History
Comments
Current Medications
Comments
Current Symptoms
Comments
Physical Examination
Comments
Laboratory Data
Comments
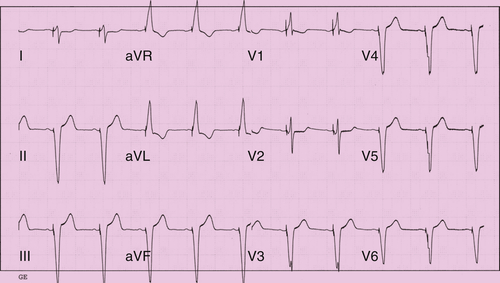
Electrocardiogram
Findings
Comments
Echocardiogram
Findings
Comments
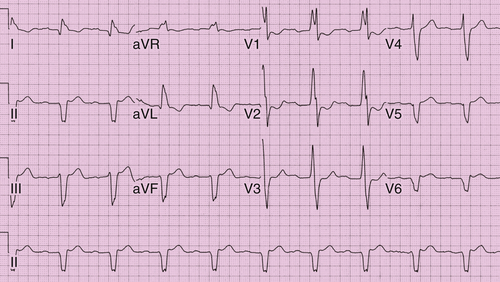
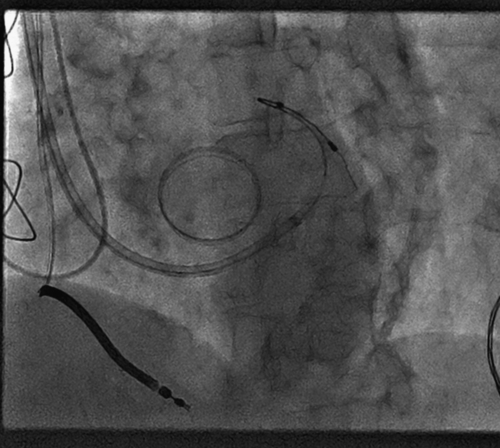
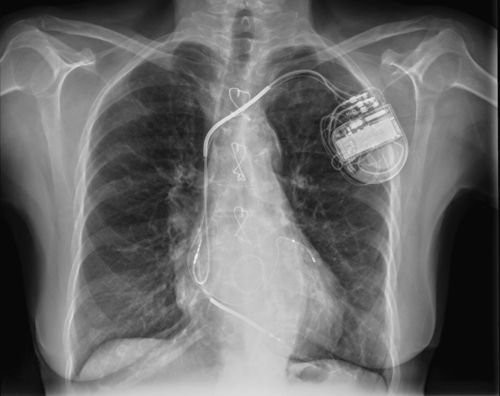
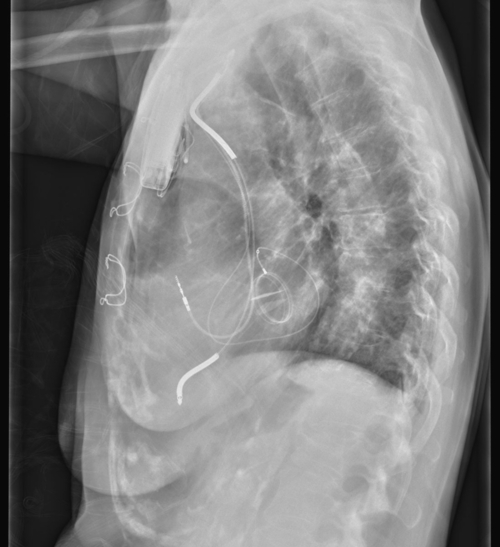
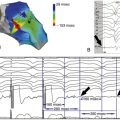
FIGURE 3-1
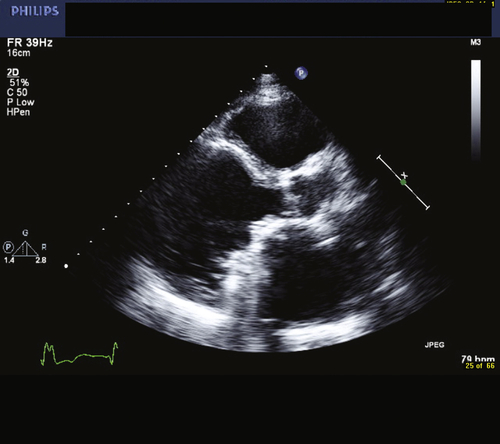
FIGURE 3-2
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
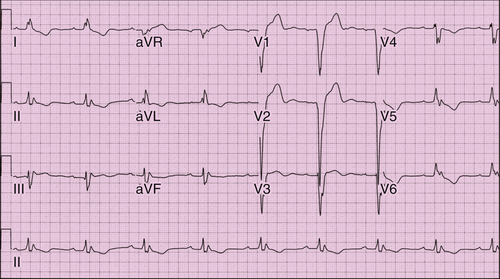
FIGURE 3-3
TABLE 3-1
Trials That Analyzed the Efficacy of Biventricular Pacing Depending on QRS Morphology
| Trial | No. | Control | Intervention | End Point | RRR Overall (%) | RRR in Non-LBBB |
| COMPANION | 1520 | Medical therapy | Biventricular pacemaker/ICD | Death from or hospitalization from heart failure | Biventricular pacemaker 34% Biventricular ICD 40% |
No difference |
| CARE-HF | 813 | Medical therapy | Biventricular pacemaker | Death or hospitalization for a major cardiovascular event | 37% | No difference |
| RAFT | 1798 | ICD | Biventricular ICD | Death or heart failure hospitalization | 25% | No difference∗ |
| MADIT-CRT | 1820 | ICD | Biventricular ICD | Death or heart failure event | 44% | No difference |
CARE-HF, Cardiac Resynchronization in Heart Failure trial; COMPANION, Comparison of Medical Therapy, Pacing and Defibrillation in Heart Failure trial; ICD, implantable cardioversion defibrillator; LBBB, left bundle branch block; MADIT-CRT, Multicenter Automatic Defibrillator Implantation with Cardiac Resynchronization Therapy study; RAFT, Resynchronization–Defibrillation for Ambulatory Heart Failure Trial; RRR, Relative Risk Reduction.
∗ See text: Overall no difference was found in the RBBB group, but when analyzed by QRS width, patients with RBBB and a QRS >160 msec derived benefit from biventricular ICD therapy.
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
FIGURE 3-4
FIGURE 3-5
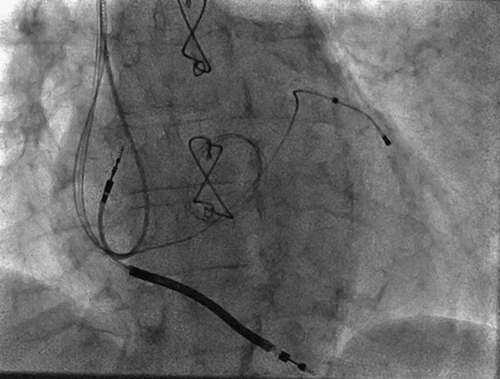
FIGURE 3-7
Selected References
1. Birnie D.H., Ha A., Higginson L. et al. Importance of QRS duration and morphology in determining response to cardiac resynchronization therapy: results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). Heart Rhythm,. 2012;9(Suppl 5):S295–S296.
2. Bristow M.R., Saxon L.A., Boehmer J. et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150.
3. Cleland J.G., Daubert J.C., Erdmann E. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549.
4. Gervais R., Leclercq C., Shankar A. et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11:699–705.
5. Moss A.J., Hall W.J., Cannom D.S. et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338.
6. Tang A.S., Wells G.A., Talajic M. et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395.
7. Zareba W., Klein H., Cygankiewicz I. et al. Effectiveness of cardiac resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT). Circulation. 2011;123:1061–1072.