History
The patient was a 76-year-old man with heart failure, left bundle branch block (LBBB), and paroxysmal atrial fibrillation.
Comments
The patient with symptomatic heart failure (New York Health Association [NYHA] III) was referred for cardiac resynchronization therapy defibrillator (CRT-D) implantation. He had a dilated cardiomyopathy with left ventricular disfunction (ejection fraction <35% documented by echocardiography) and prolonged QRS duration (>120 ms) with LBBB morphology. He was on optimal medical therapy for heart failure, including angiotensin-converting enzyme (ACE) inhibitors and beta blockers. In addition, the patient was on oral anticoagulation therapy with warfarin because of recurrent episodes of atrial fibrillation. A coronary angiography was performed and ruled out an ischemic cause of the cardiomyopathy.
On May 5, 2010, he was implanted with a CRT-D device; the left ventricular lead was positioned into the midlateral vein through the coronary sinus. The best left ventricular capture threshold was 1.5 V at 0.4 ms with left ventricular tip to left ventricular ring vector polarity. No phrenic nerve stimulation was observed, and no complications occurred during and soon after the procedure.
The CRT-D was programmed with left ventricular tip to left ventricular ring polarity, and an algorithm to measure the left ventricular threshold and adapt left ventricular output was activated (LV Capture Management, Medtronic, Minneapolis, Minn.).
A CareLink monitor (Medtronic) for remote monitoring was given to the patient because he lived alone, far from the clinic, and was not able to attend all of the scheduled follow-up visits.
Current Medications
The patient was taking warfarin, optimal medical therapy for heart failure.
Current Symptoms
Exacerbation of cardiac heart failure symptoms with worsening of NHYA class (II to III).
Electrocardiogram
The CareLink monitor transmission for July 3, 2010 was as follows:
• CareLink alert for burden atrial fibrillation (>6 hours) and for increased left ventricular pacing threshold
• Left ventricular pacing threshold 4.0 V at 0.4 ms (+1.75 V compared to that on June 25, 2010)
• Percent V pacing 99.1%
• Leadless electrogram (ECG) shows a changed axis compared to May 2012, 2010 recording
• Left ventricular lead is still pacing (but probably moved back a little)
Findings
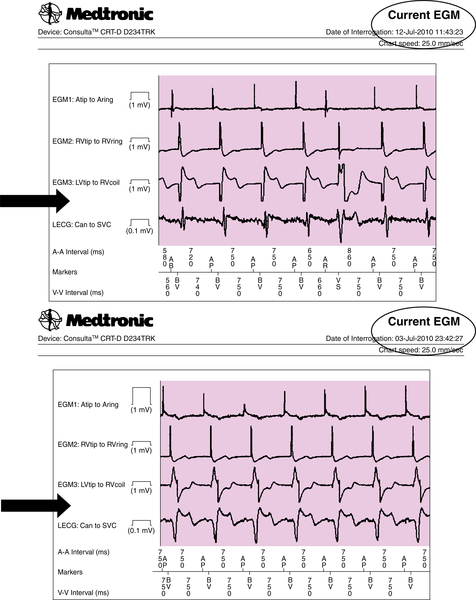
Two months after the CRT device implant (July 3, 2010), an automatic CareLink transmission was generated by two different alerts (burden atrial fibrillation longer than 6 hours and increased left ventricular pacing threshold [+1.75 V vs. that on June 25, 2010]) (Figures 24-1 and 24-2). The patient had a history of paroxysmal atrial fibrillation and was already on anticoagulant therapy, so the single atrial fibrillation event was not unexpected. On the leadless ECG transmitted (three electrograms plus high-resolution digital leadless ECG and Active Can-superior vena cava (SVC) Coil [Medtronic] corresponding to lead DI surface ECG), a variation of paced QRS was noted in contrast to the transmission on May 12, 2010 (Figure 24-3, black arrows). The patient was contacted by telephone. He was completely asymptomatic but unable to reach the medical center for clinical follow-up.
The CareLink monitor transmission for October 9, 2010 was as follows:
• CareLink alert for burden FA, OptiVol, and for high left ventricular pacing threshold on October 8, 2010
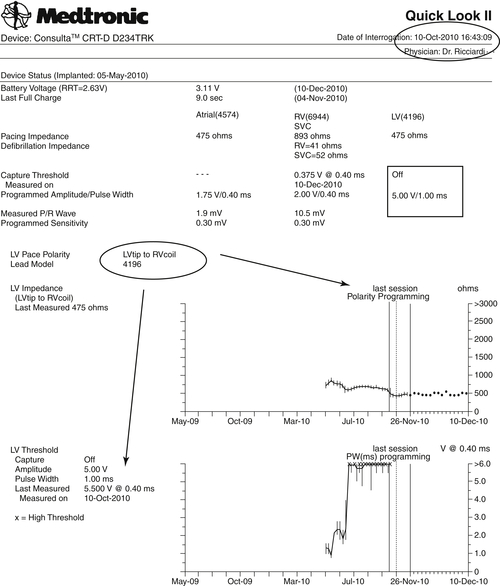
• Left ventricular pacing threshold 6.0 V at 0.4 ms, device unable to maintain an appropriate safety margin (more than 1 V of the pacing threshold)
• Three episodes of nonsustained ventricular tachycardia
• Leadless ECG shows same axis but larger than on July 7, 2010 (queried partial capture)
• Patient asked to go to the clinic the next day
Findings
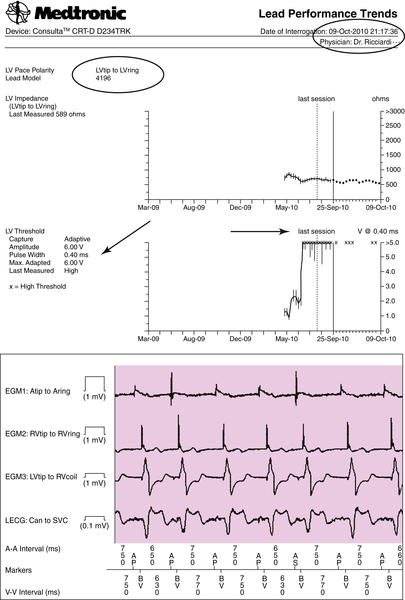
On October 9, 2010 an automatic CareLink transmission was generated by three alerts (burden AF, OptiVol, and high pacing threshold, with the Automatic Capture Management algorithm unable to mantain the programmed +1 V safety margin) (Figures 24-4 and 24-5). A further increase in left ventricular pacing threshold was observed associated with an increased QRS paced width, and the axis was unchanged in contrast to that from July 3, 2010; see Figure 24-5). The OptiVol alert suggested an initial accumulation of interstitial liquid, probably as a result of an intermittent capture of biventricular pacing.
The patient was contacted for a follow-up visit on the next day (October 10, 2010). Changing the polarity from tipLV/ringLV to tipLV/coilRV obtained a lower left ventricular pacing threshold (3.5 V at 1.0 ms), left ventricular pacing output was set to 5.0 V at 1.0 ms, programming off (monitoring only) the Automatic Capture Management function to ensure continuous biventricular pacing at higher output.
The CareLink monitor transmission for December 10, 2010 was as follows:
• Reprogrammed polarity from tipLV/ringLV to tipLV/coilRV (left ventricular pacing threshold 3.5 V at 1.0 ms) on October 10, 2010 patient ambulatory visit
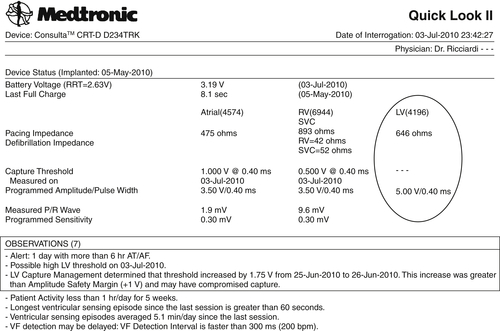
FIGURE 24-1
• Increased left ventricular pulse width from 0.4 ms to 1.0 ms
• Automatic Capture Management programmed off (monitoring only) to have continuous left ventricular pacing at 5.0 V at 1.0 ms output.
Findings
On December 10, 2010 (Figure 24-6), we received a programmed CareLink transmission with data indicating a satisfactory situation. The patient activity level was considerably increased, with only a few episodes of paroxysmal atrial fibrillation, as was usual for the patient.
On May 7, 2011, we received another programmed CareLink Transmission with a good clinical situation and a stable biventricular pacing (Figure 24-7). The patient did not experience any worsening heart failure episodes.
Focused Clinical Questions and Discussion Points
Question
The first CareLink automatic transmission (July 3, 2010; see Figures 24-1 to 24-3) reported an increased left ventricular pacing threshold. Should it be considered a “normal” consequence of the chronic phase of the lead maturation process?
Discussion
Elevated thresholds that occur during the first several weeks after implantation were common, although the introduction of steroid eluting electrodes and other electrode materials and designs has markedly decreased this problem.
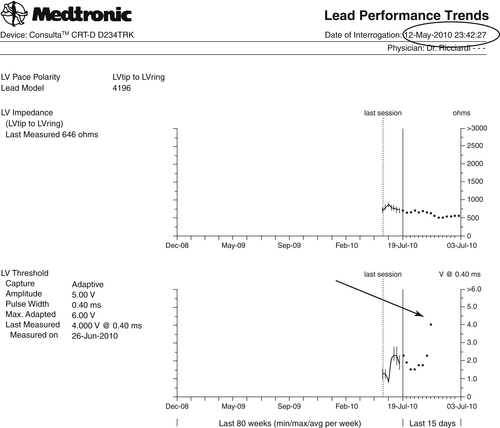
FIGURE 24-2
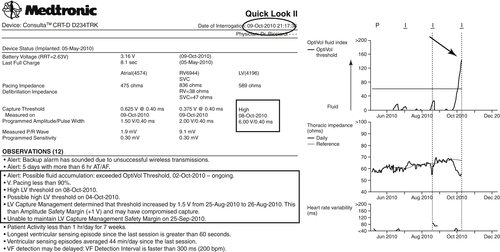
FIGURE 24-4
The rise in capture threshold usually has a peak between 2 and 6 weeks after implantation and may be attributed to an inflammatory reaction around the electrode. An elevation observed more than 6 weeks after implantation, as in this case, usually is considered to be in the chronic phase of the lead maturation process. It was also noted that biventricular pacing was still effective but with a different left ventricular activation and consequently a different QRS paced axis, probably as a result of a minimal left ventricular lead backdown. However, the CareLink System provided the opportunity to detect the increased pacing threshold and further monitor the evolution of the clinical situation without unscheduled follow-up visits.
Question
On October 9, 2010 a new automatic CareLink transmission was generated by three different alerts: burden atrial fibrillation, OptiVol, and high pacing threshold. Should the OptiVol alert be considered a consequence of atrial fibrillation and reduced biventricular pacing or of intermittent loss of left ventricular capture?
Discussion
The OptiVol alert suggested an initial accumulation of interstitial liquids and worsening of the heart failure condition, probably as a result of an intermittent loss of biventricular pacing.
The percentage of biventricular pacing was reduced because of an increased ventricular rate during atrial fibrillation. Moreover, likely there was not constant left ventricular capture because the Automatic Capture Management algorithm was unable to maintain the programmed safety margin for left ventricular output. Therefore the patient was contacted for a clinical follow-up visit.
Question
Was the rise in left ventricular pacing threshold the result of a gross dislodgment of the left ventricular lead or of chronic pacing threshold elevation?
Discussion
Changing the polarity from tipLV/ringLV to tipLV/coilRV restored left ventricular capture, ensuring continuous biventricular pacing at higher output. Thus the rise in the left ventricular pacing threshold until the loss of capture was not due to a gross dislodgment of the left ventricular lead.
Final Diagnosis
The final diagnosis for this patient was the necessity for an increased left ventricular pacing threshold, as identified by monitoring with the CareLink system.
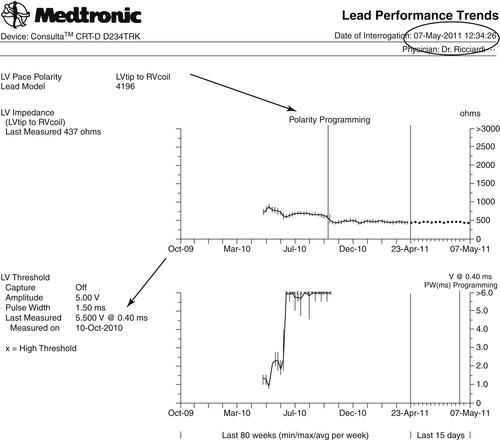
FIGURE 24-7
Plan of Action
The plan of action was monitoring of the increasing left ventricular pacing threshold using the CareLink system without unscheduled follow-up visits.
Intervention
Left ventricular output and pacing configuration were reprogrammed only when loss of capture had been detected by the monitoring system.
Outcome
The intervention resulted in restoration of effective biventricular pacing.