History
Comments
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
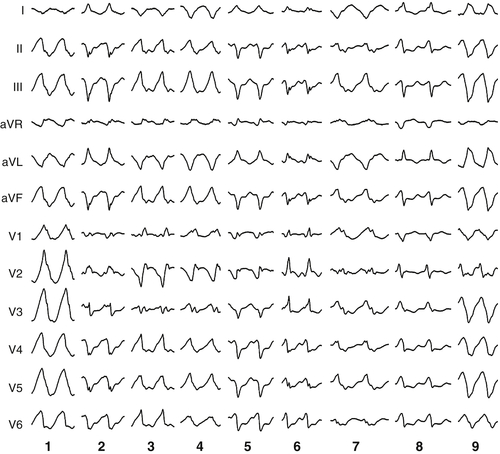
Electrocardiogram
Findings
Echocardiogram
Findings
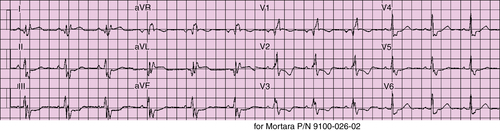
FIGURE 26-1
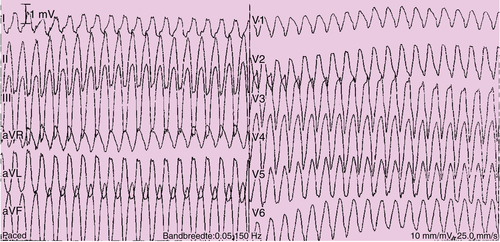
FIGURE 26-2
Magnetic Resonance Imaging
Findings
Comments
Catheterization
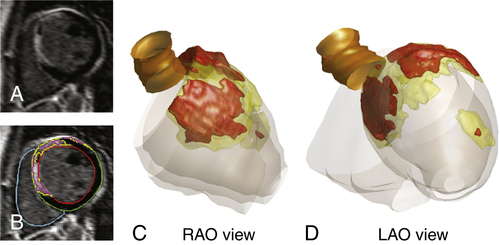
FIGURE 26-3 Based on short axis contrast-enhanced MRI slice (panel A) and semi-automatic detection of late enhancement (panel B), a 3-dimensional scar reconstruction was created (panels C and D). The core scar is displayed in red, borderzone in yellow. LAO denotes left anterior oblique; RAO, right anterior oblique.
Focused Clinical Questions and Discussion Points
Question
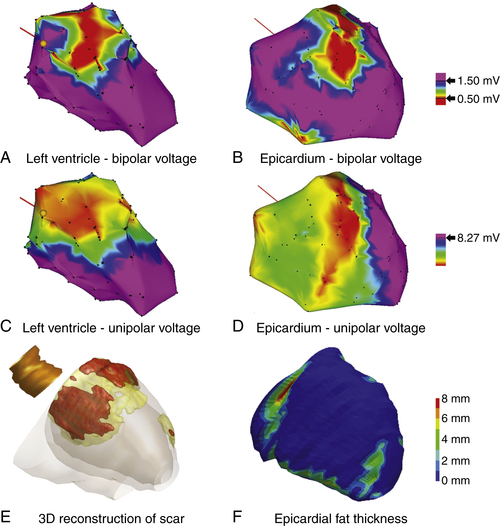
FIGURE 26-4 Electroanatomic maps of the left ventricle and epicardium, color-coded for bipolar and unipolar voltage. A low bipolar and unipolar voltage area is present in the left ventricular basal anteroseptal and anterior wall (A to D). The part of the epicardial low unipolar voltage area that was overlying the right ventricle is likely to be caused by the thinner right ventricular wall. The magnetic resonance imaging–derived three-dimensional scar reconstruction reveals scar in this area (E). The anterior basal wall is covered by no or little epicardial fat as visualized from the computed tomography–derived three-dimensional reconstruction of epicardial fat distribution (F).
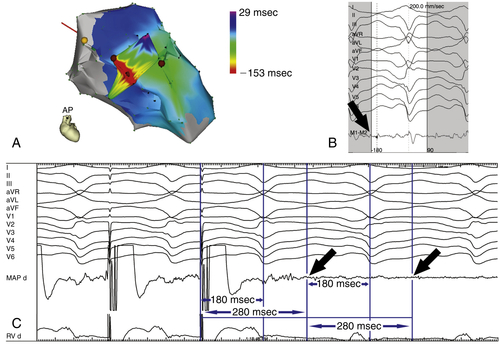
FIGURE 26-6 Electroanatomic maps of the left and right ventricles during sustained monomorphic ventricular tachycardia number 5 (A; for 12-lead electrocardiograph morphology, see Figure 36-4). The earliest activated region is displayed in red. A diastolic potential was found at the mid-septum remote from the bundle of His, as indicated by the yellow tag (B). Entrainment mapping was performed, demonstrating concealed entrainment with a postpacing interval of 280 ms, equaling the tachycardia cycle length (C). The 180-msec interval between the stimulus and the peak of lead II suggests that a small potential (black arrows) slightly before the larger potential has been captured. A radiofrequency energy application at this site resulted in slowing of the ventricular tachycardia and termination after 7 seconds.
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Findings
Comments
Selected References
1. Arya A., Bode K., Piorkowski C. et al. Catheter ablation of electrical storm due to monomorphic ventricular tachycardia in patients with nonischemic cardiomyopathy: acute results and its effect on long-term survival. Pacing Clin Electrophysiol. 2010;33:1504–1509.
2. Carbucicchio C., Santamaria M., Trevisi N. et al. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008;117:462–469.
3. Haqqani H.M., Tschabrunn C.M., Tzou W.S. et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization, and implications. Heart Rhythm. 2011;8:1169–1176.
4. Kindermann M. et al. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol. 2006;47:1927–1937.
5. Nakahara S., Tung R., Ramirez R.J. et al. Characterization of the arrhythmogenic substrate in ischemic and nonischemic cardiomyopathy implications for catheter ablation of hemodynamically unstable ventricular tachycardia. J Am Coll Cardiol. 2010;55:2355–2365.
6. Sweeney M.O., Hellkamp A.S., Ellenbogen K.A. et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937.
7. Thijssen J., Borleffs C.J., Delgado V. et al. Implantable cardioverter-defibrillator patients who are upgraded and respond to cardiac resynchronization therapy have less ventricular arrhythmias compared with nonresponders. J Am Coll Cardiol. 2011;58:2282–2289.
8. Tops L.F., Schalij M.J., Bax J.J. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol. 2009;25(54):764–776.
9. Yu C.M., Chan J.Y., Zhang Q. et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361:2123–2134.
10. Wilkoff B.L., Cook J.R., Epstein A.E. et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–3123.