CHAPTER 24. Thermoregulation
Vallire D. Hooper
OBJECTIVES
At the conclusion of this chapter, the reader will be able to:
1. Describe the physiology of thermoregulation.
2. Identify two complications of altered thermoregulation in the perianesthesia/perioperative setting.
3. Define unplanned perioperative hypothermia.
4. List three common causes of perioperative hypothermia.
5. Identify four adverse outcomes related to perioperative hypothermia.
6. Describe phase-specific recommendations for the management of perioperative hypothermia.
7. Define the pathophysiology of malignant hyperthermia (MH).
8. Identify the signs and symptoms of MH.
9. Describe the treatment of MH.
I. BASIC TERMS AND DEFINITIONS
A. Thermal compartments
1. Core thermal compartment
a. Well-perfused tissues with temperature remaining relatively uniform
b. Consists of organs of:
(1) Trunk
(2) Head
c. Comprises 50% to 60% of body mass
2. Peripheral thermal compartment
a. Consists of arms and legs
b. Temperature nonhomogeneous and varies over time
(1) Temperature usually 2° C to 4° C lower than core temperature
(2) Difference can be larger in more extreme thermal and/or physiological circumstances.
(a) Lower core-to-peripheral gradients
(i) Warm environment
(ii) Vasodilation in response to an increased metabolic heat (generated in the core)
(b) Higher core-to-peripheral gradients
(i) Cold environment
(ii) Vasoconstriction in an attempt to shift metabolic heat to the core
B. Temperature
1. Core temperature
a. Temperature of core thermal compartment
b. Most accurate core temperature measurement sites
(1) Pulmonary artery (PA)
(a) Obtained using a PA catheter
(b) Most accurate because the artery brings blood directly from the core and its surroundings
(c) Affected by:
(i) Large, rapid infusions of warmed or cold fluids
(ii) Respiratory cycles
(iii) Lower limb pneumatic compression devices
(2) Distal esophagus
(a) Best alternative to PA site
(b) Affected by:
(i) Active cooling phase of cardiopulmonary bypass
(ii) Surgery involving an open thorax or exposure of the diaphragm
(3) Nasopharynx
(a) Used to monitor brain temperature
(b) Not recommended with:
(i) Substantial anticoagulation
(ii) Manipulation of nasal mucosa
(4) Oral
(a) Temperature readings vary dependent on placement in oral cavity (Figure 24-1).
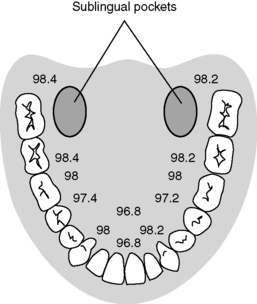 |
| FIGURE 24-1
Temperature variations in the oral cavity.
(From Nicoll LH: Heat in motion: Evaluating and managing temperature. Nursing 32:s1-s12, 2002.)
|
(b) Accurate reflection of core temperature when taken in left or right posterior sublingual (buccal) pocket
(c) Site is dependable even in presence of:
(i) Oxygen therapy
(ii) Warmed and cooled inspired gases
(iii) Varied respiratory rates
(d) Do not use in patients who are:
(i) Disoriented
(ii) Shivering
(iii) Having seizures
c. Other temperature measurement sites used in the perianesthesia setting
(1) Tympanic membrane (Figure 24-2)
(a) Accuracy of reading dependent on:
(i) Operator technique
(ii) Patient anatomy
(iii) Accurate calibration
(iv) Inherent instrument error of instrument used
(b) Shown to be inaccurate to true core temperature measurements
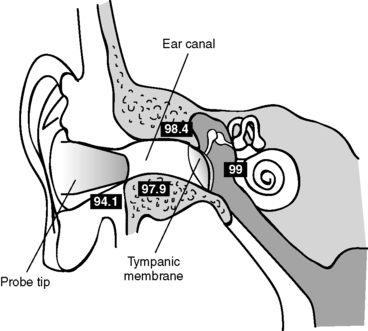 |
| FIGURE 24-2
Tympanic temperature monitoring.
(From Nicoll LH: Heat in motion: Evaluating and managing temperature. Nursing 32:s1-s12, 2002.)
|
(2) Temporal artery
(a) Favorably compares to tympanic and rectal measurements in children
(b) Less reliable in adults
(c) Lack of evidence to support accuracy to core temperature measurements in adults
(3) Axillary
(4) Bladder
(a) Subject to thermal lag in unsteady thermal states
(b) Continuous urinary drainage required
(c) Useful indicator of total body warming
(5) Rectum
(a) Subject to thermal lag in unsteady thermal states
(b) Potential for probe to be inserted into stool
(6) Skin
2. Normothermia: core temperature of 36° C to 38° C (96.8° F to 100.4° F)
3. Hypothermia: core temperature less than 36° C (96.8° F)
4. Hyperthermia: core temperature greater than 38° C (100.4° F)
C. Unplanned perioperative hypothermia
1. Active warming measures
a. Forced air convective warming
b. Circulating mattresses
c. Resistive heating blankets
d. Radiant warmers
e. Negative-pressure warming systems
f. Warmed humidified inspired oxygen
2. Passive thermal care measures
a. Warmed cotton blankets
b. Reflective blankets
c. Circulating water mattress
d. Socks
e. Head covering
f. Limited skin exposure
3. Prewarming
a. Warming of peripheral tissues or surface skin before anesthesia induction
4. Risk factor
a. Independent predictor, not an associated factor of an untoward event
5. Thermal comfort
a. Patient perception that they are neither too warm or too cold
D. MH
1. Hereditary abnormality of muscle metabolism
a. Caused by certain triggering agents
b. Results in a life-threatening pharmacogenetic disorder
2. Must have specific genes for MH to occur
a. Relatives of patient who has had an MH crisis are at risk.
(1) Siblings
(2) Parents
(3) Children
b. Inheritance by autosomal dominant pathway
(1) Risk diminishes as relationship becomes further removed.
3. Characterized by muscular hypercatabolic reactions
a. Level of intracellular calcium reuptake is impaired producing:
(1) Muscle tetany
(2) Increased production of:
(a) Heat
(b) Carbon dioxide
(c) Lactate
II. THERMOREGULATION PHYSIOLOGY
A. Most of body’s heat provided by basal metabolic rate
1. Core body temperature remains fairly constant.
2. Skin and extremity temperatures may vary with:
a. Environmental changes
b. Thermoregulatory responses
B. Temperature regulation in conscious adults mediated by the hypothalamus (Figure 24-3) through a combination of behavioral and physiological responses
1. Hypothalamus
a. Nestled at base of the brain
b. Primary temperature control center
(1) Maintains normothermia by regulating heat loss with heat production
(2) Receives input via spinal cord from thermoreceptors located in:
(a) Skin
(b) Nose
(c) Oral cavity
(d) Thoracic viscera
(e) Spinal cord
c. Generates conscious and unconscious responses to maintain normothermia
2. Mechanisms of temperature regulation
a. Behavior
(1) Adding or removing clothing or covering
(2) Changing location
(3) Adjusting temperature of dietary intake
(4) Adjusting environmental temperature
b. Endocrine
(1) Hormones released in response to hypothalamic stimulation
(2) Initiates organ and tissue responses in all systems
c. Autonomic
(1) Changes in peripheral circulation
(2) Peripheral shell expands or contracts in response to peripheral and core temperature changes (Figure 24-4).
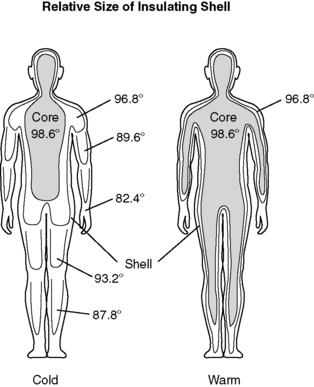 |
| FIGURE 24-4
Relative size of the insulating shell in response to temperature changes.
(From Pressley TA: Temperature regulation, 2000. Available at: http://phy025.lubb.ttuhsc.edu/Pressley/Course/Temp-Reg.htm. Accessed March 2003.)
|
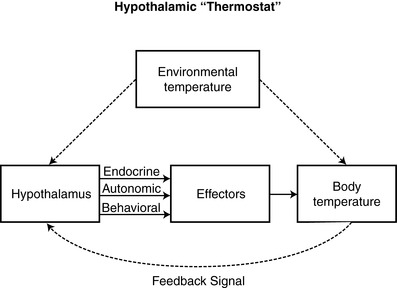 |
| FIGURE 24-3
The hypothalamic thermostat.
(From Pressley TA: Temperature regulation, 2000. Available at: http://phy025.lubb.ttuhsc.edu/ Pressley/Course/Temp-Reg.htm. Accessed March 2003.)
|
C. Mechanisms of heat production and loss
1. Mechanisms of heat production
a. Body tissues produce heat in proportion to their metabolic rates.
(1) Metabolism is the only natural internal source of heat.
(2) Brain and major organs (core thermal compartment)
(a) Most metabolically active
(b) Generate more metabolic heat than skeletal muscle at rest
(3) Skeletal muscle can briefly exceed the basal metabolic rate by a factor of 10.
b. Increased metabolism related to work or physical exercise
c. Thermogenesis
(1) Accomplished by shivering and nonshivering means
(2) Nonshivering
(a) Limited physiologic response of newborn infant to hypothermia
(b) Involves catabolism of brown fat, which is not coupled with adenosine triphosphate formation
(c) Releases energy in the form of heat
(3) Shivering
(a) Can increase heat production by up to 500%
(b) Accompanied by increased:
(i) Metabolic rate
(ii) Oxygen demand
2. Mechanisms of heat loss
a. Processes controlling heat transfer (Figure 24-5)
(1) Radiation
(a) Loss of energy through radiant electromagnetic waves in the infrared spectrum
(b) Involves no direct contact between the objects involved
(i) Energy (or heat) radiates from warmer object to cooler one.
(ii) Uncovered skin in operative patient will radiate energy away from patient, reducing the body temperature.
(c) Accounts for 40% to 60% of all heat loss
(d) Accentuated in the elderly and neonates
(2) Convection
(a) Loss of body heat by means of transfer to surrounding cooler air
(b) Need a temperature gradient between the body and surrounding air
(c) Heat transfer may occur in two ways
(i) Passive movement
[a] Warm air rises.
[b] Loss of body heat as a result of basic skin exposure
(ii) Active movement
[a] Fan or wind blowing across the body surface
[b] Facilitated by laminar flow systems in operating room (OR)
(d) Accounts for 25% to 35% of heat lost and 10 kilocalories/hour (kcal/h)
(3) Conduction
(a) Transfer of heat energy through direct contact between objects
(b) Heat loss occurs with contact with any of the following:
(i) Cold OR table
(ii) Skin preparation solutions
(iii) Intravenous (IV) fluids
(iv) Irrigants
(v) Cold sheets and drapes
(c) Causes core body heat to move out to cooler periphery
(d) Accounts for up to 10% of heat loss
(i) With IV fluid infusion, 16 kcal/h loss
(ii) With blood infusion, 30 kcal/h loss
(4) Evaporation
(a) Transfer of heat that occurs when a liquid changed into a gas
(b) Routes of heat loss
(i) Perspiration (12-16 kcal/h)
(ii) Evaporation (12-16 kcal/h)
(iii) Exposed viscera during surgery or trauma
[a] The larger the wound, the greater the heat loss
[b] Can result in a 400 kcal/h loss
(c) May account for up to 25% of heat loss
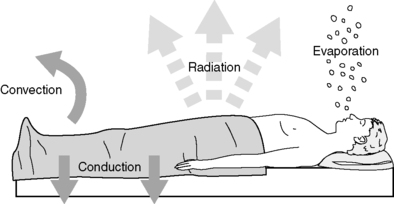 |
| FIGURE 24-5
Mechanisms of heat loss.
(From Sessler DI: Perioperative heat balance. Anesthesiology 92:583, 2000.)
|
b. Other routes of heat loss in perioperative setting
(1) Infusion of IV fluids that are cooler than body temperature
(a) A mass is added to the body that is cooler than current body temperature.
(b) Average body temperature falls.
(c) Fluid exits the body as urine or blood after being warmed to body temperature.
(d) Net loss of heat energy occurs.
(2) Ventilation with dry gas
(a) Gas is cooler than body temperature.
(b) Warmed, heated, and humidified in tracheobronchial tree
(c) Warmed and saturated with water vapor, the gas is exhaled at body temperature.
(d) Significant heat energy loss may occur over time.
D. Physiological responses to changes in environmental temperature
1. Cold environment
a. Physiological goal is to minimize heat loss while maximizing heat production.
b. Sympathetic stimulation
(1) Increases thickness of insulating shell through vasoconstriction
(2) Stimulates nonshivering thermogenesis
(3) Initiates piloerection
c. Shivering thermogenesis initiated
d. Long-term exposure also results in release of thyrotropin-releasing hormone from the hypothalamus.
2. Hot environment
a. Physiological goal is to maximize heat loss.
b. Vasodilation shrinks insulating shell.
c. Sudomotor response (stimulation of sweat glands)
(1) Regulates sensible evaporative heat loss
(2) Increases activity of cholinergic pathways
(3) Critical for cooling in an environment that is hotter than the body
(4) May also promote vasodilation
d. Decreased heat production
e. Long-term exposure
(1) Increase in sweating capacity of sweat glands
(2) Aldosterone-mediated increase in sodium retention
III. PERIOPERATIVE THERMOREGULATION
A. Unless actively warmed, patients receiving an anesthetic become hypothermic.
1. Usual temperature drop is 1° C to 3° C.
2. Temperature loss depends on:
a. Type and dose of anesthetic
b. Amount of surgical exposure
c. Ambient temperature
3. Normal physiological responses used to regulate the core temperature impaired by anesthetic agents
a. Patient tends to become poikilothermic.
b. Body takes on temperature of environment.
B. Redistribution hypothermia occurs.
1. Mechanisms of redistribution
a. General anesthesia reduces the vasoconstriction threshold.
(1) Threshold drops well below normal core temperature.
(2) Centrally mediated thermoregulatory constriction is inhibited.
b. General and regional anesthesia also cause peripheral vasodilation.
(1) Blood flow to skin increased
(2) Core heat lost through peripheral tissues
2. Both mechanisms result in a core-to-peripheral redistribution of body heat (Figure 24-6).
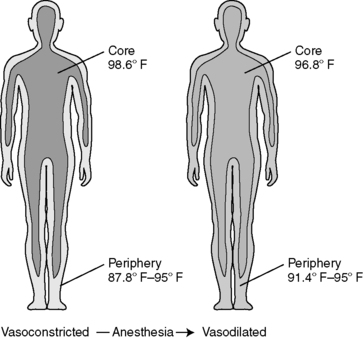 |
| FIGURE 24-6
Core-to-peripheral redistribution after the administration of anesthesia.
(From Sessler DI: Perioperative heat balance. Anesthesiology 92:581, 2000.)
|
C. Typical patterns of heat loss during a surgical case (Figure 24-7)
1. Core temperature drop of 1° C to 1.5° C occurs during first hour of surgery.
a. Caused by core-to-peripheral redistribution
b. Affected by other factors
(1) Initial body heat content
(2) Body morphology
(3) Amount of systemic heat loss
2. Initial heat loss followed by 2 to 3 hours of a slower, more linear drop
a. Metabolic rate drops 15% to 40% with administration of general anesthesia.
b. Heat loss exceeds metabolic heat production.
c. Heat loss mediated by the four fundamental mechanisms of heat loss (Figure 24-6)
(1) Radiation
(2) Convection
(3) Conduction
(4) Evaporation
3. Patient enters a plateau phase where the core temperature stabilizes.
a. Usually develops 2 to 4 hours into surgery
b. Characterized by a constant core temperature
c. May be passively or actively maintained
(1) Passive plateau
(a) Metabolic heat production equals heat loss without activating thermoregulatory defenses.
(b) Commonly seen in small operations in which patients are well covered with effective insulators
(2) Active plateau
(a) Patient becomes hypothermic enough to trigger thermoregulatory vasoconstriction.
(b) To occur with anesthetics, core temperature drops to 34° C to 35° C (93.2° F to 95° F)
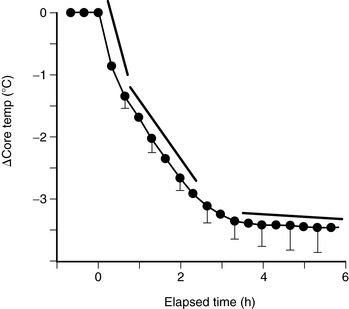 |
| FIGURE 24-7
Perioperative heat loss over time.
(From Sessler DI: Perioperative heat balance. Anesthesiology 92:580, 2000.)
|
D. Postoperative return to normothermia
1. Brain anesthetic concentration decreases to allow for triggering of normal thermoregulatory responses.
2. Can be impaired by:
a. Residual anesthetics
b. Postoperative opioids
3. May take 2 to 5 hours
4. Can be affected by:
a. Degree of hypothermia
b. Age of patient
IV. PLANNED PERIOPERATIVE HYPOTHERMIA
A. Hypothermia may be intentionally induced in some surgical cases to prevent intraoperative complications.
1. Cardiac ischemia
2. Cerebral ischemia
B. Most common cases include:
1. Cardiac surgery requiring cardiopulmonary bypass
a. Decreases amount of oxygen required by myocardial cells
b. Slows metabolic demands
2. Neurosurgical procedures
a. Decreases intracranial pressure
b. Decreases amount of bleeding
C. Intentionally induced hypothermia has been shown to be effective in:
1. Acute stroke
2. Perinatal asphyxia
3. Neurological outcome after cardiac arrest
4. Severe head injury
V. UNPLANNED PERIOPERATIVE HYPOTHERMIA (UPH)
A. Unexpected core temperature decrease to less than 36° C (96.8° F) as a result of surgery or other procedure.
B. May be present regardless of patient’s temperature if:
1. Patient complains of feeling cold.
2. Patient presents with common signs and symptoms.
a. Shivering
b. Peripheral vasoconstriction
c. Piloerection
C. Risk factors
1. Every patient undergoing surgery
2. Risk factors supported by weak evidence:
a. Age
b. Female gender
c. Systolic blood pressure
d. Level of spinal block
3. Risk factors supported by insufficient (mixed) evidence
a. Body Mass Index (BMI) below normal
b. Normal BMI
c. Procedure duration
d. Body surface/wound area uncovered
e. Anesthesia duration
f. History of diabetes with autonomic dysfunction
D. Negative effects associated with UPH
1. Patient discomfort related to:
a. Shivering
b. Unpleasant sensation of being cold
2. Adrenergic stimulation resulting in an increase in serum catecholamine levels
3. Untoward cardiac events
a. Increased catecholamines may cause myocardial ischemia.
b. Cardiac function directly impaired at temperatures less than 33° C (91.4° F)
c. Threshold for dysrhythmias around 31° C (87.8° F)
d. Ventricular fibrillation likely at 30° C (86° F)
4. Coagulopathy
a. Platelet function reduced
b. Clotting cascade slowed
c. Blood loss increased
5. Altered drug metabolism
a. Elimination of injectable drugs prolonged
b. Duration of anesthetic agents prolonged
6. Impaired wound healing/surgical site infection
a. Tissue oxygenation decreased
b. Immunity and collagen production impaired
c. Infection rates increased
(1) 19% in a hypothermic patient
(2) 6% in a normothermic patient
7. Increased hospital costs
a. Hypothermia of 1.5° C below normal results in a $2500 to $7000 increase.
b. Elevated costs related to:
(1) Increased length of stay in:
(a) Post anesthesia care unit (PACU)
(b) Intensive care unit (ICU)
(c) Hospital
(2) Increased use of:
(a) Red blood cells
(b) Plasma
(c) Platelets
(3) Increased need for mechanical ventilation
(4) Management of adverse cardiac events
E. Perioperative patient management
1. Preoperative (Figures 24-8 and 24-9)
a. Assessment
(1) Identify patient’s risk factors for UPH.
(2) Measure patient’s temperature.
(3) Determine patient’s thermal comfort level.
(4) Assess for other signs and symptoms of hypothermia.
(5) Document and communicate all risk factor assessment findings to all members of the anesthesia/surgical team
b. Interventions
(1) Implement passive thermal care measures.
(2) Maintain ambient room temperature at or above 24 º C (75 º F).
(3) Institute active warming for hypothermic patients.
(4) Consider preoperative warming to reduce the risk of intra/postoperative hypothermia.
(a) Minimum of 30 minutes of prewarming may reduce the risk of subsequent hypothermia.
c. Outcome
(1) Patient will express thermal comfort.
(2) Nonemergent patients will be normothermic before going to the OR/procedure area.
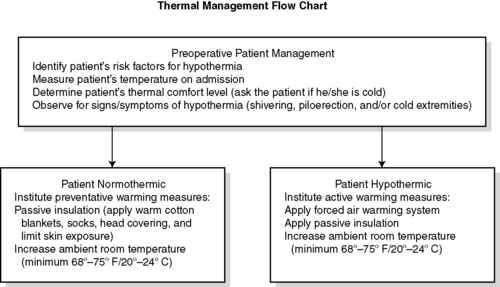 |
| FIGURE 24-8
Preoperative patient management.
(From American Society of PeriAnesthesia Nurses: Clinical guideline for the prevention of unplanned perioperative hypothermia. In 2008-2010 Standards of perianesthesia nursing practice, Cherry Hill, NJ, 2008, American Society of PeriAnesthesia Nurses, p 24.)
|
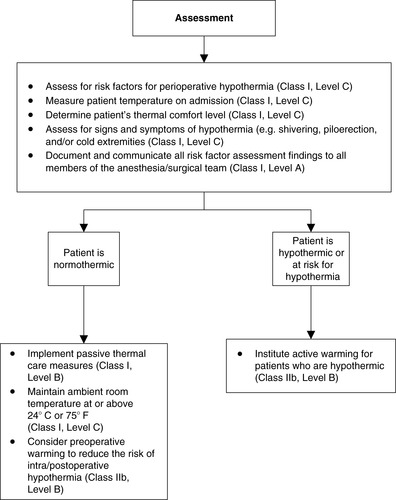 |
| FIGURE 24-9
Preadmission/Preoperative recommendations.
(From American Society of PeriAnesthesia Nurses: Preadmission/preoperative recommendations. Journal of PeriAnesthesia Nursing, in Press.)
|
2. Intraoperative (Figures 24-10 and 24-11)
a. Assessment
(1) Identify patient’s risk factors for UPH.
(2) Consider frequent temperature monitoring in all cases.
(3) Determine patient’s thermal comfort level.
(4) Assess for signs and symptoms of hypothermia.
(5) Document and communicate all risk factor assessment findings to all members of the anesthesia/surgical/nursing team.
b. Interventions
(1) All patients
(a) Passive warming measures
(b) Maintain ambient room temperature as per Association of periOperative Registered Nurses (AORN) and architectural recommendations.
(c) Limit skin exposure.
(2) Procedures longer than 30 minutes
(a) Forced air warming
(3) Alternative warming measures that may be used alone or in combination with forced air
(a) Warmed IV fluids
(b) Warmed irrigation fluids
(c) Circulating water garments
(d) Circulating water mattress
(e) Radiant heat
(f) Gel pad (Artic Sun©) surface warming
(g) Resistive heating
c. Patient will be normothermic on discharge from the OR/procedure area
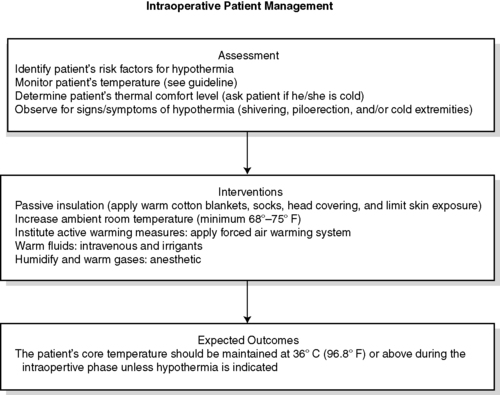 |
| FIGURE 24-10
Intraoperative patient management.
(From American Society of PeriAnesthesia Nurses: Clinical guideline for the prevention of unplanned perioperative hypothermia. In 2008-2010 Standards of perianesthesia nursing practice, Cherry Hill, NJ, 2008, American Society of PeriAnesthesia Nurses, p 24.)
|
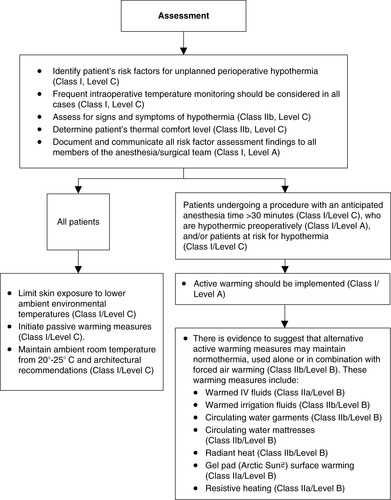 |
| FIGURE 24-11
Intraoperative recommendations.
(From American Society of PeriAnesthesia Nurses: Preadmission/preoperative recommendations. Journal of PeriAnesthesia Nursing, in Press.)
|
3. Postoperative patient management: phase I PACU (Figures 24-12 and 24-13)
a. Assessment
(1) Identify patient’s risk factors for UPH.
(2) Assess temperature on admission to phase I PACU.
(a) If hypothermic:
(i) Monitor serial temperatures at least every 15 minutes.
(ii) Monitor until normothermia reached.
(b) If normothermic, assess temperature:
(i) At least hourly
(ii) Before discharge
(iii) As ordered or indicated
(3) Determine patient’s thermal comfort level.
(4) Assess for signs and symptoms of hypothermia.
b. Interventions
(1) If normothermic:
(a) Institute thermal comfort measures.
(b) Maintain ambient room temperature at or above 24 º C (75 º F).
(c) Assess patient’s thermal comfort level.
(i) On admission
(ii) Discharge
(iii) As indicated
(d) Observe for signs and symptoms of hypothermia.
(e) Reassess temperature:
(i) If patient’s thermal comfort level decreases
(ii) If patient shows signs or symptoms of hypothermia
(f) Measure patient’s temperature before discharge.
(2) If hypothermic:
(a) Initiate active warming measures.
(b) Consider adjuvant measures.
(i) Warm IV fluids.
(ii) Humidify and warm oxygen.
(c) Assess every 15 minutes until normothermia reached.
(i) Temperature
(ii) Thermal comfort level
(3) Discharge teaching (Figure 24-14)
(a) Instruct patient and responsible adult in methods to maintain normothermia after discharge
(i) consumption of warm liquids
(ii) application of blankets, socks, and other warm clothing
(iii) increased room temperature
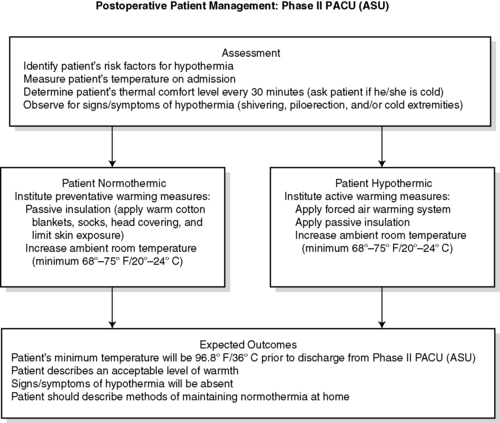 |
| FIGURE 24-14
Postoperative patient management: Phase II PACU.
(From American Society of PeriAnesthesia Nurses: Clinical guideline for the prevention of unplanned perioperative hypothermia. In 2008-2010 Standards of perianesthesia nursing practice, Cherry Hill, NJ, 2008, American Society of PeriAnesthesia Nurses, p 26.)
|
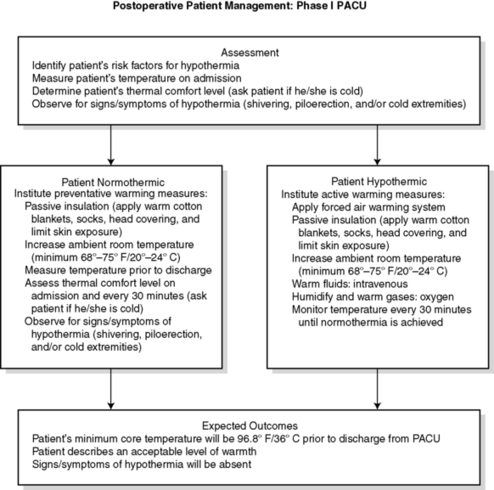 |
| FIGURE 24-12
Postoperative patient management: PACU.
(From American Society of PeriAnesthesia Nurses: Clinical guideline for the prevention of unplanned perioperative hypothermia. In 2008-2010 Standards of perianesthesia nursing practice, Cherry Hill, NJ, 2008, American Society of PeriAnesthesia Nurses, p 25.)
|
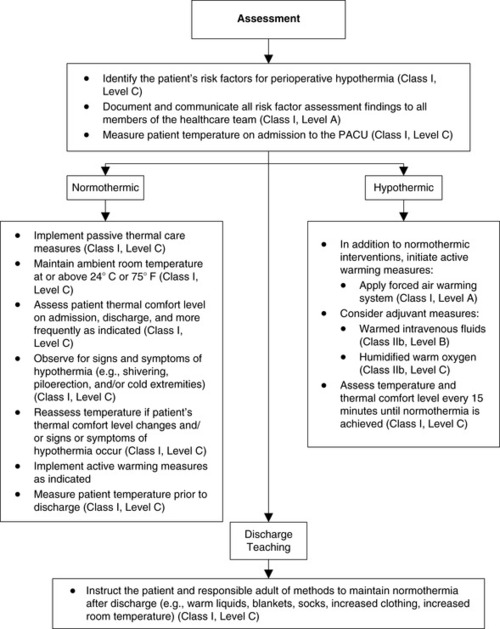 |
| FIGURE 24-13
Phase I/II PACU postoperative patient management recommendations.
(From American Society of PeriAnesthesia Nurses: Preadmission/preoperative recommendations. Journal of PeriAnesthesia Nursing, in Press.)
|
VI. MALIGNANT HYPERTHERMIA
A. Incidence of MH
1. More common in children
a. Children: 1:15,000 anesthetics administered
b. Adults: 1:20,000 to 1:50,000 anesthetics administered
2. Many cases undetected
a. Never anesthetized
b. Short anesthetic period
c. Effects of triggering agent may be modified by preceding with use of nontriggering agents.
(1) Thiobarbiturates
(2) Nondepolarizing muscle relaxants
(3) Hypothermia
d. Many cases mild, not diagnosed
B. Mortality significantly reduced since availability of dantrolene in late 1970s
1. Before 1970: 70%
2. 1976: 28%
3. Has remained 6% to 7% since the late 1980s
4. Most deaths still occur in otherwise healthy children and adults.
C. Triggering agents
1. Pharmacological
a. Succinylcholine
b. All volatile inhalation agents
(1) Halothane
(2) Enflurane
(3) Isoflurane
(4) Sevoflurane
(5) Desflurane
(6) Chloroform (trichloromethane, methyltrichloride)
(7) Trichloroethylene
(8) Xenon (rarely used)
D. Safe anesthetic agents (Table 24-1)
| MHS, Malignant hyperthermia syndrome. | ||
| Barbiturates/Intravenous Anesthetics | Opioids | Anxiety-Relieving Medications |
|---|---|---|
|
Diazepam
Etomidate (Amidate)
Hexobarbital
Ketamine (Ketalar)
Methohexital (Brevital)
Midazolam
Narcobarbital
Propofol (Diprivan)
Thiopental
|
Alfentanil (Alfenta)
Anileridine
Codeine (Methyl Morphine)
Diamorphine
Fentanyl (Sublimaze)
Hydromorphone (Dilaudid)
Meperidine (Demerol)
Methadone
Morphine
Naloxone
Oxycodone
Phenoperidine
Remifentanil
Sufentanil (Sufenta)
|
Ativan (Lorazepam)
Centrax
Dalmane (Flurazepam)
Halcion (Triazolam)
Klonopin
Librax
Librium (Chlordiazepoxide)
Versed (Midazolam)
Paxipam (Halazepam)
Restoril (Temazepam)
Serax (Oxazepam)
Tranxene (Clorazepate)
Valium (Diazepam)
|
| INHALED NONVOLATILE GENERAL ANESTHETIC | SAFE MUSCLE RELAXANTS | LOCAL ANESTHETICS |
| Nitrous oxide |
Arduan (Pipecuronium)
Curare (active ingredient is Tubocurarine)
Gallamine
Metocurine
Mivacron (Mivacurium)
Neuromax (Doxacurium)
Nimbex (Cisatracurium)
Norcuron (Vecuronium)
Pavulon (Pancuronium)
Tracrium (Atracurium)
Zemuron (Rocuronium)
|
Amethocaine
Articaine
Bupivacaine
Dibucaine
Etidocaine
Eucaine
Lidocaine (Xylocaine)
Levobupivacaine
Mepivacaine (Carbocaine)
Procaine (Novocain)
Prilocaine (Citanest)
Ropivacaine
Stovaine
|
E. Preoperative detection
1. History
a. Patient with previous MH episode
b. Fifty percent with MH have had previous anesthesia without a problem.
c. Family member with MH who has had crisis provides warning.
d. History of family member who died during surgery and anesthesia
2. Examination
a. Usually reveals nothing
b. Muscle weakness and myopathies associated with MH-like syndromes
(1) Duchenne muscular dystrophy
(2) Central core disease
(3) Myotonia
(4) Other unusual myopathies
3. Laboratory tests
a. Caffeine-halothane contracture test
(1) Most reliable test for preoperative diagnosis
(2) Few hospitals (about eight) in United States and Canada can perform this test.
(3) Requires muscle biopsy
(a) Must be performed at one of the testing hospitals
(b) Cannot be mailed to testing center
(4) Costly
b. Molecular genetic testing
(1) Detects 30% to 50% of patients at risk for MH
(2) Can be obtained with a simple blood sample
F. Signs and symptoms of MH
1. Increasing end-tidal carbon dioxide
2. Muscle rigidity
a. Masseter muscle spasm after administration of succinylcholine
b. Generalized trunk or total body rigidity
3. Tachycardia/tachypnea
4. Mixed respiratory and metabolic acidosis
5. Temperature elevation (often a late sign)
6. Myoglobinuria
G. Treatment (Box 24-1)
1. Immediate treatment
a. Discontinue anesthesia and surgery immediately.
b. Administer 100% oxygen.
c. Halt procedure as soon as possible.
2. Administer dantrolene (Dantrium) 2.5 mg/kg rapidly through a large-bore IV.
a. Dantrolene supplied in 20-mg vials
(1) Reconstitute with 60 mL of preservative-free sterile water.
(2) Shake vigorously.
(3) Warming bottle of solution may hasten mixing.
b. Side effects of dantrolene
(1) Difficulty in walking
(2) Fatigue
(3) Muscle weakness
(4) Dizziness
(5) Blurred vision
(6) Nausea
(7) Thrombophlebitis (late problem)
3. Bicarbonate for metabolic acidosis
4. Initiate patient cooling.
a. IV infusion of iced sodium chloride (NaCl)
b. Surface cooling for all patients
(1) Ice packs to groin, axillae, head
(2) Cooling blankets
(3) Immersion in container of ice
c. Lavage stomach, bladder, and rectum with cold saline
d. Lavage with cold saline if peritoneal cavity open
e. Extracorporeal cooling by heart-lung machine in exceptional cases
f. Discontinue cooling interventions when temperature decreases to 38° C (100.4° F).
g. Effective treatment in most situations includes:
(1) Treatment with dantrolene
(2) Discontinuation of anesthetic
(3) Lavage of:
(a) Stomach
(b) Bladder
(4) Cover exposed surfaces.
(a) Iced cold towels
(b) Cooling blankets
5. Maintain fluid and electrolyte balance.
a. Monitor arterial blood gases frequently.
b. To guide fluid therapy, monitor:
(1) Central venous pressure
(2) PA catheter
c. Use indwelling urinary catheter to monitor urine output.
d. Administer IV fluids as ordered.
e. Administer furosemide and mannitol as ordered.
f. For hyperkalemia, administer:
(1) Glucose (or dextrose)
(2) Insulin
(3) Calcium
6. Monitor cardiac output.
a. Maintain continuous cardiac monitoring.
b. Treat ventricular dysrhythmias.
(1) Procainamide
(2) Lidocaine
(3) Do NOT use calcium channel blockers.
BOX 24-1
SUGGESTED EQUIPMENT AND DRUGS TO BE USED IN TREATMENT OF ACUTE MALIGNANT HYPERTHERMIA
Equipment Needed
Intravenous lines with assorted cannula gauges
Central venous pressure sets (2)
Transducer kits for arterial and central venous cannulation
Esophageal or other core temperature probes
Pulmonary artery catheter
Laboratory test tubes for blood chemistry analysis
Syringes (60 mL x 5) to dilute dantrolene
Crystalloid solution (ten 1000-mL bottles), labeled for hyperthermia only and stored in PACU refrigerator
Bucket of cracked ice, labeled for hyperthermia only and stored in freezer of PACU refrigerator
Cooling blanket
Nasogastric tubes
Urine meter (1)
Irrigation tray with piston syringe
Fan
Large, clear plastic bags for ice
Drugs Needed
Sodium bicarbonate (8.4%): 50 mL x 5
Furosemide: 40 mg/amp x 4 ampules
Calcium chloride (10%): 10-mL vial x 2
Glucose (2 bottles of 50% strength)
Iced intravenous saline solution (ten 1000-mL bottles in refrigerator)
Lidocaine for injection: 100 mg/5 mL or 100 mg/10 mL in preloaded syringes (3)
Amiodarone is also acceptable (ACLS protocol for treatment of cardiac dysrhythmias)
Regular insulin (1 ampule of 100 units; refrigerated)
Dantrolene (Dantrium) intravenous: 36 vials of lyophilized powder with at least 2200 mL of sterile water for injection, USP (without a bacteriostatic agent), to reconstitute dantrolene
ACLS, Advanced cardiac life support; PACU, post anesthesia care unit; USP, United States Pharmacopeia.From Drain C, Odom-Forren J: Perianesthesia nursing: A critical care approach, ed 5, Philadelphia, 2009, Saunders.
H. Follow-up after initial treatment
1. Repeat IV or oral dantrolene every 4 to 6 hours for up to 48 hours.
2. Monitor for recurrence for 24 to 48 hours postoperatively in ICU.
3. Monitor for development of disseminated intravascular coagulation.
4. Follow serum creatine kinase levels for several days until normalized.
I. Miscellaneous issues
1. MH-susceptible ambulatory patients
a. May be discharged after 4 hours in PACU
b. Uneventful surgery
2. For inpatients
a. Label patients’ charts as “MH risk—do not use succinylcholine.”
b. MH patients who have required resuscitation have been given succinylcholine.
J. Preparing for an MH crisis
1. Maintain MH cart.
a. Drugs and fluids required to treat acute MH episode
b. May share with OR
2. Keep clear instructions with MH cart at all times.
3. Post MH treatment protocol in highly visible place.
4. Develop a detailed MH crisis response plan.
a. Specify the roles of each staff member.
b. Monitor and update education of all staff.
c. Provide updates at least annually.
d. Conduct mock MH crisis drills.
5. Have dantrolene immediately available.
a. At least 36 vials
b. Not in locked cabinet or stored in pharmacy
6. Have arterial blood gas laboratory immediately available.
7. Information sources
a. Malignant Hyperthermia Association of the United States (MHAUS)
(1) PO Box 1069, 1139 East State St., Sherburne, NY 13460-1069
(2) Phone
(a) 1-607-674-7901
(b) 1-800-98-MHAUS
(c) 1-800-MH-HYPER (MH hotline)
(3) www.mhaus.org
b. North American Malignant Hyperthermia Registry
(1) 1-888-274-7899
BIBLIOGRAPHY
1. AANA, Scope and standards for nurse anesthesia practice. ( 2007) ; Available at:www.aana.com/uploadedFiles/Resources/Practice_Documents/scope_stds_nap07_2007.pdf; Accessed September 11, 2008.
2. ASA, Standards for basic anesthetic monitoring. ( 2005) ; Available at:www.asahq.org/publicationsAndServices/standards/02.pdf; Accessed September 11, 2008.
3. ASPAN, Clinical guideline for the prevention of unplanned perioperative hypothermia. ( 2001) ; Available at:www.aspan.org/PDFfiles/HYPOTHERMIA_GUIDELINE10–02.pdf; Accessed March 5, 2007.
4. Dybwik, K.; Nielsen, E.W., Infrared temporal thermometry, Tidsskr Nor Laegeforen 6 (21) ( 2003) 3025–3026.
5. Girard, T.; Treves, S.; Voronkov, E.; et al., Molecular genetic testing for malignant hyperthermia susceptibility, Anesthesiology 100 (5) ( 2004) 1076–1080.
6. Good, K.K.; Verble, J.A.; Secrest, J.; et al., Postoperative hypothermia: The chilling consequences, AORN J 83 (5) ( 2006) 1054–1066.
7. Greenes, D.S.; Fleisher, G.R., Accuracy of a noninvasive temporal artery thermometer for use in infants, Arch Pediatr Adolesc Med 155 (3) ( 2001) 376–381.
8. Holtzclaw, B.J., Circadian rhythmicity and homeostatic stability in thermoregulation, Biol Res Nurs 2 (4) ( 2001) 221–235.
9. Hooper, V.D., In: Unplanned perioperative hypothermia: The state of the sciencePresented at the ASPAN 27th National Conference, Grapevine, Texas. ( May 6, 2008).
10. Hooper, V.D.; Andrews, J.O., Accuracy of noninvasive core temperature measurement in acutely ill adults: The state of the science, Biol Res Nurs 8 (1) ( 2006) 24–34.
11. Mahoney, C.; Odom, J., Maintaining intraoperative normothermia: A meta-analysis of outcomes with costs, AANA J 67 (2) ( 1999) 155–163.
12. Malignant Hypothermia Association of the United States, Available at:www.mhaus.org ( 2008); Accessed December 3,.
13. Moran, D.S.; Mendal, L., Core temperature measurement: Methods and current insights, Sports Med 32 (14) ( 2002) 879–885.
14. Naecsu, A., Malignant hyperthermia, Nurs Stand 20 (28) ( 2006) 51–57.
15. Nicoll, L.H., Heat in motion: Evaluating and managing temperature, Nursing 32 (2002) s1–s12.
16. Ostrowsky, B.; Ober, J.; Wenzel, R.; et al., The case of the cold thermometers, Am J Infect Control 31 (1) ( 2003) 57–59.
17. Rosenburg, H., Malignant hyperthermia syndrome. ( 2006) ; Available at:www.mhaus.org/NonFB/Slideshow_eng/SlideShow_ENG_files/frame.htm; Accessed March 15, 2007.
18. Rupp, M.E.; Heermann, J.; Uphoff, M.E., Need for a reliable system to measure body temperature, Am J Infect Control 32 (3) ( 2004) 184.
19. Sessler, D.I., Perioperative heat balance, Anesthesiology 92 (2) ( 2000) 578–596.
20. Sessler, D.I., Complications and treatment of mild hypothermia, Anesthesiology 95 (2) ( 2001) 531–543.
21. Suleman, M.I.; Doufas, A.G.; Akca, O.; et al., Insufficiency in a new temporal-artery thermometer for adult and pediatric patients, Anesth Analg 95 (1) ( 2002) 67–71.
22. Wagner, D.V., Unplanned perioperative hypothermia, AORN J 83 (2) ( 2006) 470; 473–476.
23. Welch, T.C., AANA journal course, AANA J 70 (3) ( 2002) 227–231.