History
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
Electrocardiogram
Findings
Echocardiogram
Findings
Physiologic Tracings
Findings
Focused Clinical Questions and Discussion Points
Question
Discussion
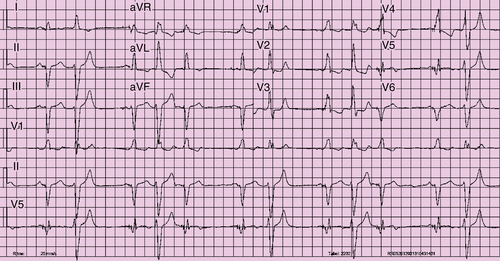
FIGURE 27-1
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
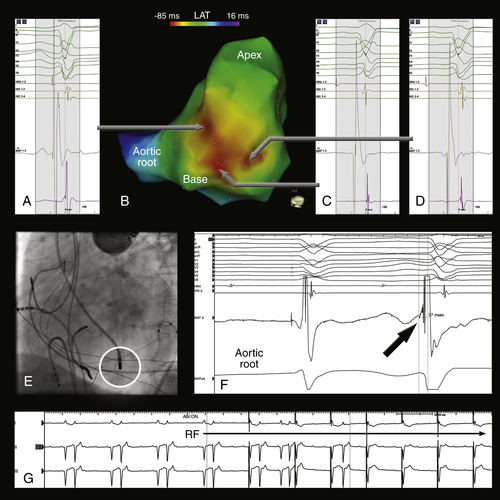
FIGURE 27-2 Electroanatomic activation map of the left ventricle color-coded for local activation time during premature ventricular contractions. The sites with earliest activation are located around the base of the papillary muscle (B, red area). The local activation times around the papillary muscle were similar (A, C, and D). Note the subtle differences in the 12-lead electrocardiogram morphology of the premature ventricular contraction, suggestive for slight alterations of the origin itself or the preferential conduction from the origin. The location of the ablation catheter at the successful ablation site on fluoroscopy is displayed in E. At this site, the local activation time was 37 msec before the onset of the QRS-complex (F). After 8 seconds, the premature ventricular contractions disappeared (G).
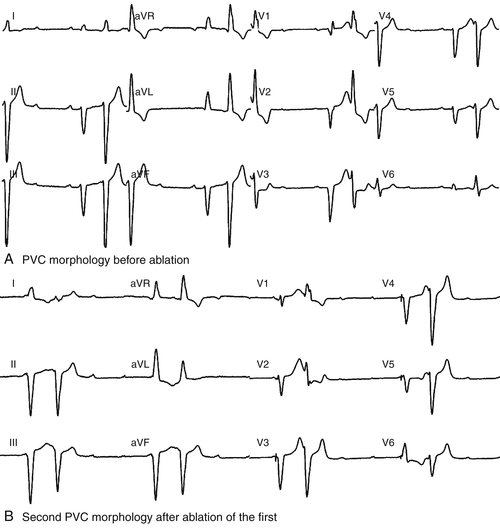
FIGURE 27-3 After a radiofrequency application based on activation mapping of the initial premature ventricular contraction (PCV) morphology, a change in the 12-lead ECG morphology of the premature ventricular contractions was observed, with a dominant S wave in V6 suggesting a shift of the PVC exit site.
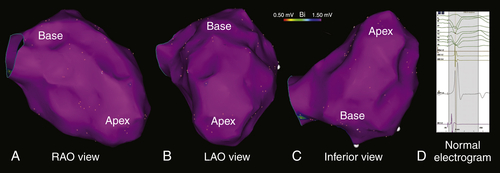
FIGURE 27-4 A to C, Electroanatomic map of the left ventricle during right ventricular pacing, color-coded for bipolar voltage (bipolar voltage >1.50 mV is considered normal and displayed in purple). The bipolar voltages and the electrogram morphologies were normal (D).
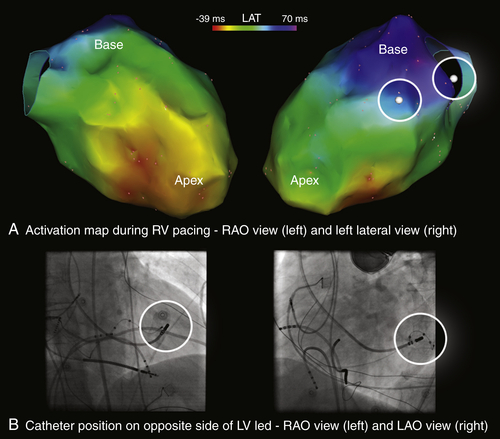
FIGURE 27-5 Electroanatomic map of the left ventricle during right ventricular pacing, now color-coded for local activation time. The sites that were opposite to the left ventricular pacing lead were identified based on fluoroscopy (B, circles) and subsequently tagged on the map (A, white tags with circles in right upper panel). The left ventricular pacing lead is located in close proximity to the latest activated site.
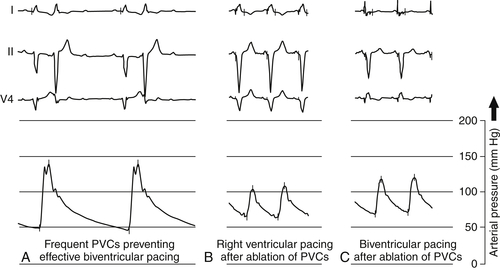
FIGURE 27-6 The frequent premature ventricular contractions (PVCs) before ablation did not result in significant cardiac output (A). After ablation of the PVC, right ventricular and biventricular pacing resulted in an almost tripled effective heart rate (B and C). Of note, the blood pressure was higher during biventricular pacing in contrast to only right ventricular pacing.
Outcome
Selected References
1. Barold S.S., Ovsyshcher I.E. Pacemaker-induced mitral regurgitation. Pacing Clin Electrophysiol. 2005;28:357–360.
2. Bogun F., Crawford T., Reich S. et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm. 2007;4:837–863.
3. Bogun F., Desjardins B., Crawford T. et al. Post-infarction ventricular arrhythmias originating in papillary muscles. J Am Coll Cardiol. 2008;51:1794–1802.
4. Bohnen M., Stevenson W.G., Tedrow U.B. et al. Incidence and predictors of major complications from contemporary catheter ablation to treat cardiac arrhythmias. Heart Rhythm. 2011;8:1661–1666.
5. Kindermann M., Hennen B., Jung J. et al. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol. 2006;47:1927–1937.
6. Vardas P.E., Auricchio A., Blanc J.-J. et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J. 2007;28:2256–2295.
7. Yamada T., Doppalapudi H., McElderry H.T. et al. Electrocardiographic and electrophysiological characteristics in idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: relevance for catheter ablation. Circ Arrhythm Electrophysiol. 2010;3:324–331.