Venom-induced consumption coagulopathy (VICC) results from a prothrombin activator in the snake venom converting prothrombin (Factor II) to thrombin which leads to consumption of Factors V, VIII and fibrinogen, associated with a massive increase in fibrinogen degradation products [5]. Most dangerous Australian snakes contain such a prothrombin activator including brown snakes, snakes in the tiger snake group and taipans [5]. VICC develops rapidly within 15–60 min and the onset may coincide with the initial collapse seen with major envenoming by brown snakes and taipans [1]. Recovery usually takes 12–18 h [5].
Anticoagulant coagulopathy
Anticoagulant coagulopathy occurs in black snake envenoming, including mulga and red-bellied black snakes and is characterized by an abnormal activated partial thromboplastin time (aPTT) [6,7]. It is unlikely to result in haemorrhage and of itself is rarely of clinical importance. However, anticoagulant coagulopathy is a useful marker of envenoming and is rapidly reversed with antivenom [6].
Neurotoxicity
Paralysis is a classic effect of snakebite and is due to mainly presynaptic neurotoxins that occur in almost all Australian elapids. Presynaptic neurotoxins disrupt neurotransmitter release from the terminal axon and are associated with cellular damage. This type of neurotoxicity does not respond to antivenom treatment and may take days to weeks to resolve in severe cases. Neurotoxic envenoming manifests as a progressive descending flaccid paralysis. The first sign is usually ptosis followed by facial and bulbar involvement and progressing to paralysis of the extraocular muscles, respiratory muscles and peripheral weakness in severe cases.
Myotoxicity
Some Australian snakes contain myotoxins that cause damage to skeletal muscles resulting in local and/or generalized muscle pain, tenderness and weakness, associated with a rapidly rising creatine kinase and myoglobinuria. In rare severe cases, secondary renal impairment can occur.
Renal toxicity
Renal impairment or acute kidney injury can occur in association with thrombotic microangiopathy, secondary to severe myolysis or, more rarely and to a minor degree, in isolation with brown snake envenoming. Thrombotic microangiopathy occurs in snakebites associated with VICC and is characterized by severe thrombocytopaenia worse 3–4 days after the bite, acute renal failure that may last 2–8 weeks and require dialysis and microangiopathic haemolytic anaemia [8]. It is most common with brown snake envenoming, but also reported with all snakes that cause VICC.
Local effects
Local effects vary from minimal effects with brown snakebites to local pain, swelling and, occasionally, tissue injury following black and tiger snakebites.
Most fatalities occur within hours of the bite from initial cardiac arrest and multiorgan failure [1]. Delayed deaths are now uncommon and mainly due to major haemorrhage from VICC in brown snake, tiger snake group or taipan envenoming. Respiratory failure from neurotoxicity remains a problem in Papua New Guinea where there continue to be large numbers of cases, mainly taipan bites, and a shortage of both antivenom and resources for mechanical ventilation.
Treatment
First aid
Australian snake venoms appear to be absorbed via the lymphatic system so absorption is likely to be increased by movement and exercise. The aim of first aid is to minimize movement of venom to the systemic circulation. This is achieved by a pressure bandage (elastic bandage, such as ACE) being applied over the bite site and then covering the whole limb with a similar pressure to that used for a limb sprain. The bitten limb must be immobilized as well as the whole patient or the first aid is ineffective. Immobilization consists of splinting and complete prevention of movement or exercise of the bitten part. It has been shown that movement of all limbs, not just the affected one, needs to be minimized for optimal effect [9]. Transport should be brought to the patient and walking must be avoided. Pressure bandaging is clearly impractical for bites that are not on the limbs but direct pressure with a pad and immobilization may be useful.
First aid must eventually be removed but this should take place in a resuscitation area of a facility with the means definitively to treat envenoming. The first aid is removed when:
Initial assessment and treatment
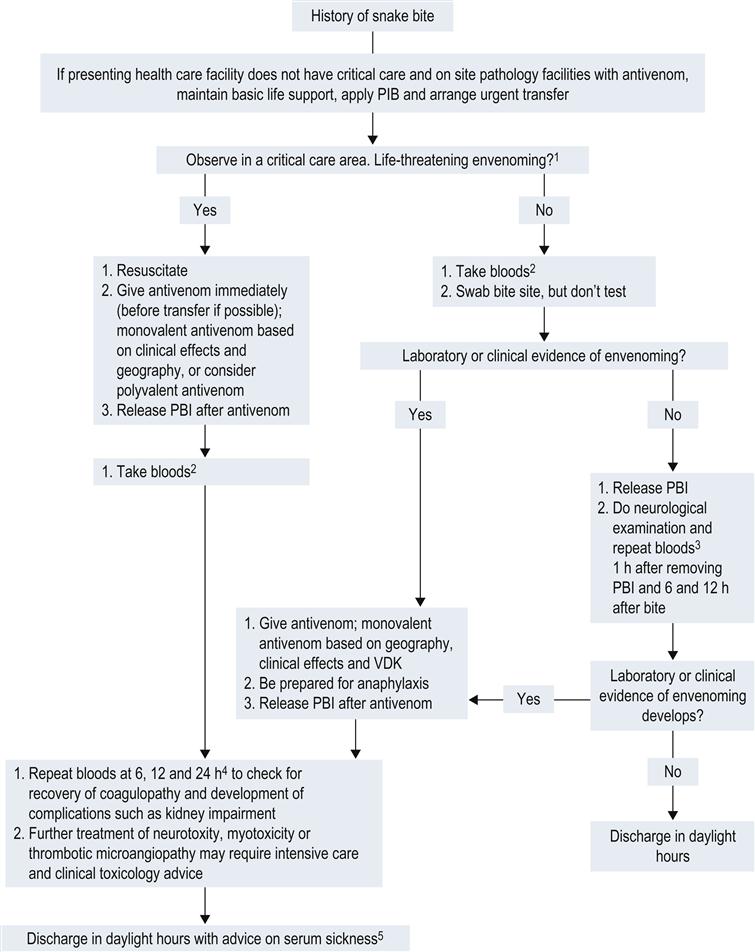
Figure 30.1.1 provides a simple approach to the management of suspected and envenomed snakebite patients. The patient is managed in an area with full resuscitation facilities. Assessment and management proceed simultaneously. The airway, breathing and circulation are assessed and stabilized. The majority of patients are not critically unwell and can have a focused neurological examination for early signs of paralysis (e.g. ptosis, drooling), examination of draining lymph nodes and general examination for signs of bleeding (oozing from the bite site, gum bleeds). Intravenous access should be established and intravenous fluids commenced.
Further management
Two major diagnostic and risk assessment issues exist for snakebite:
The majority of patients are not envenomed, but all patients must initially be assessed as if they are potentially envenomed. Asymptomatic patients, particularly those seen early after a brown snakebite, may still be severely envenomed with VICC. The diagnosis of envenoming is made on history, examination and the clinical investigations listed below. Although systemic envenoming can be ambiguous in patients with mild envenoming, the following definitions are useful for determining whether patients require antivenom:
Table 30.1.2 provides a list of relative and absolute contraindications for antivenom which can be discussed with a clinical toxicologist if there is any doubt. If there is no evidence of envenoming after clinical assessment and initial laboratory testing, the first-aid bandage can be removed. The patient requires ongoing close observation including repeated investigations 1 h after bandage removal and at 6 and 12 h after the bite (see Fig. 30.1.1).
Table 30.1.2
Absolute and relative indications for antivenom
Absolute indications
History of sudden collapse, cardiac arrest or seizure
An abnormal INR
Evidence of paralysis with ptosis and/or ophthalmoplegia being the earliest signs
Relative indications: (suggest consultation with clinical toxicologist)
Systemic symptoms (vomiting, headache, abdominal pain)
Abnormal aPTT
Creatinine kinase>1000 U/L
Leucocytosis/lymphopaenia
If the patient is envenomed, then management must proceed with antivenom. A small number of patients present in extremis, usually following collapse and in cardiac arrest and should have antivenom administered immediately as part of advanced life support.
The next step is to determine the snake group responsible for envenoming in order to allow the administration of the appropriate monovalent antivenom. This is done taking into account:
In the majority of cases, a combination of these two factors allows determination of the correct monovalent snake antivenom required. In some cases, an expert may be available to identify the snake. If the snake type cannot be determined based on geography and clinical syndrome, a snake venom detection kit (SVDK) may assist in identifying the snake. However, the results of an SVDK cannot be used in isolation from the geography, expert snake identification or clinical syndrome. If it is unclear which snake is involved then one vial of polyvalent antivenom should be administered or two vials of monovalent in regions (e.g. Victoria) where this will cover all medically important snakes – most commonly brown and tiger snake antivenoms. In Tasmania, only tiger snake antivenom is required.
Administration of antivenom
Snake antivenom should be administered by the intravenous route after being diluted 1 in 10 with normal saline and administered over 15 min. In patients with cardiac arrest or life-threatening effects, undiluted antivenom may be administered as a slow intravenous bolus. The dose of antivenom is one vial for all Australian snakes and the dose for children is the same as adults. Recovery is determined by the reversibility of effects and the time it takes for recovery once venom is neutralized. Repeat doses of antivenom are never required. Although there has been controversy over the dose of antivenom, recent studies have demonstrated that previously recommended large doses are not required [1,10].
Premedication for snake antivenom administration has previously been controversial but is no longer recommended in Australia. A recent randomized controlled trial has suggested that adrenaline is an effective premedication for snake antivenom [11], but this is more appropriate in resource poor settings where the risk of reactions is higher. Systemic hypersensitivity reactions occur in about one-fifth of antivenom administrations in Australia, but are only severe (mainly hypotension) in less than 5% of administrations [2,3]. Reactions are more common with tiger snake antivenom and polyvalent antivenom compared to brown snake antivenom [2,3]. Antivenom should always be administered in a critical care area with readily available adrenaline, intravenous fluids and resuscitation equipment.
The frequency of delayed-type reactions to antivenom or serum sickness is probably higher than acute reactions and likely to depend on the amount of horse protein administered. All patients given antivenom should be warned of serum sickness. There is no evidence for the prophylactic use of a course of oral steroids but they should be used for treatment in patients who present with serum sickness (prednisolone 50 mg/day for 5–7 days).
Other treatments
Tetanus prophylaxis should be given as appropriate but local wound care is rarely required with Australasian snakes due to minimal local effects.
A recent randomized controlled trial has shown that the use of fresh frozen plasma (FFP) appears to speed the recovery of VICC [12], but whether the decreased risk of bleeding is large enough to balance the risk of blood products remains unclear. The study also suggested that use of FFP within 6 h of the bite may be associated with a poor response to FFP. Until larger studies are undertaken, FFP should be reserved for patients with coagulopathy and active bleeding.
Clinical investigations
Assessment of the potentially envenomed requires the following investigations to be performed, usually serially:
 full blood count including a blood film looking for fragments, red cells and evidence of haemolysis
full blood count including a blood film looking for fragments, red cells and evidence of haemolysis
 urea, creatinine, electrolytes, creatine kinase (CK) and lactate dehydrogenase
urea, creatinine, electrolytes, creatine kinase (CK) and lactate dehydrogenase
 snake venom detection kit: a swab should be taken from the bite site
snake venom detection kit: a swab should be taken from the bite site
Repeat laboratory testing, particularly coagulation studies, should not be used to determine if sufficient antivenom has been given because one vial is sufficient in all cases. Such serial testing should be used to determine when the patient has recovered and can be discharged.
Snake venom detection kit
The SVDK is designed to confirm which major snake group is responsible and therefore which antivenom to give. It does not confirm or exclude envenoming and should only be included in the assessment of envenomed patients after considering geography and clinical/laboratory effects. It is best done by laboratory staff. In non-envenomed patients, the SVDK has a high false-positive rate, especially in the brown snake well and is problematic in regions where brown snakes are uncommon (e.g. Victoria) [10]. A positive SVDK on urine does not indicate systemic toxicity and, in asymptomatic patients with normal laboratory studies, it is a false-positive result. The test should not be done on blood.
Disposition
Patients with suspected snakebite but no evidence of envenoming 1 h after the removal of first aid may be admitted to an observation area. Blood tests including coagulation studies and a CK should be repeated at 1 h after first aid is removed, and 6 and 12 h post-bite and be observed for 12 h or overnight (see Fig. 30.1.1). Envenomed patients requiring ventilatory support should have continued management in ICU, but patients with coagulopathy only are commonly managed in ED observation wards.
30.2 Exotic snakebite
Julian White
Introduction
The snakebite chapter of this edition is targeted principally at the Australian snakebite experience, but snakebite is a global phenomenon, arguably with>2.5 million cases,>100000 deaths and>400000 amputations every year, so Australia accounts for only a tiny fraction of this impact.
Exotic snakebite is a worldwide problem, with increasing seizures by customs of illegally imported snakes and seizures of illegal collections by authorities in countries. Some think that the trade in exotic animals is second only to the illegal trade of drugs and weapons.
Exotic snakebite in Australia is either where an Australian snake species bites a person in a region where this snake is not usually found (e.g. pet taipan bites owner in Hobart), or where a snake, not native to Australia, bites someone in Australia. This chapter will focus on this second scenario.
This topic is vast and beyond the scope of this chapter, but similar management principles may apply. Table 30.2.1 provides a list of selected genera/species, with distribution, clinical effects and major modes of treatment.
Table 30.2.1
Selected exotic snakes; overview of clinical effects and management
| Snake | Distribution† | Clinical effects‡ | Treatment |
| Family ‘Colubridae’# | |||
| Boomslang (Dispholidus typus) | SSaf | CC, NF, BH, HF | AV, BP, IV, NC |
| Bird/vine/twig snakes (Thelotornis spp.) | SSaf | CC, NF, BH, HF | BP, IV, NC |
| Keelback & yamakagashi (Rhabdophis spp.) | SEAs, EAs | CC, BH, HF | AV, IV, BP |
| Family Elapidae | |||
| PNG small eyed snake (Micropechis ikaheka) | PNG (New Guinea) | PU, M, AC, NF | AV, IV, NC, ST |
| Bolo (Ogmodon vitianus) | PNG (Fiji) | ?LS | IV, ST |
| Bougainville coral snake (Parapistocalamas hedigeri) | PNG (Bougainville) | ?LS | IV, ST |
| Solomons coral snake (Salomonelaps par) | PNG (Solomon Islands) | ?LS | IV, ST |
| PNG forest snakes (Toxicocalamus spp.) | PNG (New Guinea) | ?LS | IV, ST |
| Asian coral snakes (Calliophis spp.) | SEAs | PU, RF | IV, ST, AC |
| Asian spitting cobras (Naja spp.) | SEAs, EAs, Ind | PN, SO, LI, HF | AV, IV, LC, AC |
| Asian cobras (Naja spp.) | SEAs, EAs, Ind, As | PN, RF, LI (some) | AV, IV, LC, AC |
| King cobra (Ophiophagus hannah) | SEAs, Ind | PN, RF, LI, HF | AV, IV, LC, AC, ST |
| Kraits (Bungarus spp.) | SEAs, EAs, Ind | PP, PN, RF, M | AV, IV, ST |
| Desert black snake (Walterinnesia aegyptia) | ME, NtAf | PN, RF | ST, IV, ?AV1 |
| Water cobras (Naja (ex Boulengerina) spp.) | SSAf | PN, RF | ST, IV |
| African spitting cobras (Naja spp.) | SSAf, NtAf, ME | SO, LI, PN, HF | AV, IV, LC |
| African cobras (Naja spp.) | SSAf, NtAf, ME | PN, RF, LI (some) | AV, IV, LC, ST, AC? |
| Mambas (Dendroaspis spp.) | SSAf | PD, RF, LI (some) | AV, IV, ST, LC |
| Rinkhals (Hemachatus haemachatus) | SSAf | LI, PN | AV, IV, LC, ST |
| African coral snakes (Aspidelaps spp.) | SSAf | PN, RF | IV, ST, AC? |
| African garter snakes (Elapsoidea spp.) | SSAf | LS | IV, ST |
| Tree cobras (Pseudohaje spp.) | SSAf | LS | IV, ST |
| Spotted harlequin snakes (Homoroselaps spp.) | SSAf | ?LS | IV, ST |
| Burrowing cobra (Paranaja spp.) | SSAf | LS | IV, ST |
| American coral snakes (Micrurus, Leptomicrurus spp.) | NtAm, CeAm, StAm | PN, PP (some), M, RF | AV, IV, ST |
| US coral snake (Micruroides euryxanthus) | NTAm | PN, RF | IV, ST |
| Sea snakes (many species) | Indo-Pacific | PN, RF, M, NF | AV, IV, ST, AC, NC |
| Family Viperidae (Viperinae; old world, non-pit-vipers/adders) | |||
| Russell’s vipers (Daboia spp.) | SEAs, EAs, Ind | CC, BH, BD, BS, NF, HF, LI, PU, RF, M | AV, IV, ST, LC, NC, BP |
| Saw scaled vipers (Echis spp.) | Ind, WAs, ME, NtAf, SSAf | CC, BH, BD, NF, HF, LI | AV, IV, ST, LC, NC, BP |
| Horned vipers (Pseudocerastes spp.) | ME, WAs | LS, PU? | IV, ST |
| Horned vipers (Cerastes spp.) | ME, NtAf | LI, CC, BH, NF, HF | AV, IV, ST, LC, NC, BP |
| Puff & Gaboon adders (Bitis spp.) | SSAf, NtAf | LI, HF, BD | AV, IV, ST, LC |
| Berg adders (Bitis atropos, etc.) | SSAf | LI, HF, PU, RF | IV, ST, LC |
| Night adders (Causus spp.) | SSAf, NtAf | LS, PU | IV, ST, LC |
| Bush vipers (Atheris, Montatheris, Proatheris spp.) | SSAf | LS, CC, HF | AV2, IV, ST, LC, BP |
| McMahon’s viper (Eristocophis mcmahoni) | WAs, ME | LI, HF, PU? | IV, ST, LC |
| Barbour’s bush viper (Adenorhinos barbouri) | SSAf | LS | IV, ST |
| Fea’s viper (Azemiops feae) | EAs, SEAs, As | LS | IV, ST |
| European adders (Vipera, Macrovipera spp.) | NtAf, EU, ME, As | LI, CC, HF, BD, PU | AV, IV, ST, LC, BP |
| Family Viperidae (Crotalinae; pit vipers) | |||
| Copperhead, cottonmouth, cantils (Agkistrodon spp.) | NtAm, CeAm | LI, CC, HF, BD, NF | AV, IV, ST, LC |
| Jumping vipers (Atropoides spp.) | CeAm | LS, HF | IV, ST, LC |
| Lancehead vipers (Bothrops spp.) | StAm, CeAm | LI, HF, CC, BD, NF, LA, RF, DV (Caribbean spp.) | AV, IV, ST, LC, NC |
| Palm pit vipers (Bothriechis spp.) | CeAm | LI, HF, BD | AV, IV, ST, LC, NC |
| Malayan pit viper (Calloselasma rhodostoma) | SEAs | LI, HF, CC, BH, BD, NF | AV, IV, ST, LC, NC |
| Montane pit vipers (Cerriphidion spp.) | CeAm | LI, HF, BD | AV, IV, ST, LC, NC |
| North American rattlesnakes (Crotalus spp.) | NtAm | LI, HF, CC, BH, BD, NF, PP & RF (few spp.) | AV, IV, ST, LC, NC, BP |
| South American rattlesnakes (Crotalus spp.) | CeAm, StAm | CC, BH, M, PP, RF, NF | AV, IV, ST, NC |
| Hundred pace viper (Deinagkistrodon acutus) | EAs | LI, HF, BD, NF | AV, IV, ST, LC, NC |
| Mamushis, etc (Gloydius spp.) | EAs, SEAs | LI, HF, CC, BD, PU, RF, M, NF | AV, IV, ST, LC, NC |
| Hump nosed vipers (Hypnale spp.) | Ind | LI, HF, CC, BH, NF | IV, ST, LC, NC |
| Bushmaster (Lachesis spp.) | CeAm, StAm | LI, HF, CC, BH, BD | AV, IV, ST, LC |
| Horned pit viper (Ophryacus spp.) | CeAm | LI, HF | IV, ST, LC |
| Montane pit vipers (Porthidium spp.) | CeAm | LI, HF | IV, ST, LC |
| Habus (Protobothrops spp.) | EAs, Ind | LI, HF, CC, BH, BD | AV, IV, ST, LC, NC |
| Pygmy rattlesnakes (Sistrurus spp.) | NtAm | LI, HF, CC, BD | AV, IV, ST, LC |
| Green tree vipers (Trimeresurus spp. incorporating spp. variously assigned to the genera Ovophis, Crypteletrops, Popeia, Parias, Viridovipera, Himalayophis, Peltopelor) | SEAs, EAs, Ind | (varies significantly between species) LI, HF, CC, BH, BD, NF | AV, IV, ST, LC, NC, BP |
| Temple pit vipers (Tropidolaemus spp.) | SEAs, Ind | LI, HF | IV, ST, LC |
| Mount Mang pit viper (Protobothrops (ex Zhaoermia) mangshanensis) | EAs | LI, HF | IV, ST, LC |
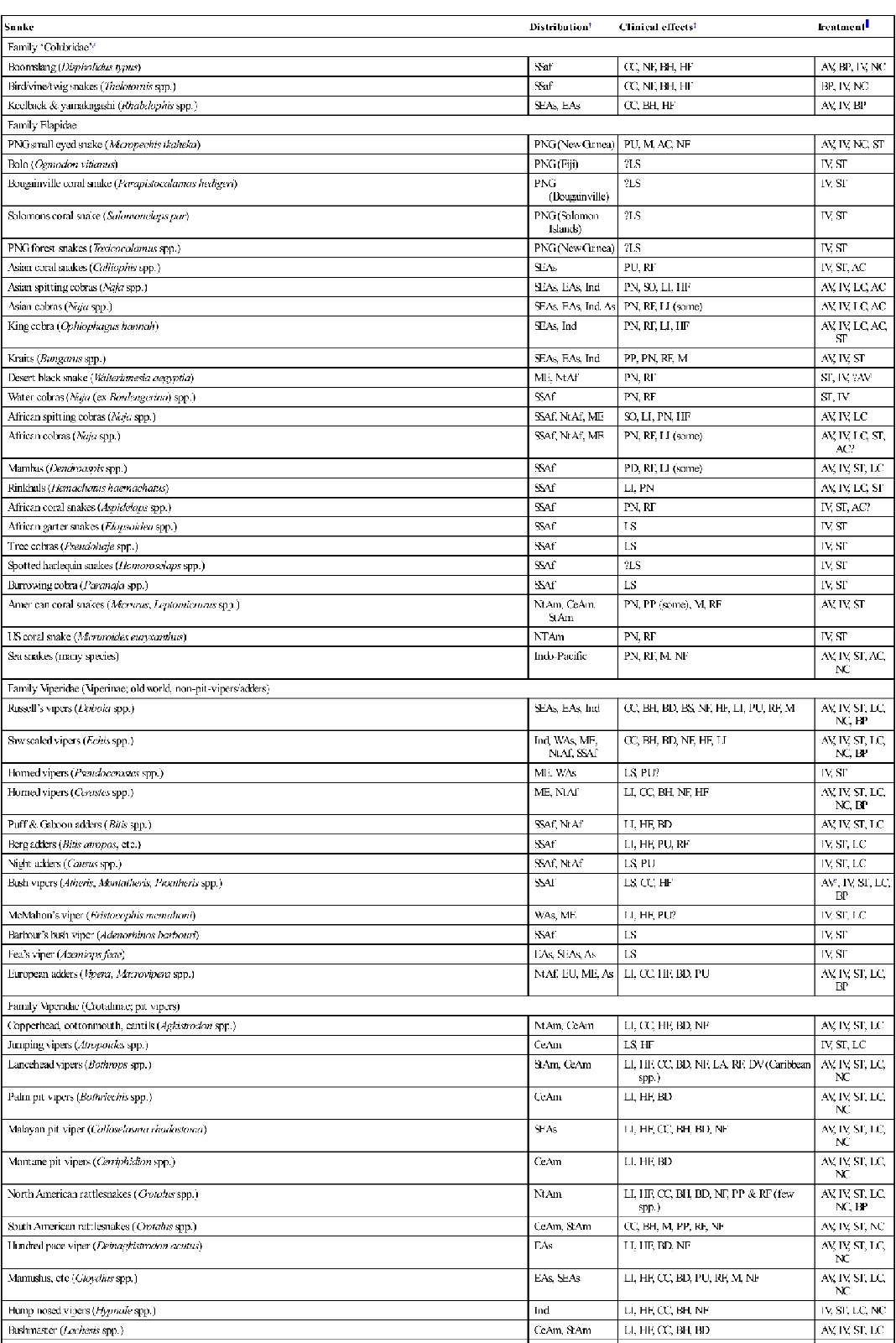

The Australian snake fauna is not listed here (see previous chapter).
 Key to treatment: AV: antivenom available (for details of available antivenoms see www.toxinology.com); LC: local wound care essential (necrosis or abscess potential); BP: consider blood products as replacement in consumptive coagulopathy with major active bleeding – if antivenom available ensure adequate antivenom given first; IV: ensure adequate IV fluid hydration, watch for & treat shock (mostly hypovolaemic); NC: particular risk of renal damage; ensure good hydration, renal output, strict fluid balance charting; AC: postsynaptic only flaccid paralysis may respond to neostigmine+atropine, if antivenom delayed or unavailable; ST: supportive treatment; may include intubation & ventilation for respiratory paralysis.
Key to treatment: AV: antivenom available (for details of available antivenoms see www.toxinology.com); LC: local wound care essential (necrosis or abscess potential); BP: consider blood products as replacement in consumptive coagulopathy with major active bleeding – if antivenom available ensure adequate antivenom given first; IV: ensure adequate IV fluid hydration, watch for & treat shock (mostly hypovolaemic); NC: particular risk of renal damage; ensure good hydration, renal output, strict fluid balance charting; AC: postsynaptic only flaccid paralysis may respond to neostigmine+atropine, if antivenom delayed or unavailable; ST: supportive treatment; may include intubation & ventilation for respiratory paralysis.
Bites by captive non-native (exotic) venomous snakes
At least in ‘Western’ countries, there is an increasing number and diversity of exotic venomous snakes being kept in captivity, especially in private collections, either legally or often illegally (in Australia only registered zoos can legally keep exotic venomous snakes).
These exotic snakes may cause quite different patterns of envenoming compared to native snakes and, if any antivenom is available, it will be different to local products and may be difficult to obtain. Doctors will likely have no training in how to manage such bites. The person bitten may have limited knowledge of the risks, appropriate first aid and, if the snakes are illegally kept, may be reluctant to seek medical attention, so delayed presentation with resultant more severe complications is common. In contrast, bites occurring in legal collections, such as in zoos, are likely to present early, with correct first aid and appropriate antivenom immediately available.
Exotic venom activity
The mix of venom toxins and corresponding clinical effects varies with species of snake, but can include one or more of: (1) paralytic neurotoxins (pre- and/or postsynaptic); (2) myotoxins (local or systemic); (3) toxins decreasing blood coagulability (many mechanisms; procoagulant, fibrin(ogen)olytic, anticoagulant, antiplatelet, etc); (4) toxins promoting clotting (cause deep vein thromboses [DVTs], etc; notably selected Caribbean pit-vipers); (5) haemorrhagins (damage vascular endothelium, promote bleeding); (6) nephrotoxins; (7) cardiotoxins; (8) local necrotoxins (cause severe local tissue injury/necrosis).
First aid
Many non-Australian venomous snakes can cause moderate to severe local effects around the bite site, including blistering, swelling, bleeding and skin necrosis (many vipers and pit-vipers, some cobras, especially spitting species) and, for these, the Australian pressure bandage and immobilization (PBI) is not recommended as it may worsen local tissue injury. Simple immobilization of the bitten limb is recommended.
For those snakes which do not cause significant local tissue injury (sea snakes, kraits, some mambas, coral snakes, South American rattlesnakes, a few other pit-vipers), the Australian PBI first aid is recommended.
Venom spit ophthalmia
Some African and Asian cobras can accurately spit venom over several metres and commonly aim for the eye. This can cause severe eye pain, corneal damage and potentially permanent blindness, but systemic envenoming does not occur. Treatment is copious irrigation of the eye, slit-lamp examination for corneal injury, standard treatment for non-infective corneal ulceration (if present) and analgesia. Topical adrenaline drops are reported as effective analgesia if standard treatments prove inadequate. Antivenom is not recommended.
Approach to hospital management
All cases should be managed as high priority and early expert clinical toxinologist advice sought (in Australia, the Toxinology Unit, Women’s & Children’s Hospital, Adelaide or the Australian Poisons Information Centres on ph: 131126). Provide early IV hydration, particularly important in bites by necrosis-causing species where massive fluid shifts into the bitten limb can cause shock and secondary renal failure.
Urgently assess for coagulation status, renal function, myolysis, flaccid paralysis (ptosis common early sign), haemodynamic status (beware hypovolaemic shock) and active major bleeding.
Antivenom use
The use of and dose of antivenom will vary depending on snake species, extent of envenoming and other patient-specific factors. Providing details of which antivenoms to use for bites by particular snakes is beyond the scope of this chapter (www.toxinology.com provides information on antivenoms for particular species).
A snake keeper at risk of future bites should not be given antivenom for minor envenoming, in most cases, as it may needlessly sensitize them, but this concern should never inhibit antivenom use if major envenoming is developing, because the earlier antivenom is given, once indicated, the more likely it will be effective.
All snake antivenoms should be given IV, preferably diluted. Adrenaline and resuscitation should be immediately available in case of adverse reactions. If an adverse reaction occurs, stop the antivenom infusion, control the reaction using a standard anaphylaxis protocol and then consider cautiously restarting the antivenom, as it is likely still needed.
Some antivenoms suggest in their PI that a pretreatment skin sensitivity test be performed. This is an outdated, useless and dangerous test and should not be performed.
Non-antivenom treatments
Some species capable of causing severe envenoming have no suitable antivenom available, so treatment must be supportive and secondary only. An example is certain ‘colubrid’ rear-fanged snakes that can cause lethal consumptive coagulopathy, where haemodynamic support and, in selected cases, use of blood products may be required. In general, antivenom is preferred to blood products in treating major coagulopathy but, if there is active severe bleeding, then blood products may be appropriate once adequate antivenom has been given (where available), otherwise blood product use is determined on a case-by-case basis based on clinical circumstances.
For some species causing postsynaptic only flaccid paralysis, if antivenom is unavailable or delayed, consider using neostigmine (+atropine) to temper the severity of paralysis, as a short-term measure (does not replace antivenom); may be helpful in selected sea snake and cobra bites, but applicability to other neurotoxic species is less certain. This treatment will not work for predominantly presynaptic paralysis.
For species causing local tissue injury in the bitten limb, in addition to preventing shock and controlling bleeding, good wound care is important. Swelling and pain may be severe and suggest compartment syndrome, but beware of injudicious fasciotomy as this can cause long-term loss of function, severe bleeding and a risk of secondary infection. Only perform fasciotomy as a last resort in cases where compartment syndrome is confirmed by pressure measurement (commonly for pressures exceeding 35–40 mm/Hg). The role of limited digit fasciotomy for bites to fingers is unclear, but some experts suggest it may reduce incidence of later digit amputation; this is currently unproven.
30.3 Spider bite
Geoffrey Isbister
Introduction
Australasia is home to a large variety of arachnids including spiders, scorpions and ticks. Spiders are the most medically important arachnids in Australasia and include redback spiders and funnel-web spiders. Funnel-web spider (FWS) envenoming occurs rarely in Eastern Australia and can cause severe and potentially life-threatening neurotoxicity. Redback spider envenoming (latrodectism) occurs throughout Australia and causes a local or regional pain syndrome associated with non-specific systemic symptoms and, less commonly, autonomic effects. Other spiders that commonly cause human bites are not associated with major medical effects and include huntsman spiders (Sparassidae), orb-weaving spiders (Araneidae), white-tail spiders (Lampona spp.), wolf spiders (Lycosidae) and jumping spiders (Salticidae) [1]. Fatalities have only occurred in Australia after being bitten by the redback and the funnel-web spider [2].
An approach to the patient with spider bite
Initially, a careful history should be taken to determine whether the patient has suffered a definite spider bite or only a suspected spider bite. The diagnosis of definite spider bite requires sighting of the spider at the time of the bite and usually some initial symptoms, such as local pain. If there is no history of bite or no spider was seen, then other diagnoses must be considered first. This is particularly important in persons presenting with ulcers or skin lesions with suspected spider bites (Table 30.3.1). It is important in these cases that appropriate investigations are done and the case treated as a necrotic ulcer of unknown aetiology. In the majority of these cases, an infective cause is found, although less commonly they are a result of pyoderma gangrenosum or a vasculitis [3].
Table 30.3.1
| A. Establish whether or not there is a history of spider bite |
| Clear history of spider bite (better if spider is caught): |
| No history of spider bite: |
| B. Clinical history and examination |
| Important considerations: |
|
|
| C. Investigations |
| Skin biopsy: |
| Laboratory investigations: may be important for underlying conditions (autoimmune conditions, vasculitis), including, but not be limited to: |
| Imaging: |
| D. Treatment |
| Local wound management |
| Treatment based on definite diagnosis or established pathology |
| Investigation and treatment of underlying conditions may be important (e.g. pyoderma gangrenosum or diabetes mellitis) |
| E. Follow up and monitoring |
| The diagnosis may take weeks or months to be established, so patients must have ongoing follow up. Continuing management: coordinated with multiple specialties involved |

From Isbister GK. Spider bite. Australian Doctor 2004 with permission.
If the patient has a definite history of a spider bite and has either captured the spider or has a good description of the spider, a simple approach can be taken. Health professionals should not attempt to identify spiders beyond the following simple classification:
The majority of redback spiders are likely to be identified correctly and, with supporting clinical features, this diagnosis is usually straightforward. The second group is only important in regions where FWS are known to occur and cause significant effects (east coast of Australia from Southern Queensland to Southern NSW). If the spider is large and black then the patient should be managed as an FWS. A pressure bandage with immobilization is the appropriate first-aid measure. Once in the emergency department (ED) they should be observed for at least 2 h after the pressure bandage has been released or after the bite in a patient without first aid. If the patient is asymptomatic at this time, they can be safely discharged. No attempt should be made to identify the spider because the distinction between some FWS and the less significant trapdoor spiders is impossible for non-experts.
The third group includes all other spiders. Despite previous concerns about particular spiders, such as the white-tail spiders, all other spiders are very unlikely to cause more than minor effects [1]. Patients can be reassured, their tetanus status confirmed and updated, if required, and symptomatic treatment with ice and analgesia can be offered. These patients do not need to be observed in hospital.
Redback spider (Latrodectus hasselti)
Distribution and taxonomy
The redback spider is a member of the widow group of spiders (Latrodectus spp.). The widow spider group is the single most medically important group of spiders worldwide [2] and belongs to the family of comb-footed spiders (Theridiidae). Widow spiders are distributed throughout the world and thrive in urban environments, ensuring that they frequently come into contact with humans. There are probably around 40 species, including the North American black widow (Latrodectus mactans), the Australian redback (L. hasselti), the New Zealand katipo (L. katipo) and the brown widow (L. geometricus), which is found on most continents including Australia. All species produce venom with similar properties, although the clinical syndrome (latrodectism) appears to differ in some cases [2]. Australia probably has the highest rate of latrodectism in the world, with at least 2000 definite bites per annum. New Zealand reports few cases of envenoming by its widow spider, the katipo. There is at least one other important genus of spiders in this family, Steatoda spp., that is responsible for human envenoming. They are black spiders with the same body shape and size as widow spiders, but without the red markings.
Venom
The components toxic to humans in the venom of widow spiders are α-latrotoxins that cause massive release of neurotransmitters and deplete synaptic vesicles at nerve endings. Recent work based on in vitro effects suggests that all widow spiders have a similar toxin. However, although the effects of the toxin are well understood at the cellular level, it remains unclear how it produces the clinical syndrome [4].
Epidemiology
Most bites occur when the spider is disturbed in human-made objects, such as clothes, shoes, gloves, furniture, building materials and sheds. Most bites are on extremities and occur during the warmer months of the year. There were at least 13 deaths in Australia prior to the introduction of antivenom. High reported mortality rates in other countries, such as the USA, are likely to be overestimates due to reporting bias [2].
Clinical features
The majority of patients bitten develop some effects from redback spider bites with pain being the most common and important symptom. Systemic effects occur in about one-third of cases. Initially, the bite may be painless or may feel like a pinprick or a burning sensation. The pain then increases over the first hour and may radiate proximally to the regional lymph nodes or the chest or abdomen. Localized sweating and, less commonly, piloerection may occur and are virtually pathognomonic of latrodectism. Regional and distant sweating can occur and bilateral below knee sweating is characteristic. Systemic effects include malaise, lethargy, nausea and vomiting, abdominal pain and headache. A summary of the clinical effects is listed in Table 30.3.2, including less common effects. Pain and systemic effects persist for 1–4 days [5]. Delayed effects or effects persisting for days to weeks have been reported but it is unclear in many cases whether the effects are a consequence of the spider bite.
Table 30.3.2
Clinical features of redback spider bite

1More common with paediatric cases. Reproduced with permission from Therapeutic Guidelines Emergency Medicine 2008.
Diagnosis
The diagnosis is clinical and based on history, typically one of persistent increasing pain that can radiate and may be associated with local sweating. There are no tests to confirm latrodectism. As the bite may not be felt, doctors should suspect the condition in circumstances where patients have been working in sheds, potting plants or where contact with widow spiders is possible.
Treatment
First aid
Local application of ice has been recommended, although its effectiveness remains unproven. Warm compresses provide relief in some cases. Pressure bandaging is not appropriate.
Analgesia
Adequate analgesia is an important part of the treatment of redback spider bite. Paracetamol and/or non-steroidal anti-inflammatories and/or oral opioids should be used initially, although intravenous opioids may be required if the pain does not respond. Failure to respond to intravenous opioids is frequently reported and further research is required to define the most appropriate analgesia in redback spider bite.
Antivenom therapy
Antivenom is available for the treatment of redback spider bite. Despite it conventionally being given by the intramuscular (IM) route, there has been increased use by the intravenous (IV) route. Two randomized controlled trials have shown no difference between IV and IM antivenom administration and the median dose used in both trials was two vials [6,7]. Fears that IV antivenom results in a higher rate of reactions are not founded and the reaction rate with diluted IV administration is less than 5% and similar to that with IM antivenom [6,8]. It is therefore reasonable to administer redback antivenom by either IM injection or slow IV infusion (diluted in 100 mL normal saline and given over 15 min). The initial dose should be two vials and premedication is not recommended. Although repeat doses of antivenom are sometimes used, there is no evidence that this is beneficial. It is essential that ongoing analgesia is provided to the patient. As for all antivenoms, the dose for children is the same as for adults.
Steatoda species (cupboard or button spiders)
There have been a number of reports of bites by Steatoda spp., mainly in Australia [9]. They appear to cause a similar syndrome to latrodectism with persistent local pain but fewer systemic features. In vitro studies of these spiders’ venom demonstrate that they cause similar but far less potent effects compared to α-latrotoxin [10]. The majority of bites by this group of spiders cause only minor effects, although the patient may have annoying pain for a period of hours [9]. Uncommonly, they can cause more severe and persistent pain, similar to widow spiders.
Funnel-web spider (Atrax and Hadronyche species)
Distribution and taxonomy
At least 39 species of funnel-web spiders occur along the east coast of Australia, including Tasmania and Adelaide. However, only six species occurring from Southern NSW to Southern Queensland have been associated with significant envenoming – Sydney FWS (Atrax robustus), the Southern Tree FWS (Hadronyche cerberea), the Northern Tree FWS (H. formidabilis), the Blue Mountains FWS (H. versuta), the Toowoomba or Darling Downs FWS (H. infensa) and the Port Macquarie FWS (H. sp 14) [11]. Historical records suggest there is an increase in bites by other species in the last few decades compared to most bites being due to the Sydney FWS in the past. This may be due to increasing population density in the area of distribution of Hadronyche species. FWS are burrowing spiders and most encounters with humans occur when males are out looking for mates.
Venom
The males have a more potent venom than the females and only males have been reported to cause significant illness in humans [4,11]. The important toxins in human envenoming appears to be α-atracotoxins which have been isolated from the venom of a number of species of FWS [4]. These are low-molecular-weight neurotoxins that prevent inactivation of sodium channels. The main effect of the neurotoxin is an autonomic storm that can be predominantly sympathetic or parasympathetic, or mixed in effect, associated with initial excitation at neuromuscular junctions, followed by paralysis [4].
Epidemiology
Although there are a large number of suspected FWS bites each year, severe envenoming is rare and only 5–10 cases requiring antivenom occur annually [11]. Many definite bites by FWS do not result in envenoming (dry bites) and the frequency of non-envenoming varies between species [11]. In addition, many cases are a result of other big black spiders that appear to be FWS and are not collected or identified.
Clinical features
The initial bite is painful due to the size of the fangs and fang marks are usually present. Severe envenoming develops rapidly and usually occurs within 30 min [11]. Initial effects include paraesthesia (local, distal extremities and perioral), local fasciculations, tongue fasciculations and non-specific systemic effects (nausea, vomiting and abdominal pain). Autonomic features are typical of systemic envenoming with hypersalivation, lacrimation and generalized sweating. Other autonomic features can include miosis, mydriasis, tachycardia or bradycardia and hypertension. Initially, the patient is usually agitated and anxious with decreased level of consciousness and coma developing as late signs [11]. Non-cardiogenic pulmonary oedema may develop and is thought to result from venom-induced capillary leakage. Prior to antivenom treatment this occurred early, but is now more commonly reported as a delayed effect.
Diagnosis
As with redback spider bite the diagnosis is clinical.
Treatment
First aid
Pressure bandaging with immobilization is the recommended first aid and, if not applied at the scene, it should be applied in hospital on arrival if antivenom is not available.
Supportive treatment
With the introduction of antivenom therapy, the requirement for intensive care therapy is less common. Attention to basic resuscitation is essential, but usually does not require more than IV fluid therapy after assessment and stabilization of the airway and ventilation. Atropine can be used to treat cholinergic features, but this is not a substitute for antivenom. The use of inotropes and other pharmacological agents is unnecessary except in the rare instance of delayed presentation with severe envenoming not responding to antivenom. If pulmonary oedema occurs, this can be treated with continuous positive airways pressure ventilation in association with antivenom therapy.
Antivenom therapy
Definitive treatment is venom neutralization with specific FWS antivenom. The antivenom is derived from rabbit serum and appears to be less antigenic to humans than horse serum antivenoms with a low reaction rate (<2%) [11]. Premedication is not recommended but antivenom must be administered in a critical care area with adrenaline available. Antivenom is indicated for systemic envenoming as defined above. Initially, two vials should be given intravenously, which can be repeated if there is no improvement after 15–30 minutes. Delayed serum sickness reactions have been reported in at least one case [11].
Mouse spiders (Missulena spp.)
Another group of spiders, the mouse spiders (Missulena spp.), are rarely reported to cause similar effects to FWS [12]. These spiders belong to the family Actinopodidae and occur in most parts of Australia. Most bites are by wandering male spiders and do not cause any major effects. The initial bite causes pain and fang marks, due to the size of the fangs. There is one report of a bite by the Eastern mouse spider (Missulena bradleyi) that caused a syndrome similar to funnel-web envenoming in a 19-month-old child [12]. However, all other reported cases have caused only local effects and, less commonly, local neurotoxic effects and/or mild non-specific systemic effects [12].
Other Australasian spiders
There are a number of other Australian spiders that cause human bites. In the majority of cases, they cause only minor effects and symptomatic treatment is all that is required. In a large study of definite spider bites, there were no cases of necrotic lesions or allergic reactions, suggesting these effects are either rare or do not occur [1]. The incidence of secondary infection is also low and occurred in less than 1% of cases in the same study [1].
Necrotic arachnidism
Necrotic arachnidism is generally defined as necrotic lesions or ulcers that occur following a spider bite and are a result of venom effects. Significant skin necrosis following bites from recluse spiders (Loxosceles species) is well reported in many parts of the world [2]. However, excepting rare reports of L. rufescens in South Australia, this group of spiders is not endemic to the country or been reported to cause necrotic ulcers from definite bites.
The white-tailed spider (Lampona cylindrata/murina group) has been implicated in the development of necrotic arachnidism. However, recent studies show that this is not the case with 130 definite bites by these spiders causing no cases of necrotic lesions [13]. Other spiders have been implicated in this condition, including wolf spiders, sac spiders and the black house spiders, but there is similarly little evidence to support this and prospective cases of definite bites by these groups of spiders have not demonstrated necrotic lesions [1]. Table 30.3.1 provides an approach to the patient with a skin ulcer attributed to a spider bite.
30.4 Marine injury, envenomation and poisoning
Peter Pereira and Jamie Seymour
Cnidaria
Only Chironex fleckeri (box jellyfish) and Carukia barnesi (‘classic’ Irukandji jellyfish) have been documented to cause deaths in Australian waters, with the Australian Resuscitation Council (ARC) attributing 80 deaths to C. fleckeri, and 2 to irukandji syndrome. Children are particularly prone to a fatal outcome and account for the last 10 C. fleckeri deaths in the Northern Territory.
Victims of C. fleckeri envenomation are readily identifiable from the characteristic cutaneous features. Stings from C. barnesi and other Irukandji syndrome-inducing jellyfish may have minimal or absent cutaneous manifestaions. Other cnidaria may cause serious envenomation, although no deaths have been recorded in Australian waters from these species.
Chironex fleckeri
Chironex fleckeri (CF), commonly referred to as the box jellyfish, is found in tropical coastal and estuarine waters of northern Australia, predominantly between November and April. It, or similarly deadly cubomedusae, are likely to be found in other tropical environments including those of Papua New Guinea (PNG), the Indonesian archipelago and SE Asia, based on similar case reports from these areas. The animals are effectively transparent in water and the sudden severe pain of a sting may be the first indication of its presence. The bodies of mature animals may be 40 cm in size with as many as 60 tentacles trailing for up to 3 m. These tentacles have a typical banded, ladder-like appearance and leave similar marks on the skin. Lethal envenomations have only been reported where more than 2.5 m of tentacles have been involved. The venom is a complex mixture of proteins ranging in size from 54 to 150 kDa; however, most have yet to be researched and only some are demonstrably antigenic to CSL box jellyfish antivenom. Known cytotoxic components include dermatonecrolysins, haemolysins, and rhabdomyolysins including cardionecrolysins.
In a prospective study of jellyfish stings presenting to the Royal Darwin Hospital over a 12-month period, of 23 patients with nematocyst proven Chironex fleckeri stings, only one required parenteral analgesia and none received antivenom. Most victims experienced minor dermatological injuries which were successfully treated as though they were burns. However, shock and loss of consciousness from cardiorespiratory depression may occur and victims, especially children, have died within minutes of being stung.
Chironex stings can be prevented by avoiding swimming in their known habitat during the dangerous months of the year, usually November to April, but varying depending on the region, swimming within netted areas on beaches (mainly in Queensland), the wearing of protective ‘stinger’ suits or pantyhose when swimming and entering the water slowly as the jellyfish may take evasive action to avoid a swimmer.
First aid
In Australia, the ARC divides its first aid advice into jellyfish stings in tropical and non-tropical regions
Tropical jellyfish stings
The ARC recommends the liberal dousing of vinegar to the affected site(s) for at least 30 seconds, as it is effective in preventing further triggering of undischarged nematocysts. However, recent in vitro research casts some doubt on its utility, as it has been demonstrated that triggered nematocysts continue to contain residual venom, which is expelled after the application of vinegar, effectively increasing the volume of expressed venom by 60%.
Pressure immobilization bandages are no longer recommended. Although previously advocated by the ARC, there is no evidence to support the application of ice packs to sting sites.
Treatment
Although the literature mainly describes cases of cardiac arrest, the vast majority of Chironex fleckeri stings cause localized pain and discomfort.
 Remove the victim from the water.
Remove the victim from the water.
 Liberally apply vinegar to the affected areas.
Liberally apply vinegar to the affected areas.
 Scrape off adherent tentacles.
Scrape off adherent tentacles.
 Treat sting as a burn. Watch for and treat any secondary infection.
Treat sting as a burn. Watch for and treat any secondary infection.
If there is cardiac arrest (usually at the beach) commence CPR and continue until adequate doses of box jellyfish antivenom are administered.
Carukia barnesi
The Irukandji jellyfish (Carukia barnesi) consists of a bell measuring up to 3 cm across, but with tentacles up to 75 cm in length. It is found in waters north of Fraser Island on the east coast of Australia to the tip of Cape York. This jellyfish is transparent and effectively invisible in the water. It was first captured in 1961 in Cairns by Dr Jack Barnes, who demonstrated causation for Irukandji syndrome by reproducing the symptoms by stinging himself, his 9-year-old son and the local lifeguard. All three were taken to hospital for treatment. The Irukandji syndrome, however, is also caused by many other jellyfish, including blue bottles (Physalia spp.) and, as such, envenomings may occur in all Australian tropical waters. Cases have been reported in northern Australia around to Exmouth in Western Australia. Cases have also been reported internationally, including PNG, Hawaii, Florida, the Caribbean and Thailand.
Patients with Irukandji syndrome often have minimal symptoms at the time of the sting. After a latent period of up to 60 minutes the ‘Irukandji syndrome’ may develop, with clinical features of catecholamine excess that include restlessness, anxiety, diaphoresis, vomiting, abdominal, chest and back pain, blood pressure lability and tachycardia. It is reported that 20% of victims develop raised cardiac markers, 6% develop echocardioghraphic evidence of myocardial dysfunction and 2% develop clinical cardiac failure. Although most patients settle within 6 hours, all patients developing cardiac dysfunction have ongoing pain. There have now been two deaths associated with Irukandji syndrome, both succumbing from intracerebral haemorrhages presumably from the associated hypertension. Recent in vitro research indicates that C barnesi venom has no direct myocardial effect, strongly supporting the notion that the observed myocardial dysfunction may be due to the characteristic catecholamine excess.
Treatment
Vinegar is indicated immediately on experiencing a sting even before the development of Irukandji syndrome.
After a characteristic delay of up to 60 minutes, these patients experience increasing agitation and severe truncal pain traditionally requiring large doses of opioids to relieve their symptoms. In a review of 62 cases of Irukandji syndrome presenting to Cairns hospitals in one year, 38 (61%) required parenteral analgesia while, in a review of cases over a 10-year period, over 90% of patients required some type of pain relief. Fentanyl has been recommended solely because it is easily titratable and has an excellent cardiac profile. Patients should be observed in hospital for 6 hours after their last dose of opioid and, if asymptomatic, may then be discharged.
As about 20% demonstrate elevations in troponin levels, patients need to have ECG and troponin levels measured, especially those with ongoing pain or high opiate requirement. A small percentage of patients will develop pulmonary oedema usually requiring positive pressure ventilator support, inotropic support or antihypertensives, depending on the degree of cardiac dysfunction. Following anecdotal success, intravenous magnesium has become a mainstay of treatment to control pain and other symptoms of catecholamine excess. However, the only randomized and blinded trial failed to demonstrate any superiority of Mg over narcotics. It is likely that the reported variation in effectiveness of magnesium may reflect different species and different toxins. There is no antivenom.
Non-tropical jellyfish stings
By far the most common type of jellyfish sting in Australia and worldwide, with symptoms mainly being of painful welts.
First aid for all non-tropical Australian jellyfish stings consists of removing the tentacles. Ice is recommended as first aid, although the evidence for this is minimal.
Vinegar is not recommended due to concerns that it may increase firing of undischarged nematocysts in temperate species. For non-tropical blue bottle (Physalia spp.) stings, hot water has been demonstrated to reduce pain. It should be applied as hot as can be tolerated (but no more than 45°C), ensuring the patients do not burn themselves.
Fish
Envenomations
Scorpinaedea: stonefish, bullrout, lionfish, scorpionfish
The most clinically significant member of the scorpinaedea are the three species of stonefish (Synanceia spp.). Fatalities have been reported in other parts of the world, however there are no confirmed Australian fatalities (although Sutherland reports the death of an Army doctor on Thursday Island in 1915). The stonefish possesses 13 dorsal fin spines, each with paired venom glands. These spines become erect when the fish is trodden on and venom is discharged deep into the wound. The venom has neurotoxic, myotoxic, vascular and myocardial effects. Because of their excellent camouflage, stonefish are rarely seen and the first indication of their presence may be the excruciating pain of a sting. The overwhelming clinical feature of the presentation is one of severe, unrelenting local pain.
The other members of the scorpinaedae can produce similar clinical presentations though the pain is more responsive to hot water and opiates. There are reports of stonefish antivenom being successful with severe, recalcitrant pain from these fish.
Treatment
Poisonings
Ciguatera poisoning
Ciguatera poisoning is endemic in the tropics and is caused by ingesting tropical fish contaminated with ciguatoxins, a group of heat and acid stable, lipid-soluble toxins that bind and open voltage sensitive Na+ channels. They originate in dinoflagellates often associated with algae on dead coral. These are consumed by herbivorous fish which, in turn, are consumed by carnivorous fish and so on, the toxins bioaccumulating up the food chain to humans. In Australia, ciguatoxic fish are particularly found between Mackay and Cairns and the syndrome is most commonly associated with ingestion of Spanish mackerel, although a number of species are known to be ciguatoxic.
Poisoning is characterized by neurological dysfunction preceded or accompanied by an acute gastrointestinal illness. The symptoms usually begin within 1 and 6 hours of eating contaminated fish. Gastrointestinal symptoms include nausea, vomiting, abdominal pain and diarrhoea and neurological symptoms include paraesthesiae, particularly perioral, cold allodynia (a burning sensation on contact with cold), myalgias, mood disorders and autonomic and cerebellar disturbances. Cardiorespiratory complications including respiratory depression, bradycardia or cardiovascular collapse are rare and respond to standard resuscitation measures. In the absence of any diagnostic tests, the diagnosis is based on clinical suspicion and exclusion of other pathology. Treatment is primarily supportive with fluids and simple analgesics. A recent randomized controlled trial has demonstrated no benefit in using mannitol. Alcohol classically exacerbates symptoms and should be avoided during the illness. The majority of victims are treated as outpatients and admission is reserved for those considered at risk for cardiorespiratory compromise. Most poisonings resolve within a week, though severe cases may have ongoing dysaesthesias for months or years. Commonly utilized pain modulators, such as gabapentin and amitriptyline, have been used to control dysaesthesias though their effectiveness remains anecdotal and unproven. Recrudescence may occur with further ingestion of contaminated fish and, as such, it is generally advised to avoid eating tropical fish for at least 6 months after symptoms have abated.
Scombroid poisoning
Scombroid poisoning is the manifestation of histamine toxicity following the ingestion of histamine laden saltwater fish. Initially described after ingestion of scombroidae species, such as tuna and mackerel, it has since been associated with non-scromboidae species, such as herrings and sardines. The toxic levels of histamine are by-products of bacterial action on histadine contained in the flesh of these fish during inadequate storage after being caught. Ingestion is soon followed by classic histamine-related symptoms mimicking allergic reactions. These include pruritus, urticaria, rhinorrhoea, bronchospasm, vomiting, diarrhoea and abdominal pain. Rarely, severe toxicity may present as an anaphylactoid reaction. Mild to moderate presentations respond to antihistamines (H1 and H2). Severe reactions require cardiorespiratory support and adrenaline.
Paralytic shellfish
Paralytic shellfish poisoning may occur following the ingestion of saxitoxin-contaminated shellfish, particularly during periods of algae bloom. Similar to ciguatoxin, saxitoxin is a heat- and acid-stable toxin produced by a dynoflagellate microorganism and is concentrated in the flesh of bivalve molluscs. Following ingestion, there may be rapid progression from vomiting and perioral paraesthesia, to generalized paraesthesia, muscular weakness, ataxia, to paralysis and death from type 2 respiratory failure. Unlike ciguatera poisoning, it would be prudent to admit all suspected cases, including those that initially only demonstrate gastrointestinal dysfunction, for at least 24 hours to observe for the development of weakness and deteriorating respiratory function. There is no antidote and aggressive and prolonged respiratory supportive may be required. Gradual recovery occurs over 2–5 days with weakness persisting sometimes for weeks.
Tetrodotoxin poisoning
Tetrodotoxin (TTX) is a paralytic toxin that occurs in the flesh, skin and viscera of puffer fish. Tetrodotoxin acts by inhibiting voltage-sensitive fast sodium channels and thus preventing conduction centrally and in peripheral motor and sensory nerves. In Australia, TTX poisoning is rare and usually occurs in those ignorant of the danger of ingesting puffer fish. In Japan, where such fish are a delicacy called ‘fugu’, numerous cases and deaths occur every year. Toxicity usually manifests rapidly after ingestion, with paraesthesia starting periorally followed by facial numbness and weakness progressing to bulbar paralysis, respiratory paralysis and death. Unlike therapeutic neuromuscular blockade, the pupils are unresponsive. Management is supportive as there is no antidote. All cases should be admitted for observation until peak clinical effects have passed. It is extremely unlikely that life-threatening effects will occur after 24 h in patients who have not already developed severe effects.
Injuries
Stingray
Stingrays possess a barbed spine with an enveloping integumentary sheath and associated venom glands on the tail. Human injury usually occurs from slashing of the tail when the animal is trodden on, resulting in a wound on the distal half of the lower limb. Although the majority of injuries are minor, three deaths have been documented in Australia, the last occurring in 2006. All died from penetrating cardiac wounds. In one of these cases, cardiac tamponade occurred 5 days post-injury, secondary to myonecrosis of myocardium at the site of the wound. Part of the radiopaque spine and its integumentary sheath is frequently left in the wound. Often a combined penetrative–lacerative injury, these wounds can appear deceptively minor. As such, all wounds require exploration, meticulous debridement and cleaning, followed by a low threshold for prophylactic antibiotics. Wounds should not be repaired initially and reviewed some days later for possible delayed primary closure. All wounds to the chest and abdomen need to be treated as a penetrating injury and admitted and appropriately investigated and observed.
Disproportionate pain lasting hours is often encountered, presumably from the accompanying venom, and immersion in hot water may be of benefit in addition to parenteral narcotics and regional anaesthesia. Local injury occurs from both direct trauma and envenoming. Systemic envenomation is rare and usually minor with nausea, headaches and lightheadedness, though there are reports of seizures and cardiovascular collapse. Treatment is symptomatic and no antivenom is available.
Molluscs
Blue-ringed octopus
Seven species of this small octopus are found along the Australian coastline. Normally brown in colour, the characteristic small bright blue rings become vividly prominent when the animal is agitated. Humans are at risk of envenoming when they disturb the animal. There are two documented deaths from the blue-ringed octopus (Hapalochlaena maculosa) in Australia and several other cases of potentially fatal envenoming that were successfully managed. The active component of the venom is tetrodotoxin which inhibits central and peripheral nerve conduction by changing the morphology of fast sodium channels. Death occurs from respiratory failure due to progressive paralysis. The bite is typically painless, but a small lesion with bleeding may occasionally be visible. There is a spectrum of envenoming, from minimal symptoms of localized neurology to rapid onset of generalized paralysis, during which time the patient remains conscious until succumbing to the effects of hypoxia.
Treatment
Cone shell
Of the many species of cone shell, about 18 have been implicated in human envenoming. The sole Australian fatality reported was caused by Conus geographus in 1936.The cone shell snail injects venom from a radular tooth harpoon carried on a proboscis that protrudes from the narrow end of the shell. The venom consists of peptitoxins called conopeptides. Pain is usually felt at the site of the bite and, in serious envenoming, evidence of muscular weakness may rapidly develop and occasionally progress to respiratory paralysis.
Treatment
Reptiles
Snakes
Sea snakes are readily distinguished from terrestrial snakes by their flat oar-like tail.
It is important to remember that terrestrial snakes may also take to the water, but swim on the surface. Sea snakes, like terrestrial snakes, are air-breathing reptiles and, in Australia, are found in tropical or temperate waters. They are only occasionally found in terrestrial situations, but bites have been recorded from handling animals that have been washed ashore.
In contrast to fish stings, the bite of a sea snake typically causes minimal local pain. Symptoms include progressive muscle pain and tenderness, with pain and stiffness on passive movement. Neuromuscular paralysis and rhabdomyolysis are common, but coagulopathy is rare. Fortunately, as for terrestrial snakes, most bitten victims are not envenomed.
Treatment
Note: CSL venom detection kit (VDK) does not detect sea snake antigens and the effectiveness of CSL polyvalent is unknown for these envenomings.