Procedure 3 Anterior Odontoid Resection
The Transoral Approach
Indications
 Generally, for the correction of irreducible, ventral compression of the cervicomedullary junction.
Generally, for the correction of irreducible, ventral compression of the cervicomedullary junction.
 Specifically, for ventral extradural, midline pathology from the lower clivus to the C2-3 disk. Anticipated dissection should not extend laterally more than 11 mm on either side of the midline, as this may result in damage to the eustachian tubes, hypoglossal nerves, or vertebral arteries.
Specifically, for ventral extradural, midline pathology from the lower clivus to the C2-3 disk. Anticipated dissection should not extend laterally more than 11 mm on either side of the midline, as this may result in damage to the eustachian tubes, hypoglossal nerves, or vertebral arteries.
 Commonly used to decompress neural elements, typically in patients with rheumatoid arthritis. Cervicomedullary neural compression may be due to
Commonly used to decompress neural elements, typically in patients with rheumatoid arthritis. Cervicomedullary neural compression may be due to
 As part of a staged procedure, may be used to excise a chordoma or other midline extradural tumor at the craniocervical junction
As part of a staged procedure, may be used to excise a chordoma or other midline extradural tumor at the craniocervical junction
 May very occasionally be used for midline intradural pathology, such as meningiomas and schwannomas, usually as part of a staged procedure.
May very occasionally be used for midline intradural pathology, such as meningiomas and schwannomas, usually as part of a staged procedure.
Examination/Imaging
 Neurologic and musculoskeletal examination
Neurologic and musculoskeletal examination
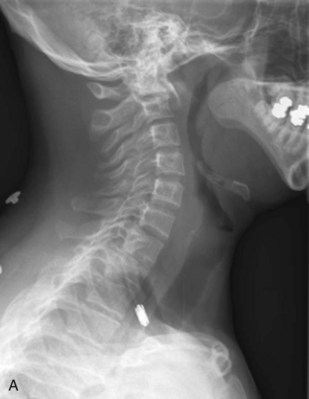
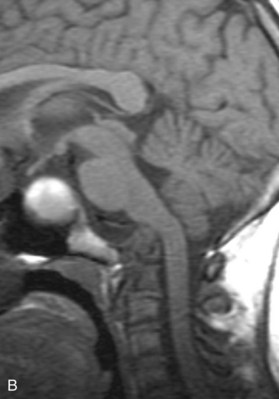
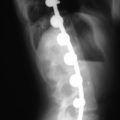
 Preoperative imaging should include multiplanar radiographs of the cervical spine, computed tomography (CT) with sagittal and coronal reformatting, and magnetic resonance imaging (MRI) to clearly define any soft tissue pathology and the degree of neural compression (Figure 3-1).
Preoperative imaging should include multiplanar radiographs of the cervical spine, computed tomography (CT) with sagittal and coronal reformatting, and magnetic resonance imaging (MRI) to clearly define any soft tissue pathology and the degree of neural compression (Figure 3-1).
 CT reformatted images provide detailed information about the bony elements and can be beneficial in planning posterior instrumentation procedures.
CT reformatted images provide detailed information about the bony elements and can be beneficial in planning posterior instrumentation procedures.
 Image guidance has been used as an adjunct for anterior odontoid resection, including frameless stereotaxy and intraoperative MRI. However, frameless stereotaxy may be inaccurate because of the mobility of the craniocervical junction.
Image guidance has been used as an adjunct for anterior odontoid resection, including frameless stereotaxy and intraoperative MRI. However, frameless stereotaxy may be inaccurate because of the mobility of the craniocervical junction.
 Magnetic resonance angiography (MRA) may be beneficial in defining the vascular anatomy and relationship of the vertebral arteries to the midline, as well as dominance of one vessel.
Magnetic resonance angiography (MRA) may be beneficial in defining the vascular anatomy and relationship of the vertebral arteries to the midline, as well as dominance of one vessel.
 In the treatment of patients with rheumatoid arthritis, it is suggested that anti-tumor necrosis factor be held 2 to 4 weeks before surgery and up to 2 weeks after. There is no definitive evidence to suggest methotrexate should be discontinued perioperatively.
In the treatment of patients with rheumatoid arthritis, it is suggested that anti-tumor necrosis factor be held 2 to 4 weeks before surgery and up to 2 weeks after. There is no definitive evidence to suggest methotrexate should be discontinued perioperatively.
Treatment Options
• Anterior odontoid resection through the transoral approach (transoral-transpharyngeal, with or without palatotomy)
• Combined anterior odontoid resection through the transoral approach, followed by posterior stabilization with possible decompression
• Standalone posterior stabilization with possible decompression
• Adjunctive traction reduction (in setting of reducible basilar invagination or atlantoaxial subluxation), followed by posterior stabilization
Surgical Anatomy
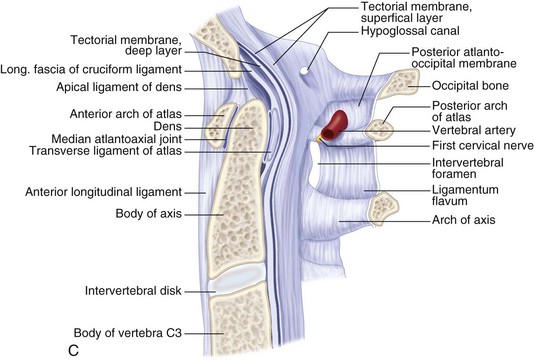
 Understanding the ligaments of the craniovertebral junction is vital when operating in this region.
Understanding the ligaments of the craniovertebral junction is vital when operating in this region.
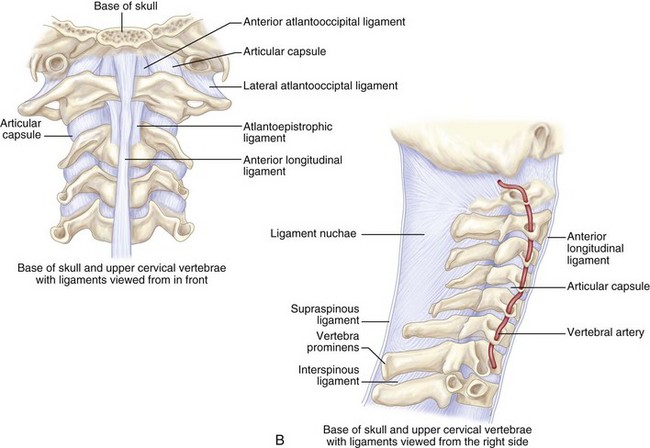
 The atlas is united to the occipital bone by the anterior and posterior atlanto-occipital membranes.
The atlas is united to the occipital bone by the anterior and posterior atlanto-occipital membranes.
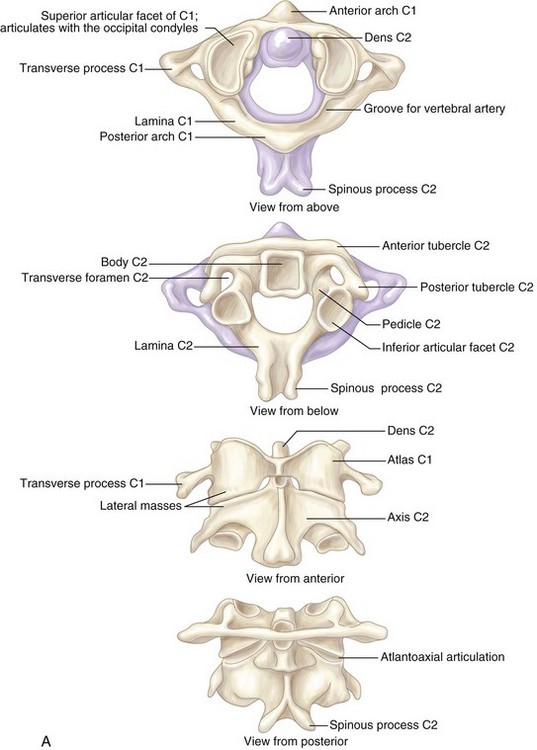
 The atlantoaxial joint consists of four articulations and two key ligaments. Two synovial joints for each lateral mass and two odontoid joints, on the anterior and posterior aspects.
The atlantoaxial joint consists of four articulations and two key ligaments. Two synovial joints for each lateral mass and two odontoid joints, on the anterior and posterior aspects.
 The alar ligament arises laterally from the odontoid to attach to the occipital condyles. The apical ligament runs from the odontoid process to the anterior margin of the foramen magnum. Disruption of any of the aforementioned ligamentous structures runs an increased risk for basilar invagination.
The alar ligament arises laterally from the odontoid to attach to the occipital condyles. The apical ligament runs from the odontoid process to the anterior margin of the foramen magnum. Disruption of any of the aforementioned ligamentous structures runs an increased risk for basilar invagination.
 The cruciate ligament runs from the atlas to the axis anteriorly. Atlantoaxial dissociation results from damage to this ligament, requiring surgical intervention.
The cruciate ligament runs from the atlas to the axis anteriorly. Atlantoaxial dissociation results from damage to this ligament, requiring surgical intervention.
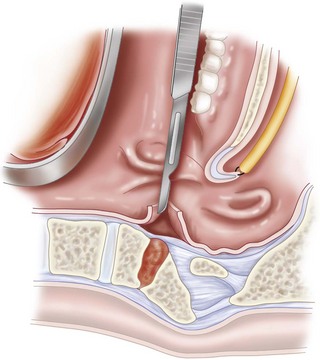
 Below the foramen magnum, the oropharynx is separated from the prevertebral fascia by a well-defined areolar plane (Figure 3-2). The oropharyngeal mucosa heals remarkably well after surgical incision and repair.
Below the foramen magnum, the oropharynx is separated from the prevertebral fascia by a well-defined areolar plane (Figure 3-2). The oropharyngeal mucosa heals remarkably well after surgical incision and repair.
 The most important bony anatomic landmarks for the transoral approach are the midline structures: rostrally, the septal attachment to the sphenoid, the pharyngeal tubercle on the clivus; and caudally, the anterior tubercle of the C1 arch. The longus colli muscles flank the dens on each side and, more laterally, the longus capitis muscles.
The most important bony anatomic landmarks for the transoral approach are the midline structures: rostrally, the septal attachment to the sphenoid, the pharyngeal tubercle on the clivus; and caudally, the anterior tubercle of the C1 arch. The longus colli muscles flank the dens on each side and, more laterally, the longus capitis muscles.
 The anterior longitudinal ligament extends caudally in the midline.
The anterior longitudinal ligament extends caudally in the midline.
Positioning
 Neurophysiologic monitoring electrodes for somatosensory-evoked potential and transcranial motor-evoked potential monitoring are placed first.
Neurophysiologic monitoring electrodes for somatosensory-evoked potential and transcranial motor-evoked potential monitoring are placed first.
 Fiberoptic nasotracheal intubation is then performed.
Fiberoptic nasotracheal intubation is then performed.
 A nasogastric tube should also be placed for intraoperative gastric drainage and postoperative feeding.
A nasogastric tube should also be placed for intraoperative gastric drainage and postoperative feeding.
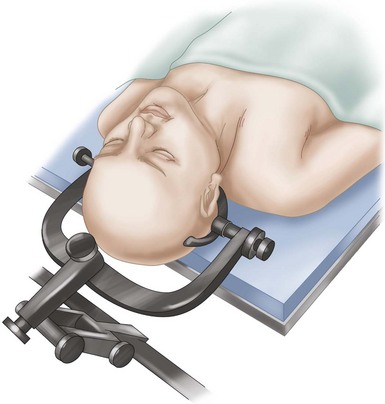
 The patient’s head can be fixed in a three-pin fixation system with slight extension. Alternatively, a horseshoe with Gardner-Wells traction or the head resting on a circular headrest can be used.
The patient’s head can be fixed in a three-pin fixation system with slight extension. Alternatively, a horseshoe with Gardner-Wells traction or the head resting on a circular headrest can be used.
 Extension should not be used in patients with fixed cervical kyphosis. Rather, they should be placed in slight Trendelenburg position to assist in the rostral extent of the dissection. One caveat to this would be the limitations in positioning caused by cervical instability or cervicomedullary compression; in this case, positioning is done cautiously with neuromonitoring.
Extension should not be used in patients with fixed cervical kyphosis. Rather, they should be placed in slight Trendelenburg position to assist in the rostral extent of the dissection. One caveat to this would be the limitations in positioning caused by cervical instability or cervicomedullary compression; in this case, positioning is done cautiously with neuromonitoring.
Positioning Pearls
• Because many patients have inherent spinal instability, perisurgical neck immobilization may be required. Halo immobilization, however, will limit neck extension and surgical exposure.
• The placement of topical 1% hydrocortisone on the oral mucosa, before and after surgery, may reduce the incidence of lip and tongue swelling.
Positioning Pitfalls
• Inability to widely open the mouth is a relative contraindication to this procedure. As a general rule, in the adult population, if you cannot place three fingers into the mouth of a patient with his or her mouth fully opened, the transoral approach should be avoided. Otherwise, splitting the mandible and tongue may be needed for adequate exposure.
• Alternatively, patients may be positioned laterally in a Mayfield clamp (Figure 3-3). The advantages of this position are that blood and washings drain out of the operative field. The head is placed in slight extension, which improves exposure. The table may be tilted laterally, allowing optimal positioning for the patient and surgeon. After the initial procedure, a posterior stabilization can be performed after reversing the lateral tilt.
• A fluoroscopy unit is then brought in following positioning to confirm adequate positioning and spinal alignment.
Portals/Exposures
 Oral swabs can be obtained for culture to identify bacterial colonization before preparation of the mouth and oropharynx with 1% Betadine or cetrimide.
Oral swabs can be obtained for culture to identify bacterial colonization before preparation of the mouth and oropharynx with 1% Betadine or cetrimide.
 The upper esophagus should be packed with a collagen sponge or gauze to minimize the ingestion of saline and blood.
The upper esophagus should be packed with a collagen sponge or gauze to minimize the ingestion of saline and blood.
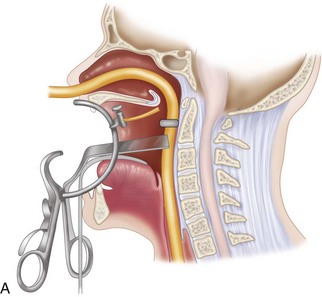
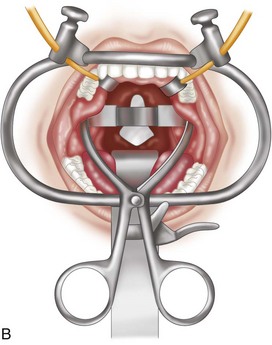
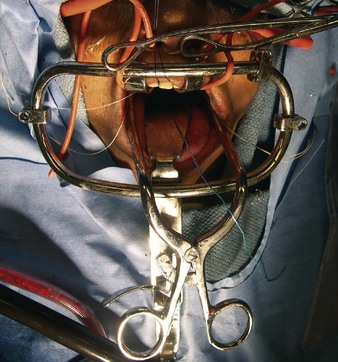
 The midlines of the oropharyngeal mucosa and soft palate are infiltrated with 1% lidocaine with epinephrine (1:100,000). A Crockard transoral retractor system (Codman, Raynham, Mass.) is used to maintain adequate exposure of the posterior oral cavity and to keep the nasotracheal and nasogastric tubes to one side, out of the surgeon’s way (Figures 3-4 and 3-5).
The midlines of the oropharyngeal mucosa and soft palate are infiltrated with 1% lidocaine with epinephrine (1:100,000). A Crockard transoral retractor system (Codman, Raynham, Mass.) is used to maintain adequate exposure of the posterior oral cavity and to keep the nasotracheal and nasogastric tubes to one side, out of the surgeon’s way (Figures 3-4 and 3-5).
 A tongue blade and soft palate retractors maximize the exposure.
A tongue blade and soft palate retractors maximize the exposure.
 To extend superior and lateral exposure, the soft palate may be split at the midline from hard palate to the uvula.
To extend superior and lateral exposure, the soft palate may be split at the midline from hard palate to the uvula.
 The uvula may be secured with a red rubber catheter and retracted along with the soft palate through the nares to avoid problems with swallowing and phonation postoperatively. After incision of the posterior pharyngeal wall, a Crockard toothed self-retaining retractor is inserted for lateral retraction to expose the underlying anterior longitudinal ligament and longus colli muscles.
The uvula may be secured with a red rubber catheter and retracted along with the soft palate through the nares to avoid problems with swallowing and phonation postoperatively. After incision of the posterior pharyngeal wall, a Crockard toothed self-retaining retractor is inserted for lateral retraction to expose the underlying anterior longitudinal ligament and longus colli muscles.
 With or without the aid of lateral fluoroscopy, the extent of the incision is from the base of the clivus to the upper border of the C3 vertebra.
With or without the aid of lateral fluoroscopy, the extent of the incision is from the base of the clivus to the upper border of the C3 vertebra.
Procedure
Step 1
 The anterior ridge or tubercle of the atlas is palpated. At this point, a confirmatory lateral localizing image may be taken. An operating microscope can then be used, or a surgeon may choose loupe magnification with directed illumination.
The anterior ridge or tubercle of the atlas is palpated. At this point, a confirmatory lateral localizing image may be taken. An operating microscope can then be used, or a surgeon may choose loupe magnification with directed illumination.
 A vertical incision is made extending approximately 2.5 cm superiorly and 2.5 to 3.0 cm inferiorly along the midline of the posterior oropharynx (Figure 3-6).
A vertical incision is made extending approximately 2.5 cm superiorly and 2.5 to 3.0 cm inferiorly along the midline of the posterior oropharynx (Figure 3-6).
 The extent of the exposure obtained with this incision will be approximately 15 to 20 mm bilaterally from the midline incision.
The extent of the exposure obtained with this incision will be approximately 15 to 20 mm bilaterally from the midline incision.
 Dissection is taken through the posterior pharyngeal mucosa, the superior constrictor muscles of the pharynx, and the anterior longitudinal ligament.
Dissection is taken through the posterior pharyngeal mucosa, the superior constrictor muscles of the pharynx, and the anterior longitudinal ligament.
 Incising the soft (and sometimes hard) palate can provide additional visualization of the lower clivus if needed.
Incising the soft (and sometimes hard) palate can provide additional visualization of the lower clivus if needed.
 Using periosteal elevators and electrocautery, a subperiosteal dissection exposes the arch of C1, as well as the anterior bodies of C2 and C3.
Using periosteal elevators and electrocautery, a subperiosteal dissection exposes the arch of C1, as well as the anterior bodies of C2 and C3.
 The longus colli and longus capitis muscles are detached medially to laterally from the cervical vertebra.
The longus colli and longus capitis muscles are detached medially to laterally from the cervical vertebra.
 In the presence of instability, there may be a large amount of granulation tissue at the level of the inferior margin of the atlas and its junction with the anterior odontoid peg. Toothed retractor blades are then used to retract the dissected soft tissues laterally. This allows excellent visualization of the midline inferior clivus, the atlas, and the axis.
In the presence of instability, there may be a large amount of granulation tissue at the level of the inferior margin of the atlas and its junction with the anterior odontoid peg. Toothed retractor blades are then used to retract the dissected soft tissues laterally. This allows excellent visualization of the midline inferior clivus, the atlas, and the axis.
Step 1 Pitfalls
• Great care should be taken to avoid cerebrospinal fluid (CSF) leakage in order to minimize the risk of postoperative meningitis. A preoperative lumbar drain should be placed when an intradural approach is anticipated. In such procedures, fat, muscle, fascia lata, or a dermal fat graft should be used in the repair of any dural opening, followed by the application of fibrin glue.
• With an incision from the inferior clivus to the superior border of C3, an operating field of 15 to 20 mm bilaterally can be exposed. Beyond that, there is an increased risk of trauma to the eustachian tube, hypoglossal nerve, vidian nerve, and vertebral artery at the C1-2 interspace.
• Given the large vascular channels and venous sinusoids in this region, postoperative hematoma formation may be a problem. This can be minimized by meticulous hemostasis, using Avitene, Surgicel, Gelfoam, or fibrin glue, and postoperative nursing in the head-up position. Bleeding from the rheumatoid pannus or small arterial feeders can be controlled with bipolar electrocautery. If an intradural procedure is performed, watertight dural closure is very important to minimize the risk of infection. Suturing or clipping the dura will rarely close the defect completely. A free dermal fat graft, pharyngeal mucosal rotation flaps, or nasal septal mucosal flaps help provide a watertight closure, and a lumbar drain may also be used.
Step 2
 A match-head burr is used to remove the anterior arch of the atlas out laterally approximately 1 cm to each side of the midline (about two thirds of the arch, exposing the shoulder of the dens bilaterally) (Figure 3-7). The odontoid mass and pannus (if present) are then resected in a rostrocaudal direction (starting at the top of the odontoid process) using a combination of drilling and curetting.
A match-head burr is used to remove the anterior arch of the atlas out laterally approximately 1 cm to each side of the midline (about two thirds of the arch, exposing the shoulder of the dens bilaterally) (Figure 3-7). The odontoid mass and pannus (if present) are then resected in a rostrocaudal direction (starting at the top of the odontoid process) using a combination of drilling and curetting.
 Alternatively, the odontoid process may be initially drilled at its base and disarticulated from the C2 body. The odontoid peg is hollowed out gradually with a 3-mm cutting burr down to the cortical bone, which is then thinned and removed with a match-head or diamond burr. The alar and apical ligaments are sharply divided, taking care not to cause a CSF leak. The proximal peg is then removed after circumferentially elevating off all soft tissue attachments. This is facilitated by grasping the odontoid peg with special forceps and pulling it down from the foramen magnum while elevating the dura off it. This allows complete removal of the dens. This technique has a greater potential for durotomy, particularly in the pediatric population, in whom the odontoid process may have a hook at its apex that can tear the dura during peg removal.
Alternatively, the odontoid process may be initially drilled at its base and disarticulated from the C2 body. The odontoid peg is hollowed out gradually with a 3-mm cutting burr down to the cortical bone, which is then thinned and removed with a match-head or diamond burr. The alar and apical ligaments are sharply divided, taking care not to cause a CSF leak. The proximal peg is then removed after circumferentially elevating off all soft tissue attachments. This is facilitated by grasping the odontoid peg with special forceps and pulling it down from the foramen magnum while elevating the dura off it. This allows complete removal of the dens. This technique has a greater potential for durotomy, particularly in the pediatric population, in whom the odontoid process may have a hook at its apex that can tear the dura during peg removal.
 The posterior longitudinal ligament is seen behind the dens, which has now been removed. The fibers of the transverse ligament are also visualized at the level of the removed C1 anterior arch. With division of these ligaments, the dura should be clearly seen. Ligament and soft tissue removal can be accomplished with a series of small angled curettes, transsphenoidal punches, and transoral bayoneted forceps. Typically, a gap exists between the ligaments and dura. Decompression is considered adequate when the dura pulsates freely and the lateral curvature of the dura is seen bilaterally. Fluoroscopy may be used to confirm adequate decompression.
The posterior longitudinal ligament is seen behind the dens, which has now been removed. The fibers of the transverse ligament are also visualized at the level of the removed C1 anterior arch. With division of these ligaments, the dura should be clearly seen. Ligament and soft tissue removal can be accomplished with a series of small angled curettes, transsphenoidal punches, and transoral bayoneted forceps. Typically, a gap exists between the ligaments and dura. Decompression is considered adequate when the dura pulsates freely and the lateral curvature of the dura is seen bilaterally. Fluoroscopy may be used to confirm adequate decompression.
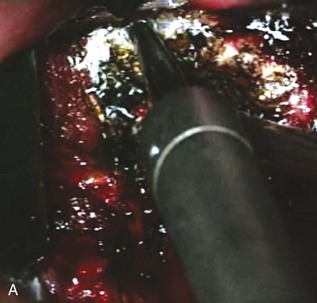
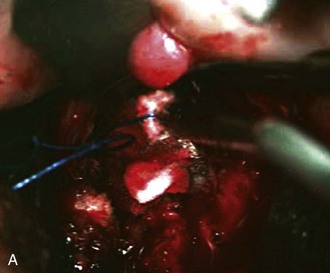
 Any venous bleeding can be controlled with Surgicel and fibrin glue (Figure 3-8, A).
Any venous bleeding can be controlled with Surgicel and fibrin glue (Figure 3-8, A).
Step 3
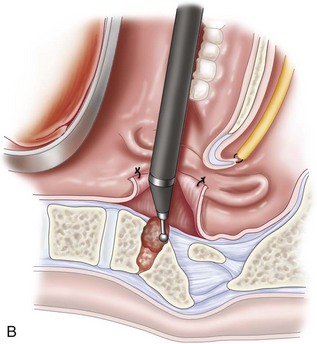
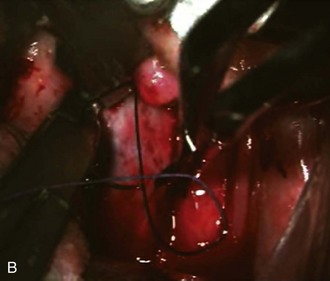
 The posterior pharyngeal wall is then closed with a two-layered closure using 3-0 Vicryl sutures (Figure 3-8, B).
The posterior pharyngeal wall is then closed with a two-layered closure using 3-0 Vicryl sutures (Figure 3-8, B).
 Despite the presence of bacterial flora in the oral cavity, a low infection rate of less than 3% is to be expected if the dura is not breeched. In the presence of a durotomy, great efforts should be made to close the dura in a watertight manner.
Despite the presence of bacterial flora in the oral cavity, a low infection rate of less than 3% is to be expected if the dura is not breeched. In the presence of a durotomy, great efforts should be made to close the dura in a watertight manner.
 A double layer closure of both the pharyngeal musculature and mucosa is less susceptible to dehiscence.
A double layer closure of both the pharyngeal musculature and mucosa is less susceptible to dehiscence.
 In the presence of durotomy, a watertight closure may be aided with the application of fat, fascia, dermal fat graft, and fibrin glue. Additionally, a lumbar drain should be used for about 5 days with regular drainage of CSF (10 to 15 mL/hr).
In the presence of durotomy, a watertight closure may be aided with the application of fat, fascia, dermal fat graft, and fibrin glue. Additionally, a lumbar drain should be used for about 5 days with regular drainage of CSF (10 to 15 mL/hr).
Postoperative Care and Expected Outcomes
 Postoperatively, the nasotracheal tube is left in place for 24 to 48 hours. It should only be removed if there is no evidence of significant labial or lingual swelling.
Postoperatively, the nasotracheal tube is left in place for 24 to 48 hours. It should only be removed if there is no evidence of significant labial or lingual swelling.
 The patient should be left in a halo vest, Minerva jacket, rigid collar, or traction if a posterior stabilization has not been performed at the initial procedure.
The patient should be left in a halo vest, Minerva jacket, rigid collar, or traction if a posterior stabilization has not been performed at the initial procedure.
 The patient should be encouraged to sit up and to ambulate, if possible, to minimize saliva pooling in the pharynx and the potential for breakdown of the incision.
The patient should be encouraged to sit up and to ambulate, if possible, to minimize saliva pooling in the pharynx and the potential for breakdown of the incision.
 The patient should take nothing by mouth for 5 days after the operation. Nasogastric feeding may commence after 5 hours.
The patient should take nothing by mouth for 5 days after the operation. Nasogastric feeding may commence after 5 hours.
 Hydrocortisone ointment should be applied to the tongue and mucosa for the first 48 hours.
Hydrocortisone ointment should be applied to the tongue and mucosa for the first 48 hours.
 In the event of a durotomy, lumbar drainage should be maintained for 5 to 10 days. Prophylactic antibiotic therapy directed against gram-positive, gram-negative, and anaerobic oral flora may be administered by some surgeons. For example, Menezes (1991) recommended CSF cultures for the initial 5 days, at which point, if the cultures remain negative, antibiotics may be stopped. Occasionally, a lumbar-peritoneal shunt may be required for persistent CSF leakage.
In the event of a durotomy, lumbar drainage should be maintained for 5 to 10 days. Prophylactic antibiotic therapy directed against gram-positive, gram-negative, and anaerobic oral flora may be administered by some surgeons. For example, Menezes (1991) recommended CSF cultures for the initial 5 days, at which point, if the cultures remain negative, antibiotics may be stopped. Occasionally, a lumbar-peritoneal shunt may be required for persistent CSF leakage.
 The main predictor of outcome is the degree of preoperative neurologic impairment. Rheumatoid patients who are unable to walk due to myelopathy (Ranawat classification IIIb) have a much higher mortality.
The main predictor of outcome is the degree of preoperative neurologic impairment. Rheumatoid patients who are unable to walk due to myelopathy (Ranawat classification IIIb) have a much higher mortality.
Complications
• Airway complications are always a concern with the transoral approach. It is the practice of the senior author to leave the endotracheal tube in place for a minimum of 24 hours following surgery. If after this time there is evidence of swelling of the tongue or oral cavity, the endotracheal tube is left in situ until the swelling subsides. The occurrence of lingual swelling may be minimized by intermittent intraoperative release of the retractor, and ensuring the tongue is not trapped between the retractor blade and the lower teeth.
• Delayed complications may include tongue swelling, meningitis, palatal/pharyngeal dehiscence, neurologic deterioration, retropharyngeal abscess, late pharyngeal bleeding, and velopalatine incompetence. Pharyngeal dehiscence may occur either early or late. Early dehiscence (during the first 7 days after surgery) is typically due to inadequate closure or starting oral feeding too early. This can be minimized by encouraging the patient to sit up and walk as soon as possible to prevent pooling of saliva at the apex or weakest point of the pharyngeal incision. If early dehiscence occurs, closure should be attempted (with the assistance of head and neck specialists if required), followed by hyperalimentation and intravenous antibiotics. In cases of late dehiscence, infection needs to be ruled out. The differential diagnosis of late dehiscence includes osteomyelitis, retropharyngeal abscess, and poor nutrition. Management of retropharyngeal abscess includes lateral drainage (rather than transoral), followed by appropriate intravenous antibiotics, hyperalimentation through a nasogastric feeding tube, and neck immobilization.
• Neurologic deterioration after transoral odontoid resection is most likely to be due to craniocervical instability. The vast majority of patients who undergo this procedure require a posterior stabilization procedure.
• In patients with altered mental status following the transoral approach, meningitis must be kept at the forefront of the differential diagnosis. This is particularly true in the elderly population with rheumatoid arthritis, in whom this diagnosis may be overlooked because confusion in this age group can be common in the critical care setting.
• Late retropharyngeal bleeding may indicate an underlying infection. Osteomyelitis and pseudoaneurysm of the vertebral artery must also be ruled out. MRI/MRA evaluation of the craniovertebral junction should be performed in addition to angiography to rule out vascular involvement. This diagnostic process also allows for potential therapeutic endovascular treatment in cases of vertebral artery compromise.
• Velopalatine incompetence (incorrect closure of the soft palate muscle during speech resulting in a nasal voice) occurs more commonly in children than in adults. It typically occurs 4 to 6 months after the transoral procedure and probably occurs secondary to contracture of the soft palate and nasopharynx. This requires otorhinolaryngologic evaluation. Usually it is treated with pharyngeal retraining, but a palatal prosthesis or a pharyngeal flap may also be used.
Apuzzo ML, Weiss MH, Heiden JS. Transoral exposure of the atlantoaxial region. Neurosurgery. 1978;3:201-207.
Crockard HA. Transoral surgery: some lessons learned. Br J Neurosurg. 1995;9:283-293.
Crockard HA, Calder I, Ransford AO. One-stage transoral decompression and posterior fixation in rheumatoid atlanto-axial subluxation. J Bone Joint Surg Br. 1990;72:682-685.
Crockard HA, Sen CN. The transoral approach for the management of intradural lesions at the craniovertebral junction: review of 7 cases. Neurosurgery. 1991;28:88-97. discussion 97-8
Fang HSY, Ong GB. Direct anterior approach to the upper cervical spine. J Bone Joint Surg Am. 1962;44:1588-1604.
Frempong-Boadu AK, Faunce WA, Fessler RG. Endoscopically assisted transoral-transpharyngeal approach to the craniovertebral junction. Neurosurgery. 2002;51(5 Suppl):S60-S66.
A review of the endoscopic transoral approach (Level IV evidence [case series of 7 patients]).
Hadley MN, Martin NA, Spetzler RF, Sonntag VK, Johnson PC. Comparative transoral dural closure techniques: a canine model. Neurosurgery. 1988;22:392-397.
Hsu W, Wolinsky J, Gokaslan Z, Sciubba DM. Transoral approaches to the cervical spine. Neurosurgery. 2010;66(Suppl. 3):119-125.
Kaibara T, Hurlbert RJ, Sutherland GR. Transoral resection of axial lesions augmented by intraoperative magnetic resonance imaging: report of three cases. J Neurosurg Spine. 2001;95:239-242.
Krauss WE, Bledsoe JM, Clarke MJ, Nottmeier EW, Pichelmann MA. Rheumatoid arthritis of the craniovertebral junction. Neurosurgery. 2010;66(Suppl. 3):83-95.
Menezes AH. Complications of surgery at the craniovertebral junction—avoidance and management. Pediatr Neurosurg. 1991;17:254-266.
Pollack IF, Welch W, Jacobs GB, Janecka IP. Frameless stereotactic guidance: an intraoperative adjunct in the transoral approach for ventral cervicomedullary junction decompression. Spine. 1995;20:216-220.
Singh H, Harrop J, Schiffmacher P, Rosen M, Evans J. Ventral surgical approaches to craniovertebral junction chordomas. Neurosurgery. 2010;66(Suppl. 3):96-103.
Youssef AS, Sloan AE. Extended transoral approaches: surgical technique and analysis. Neurosurgery. 2010;66(Suppl. 3)):126-134.