CHAPTER 22 Sleep Disorders
OVERVIEW
Sleep physiology and the diagnosis and treatment of sleep disorders are important to the practice of psychiatry. The brain regions and neurotransmitters that regulate sleep are similar to those that regulate mood and cognition, and the medications (e.g., stimulants or sedative-hypnotics) used to modulate these neurotransmitters are widely used by patients. Patients with disturbances of mood or anxiety, or with other psychiatric symptoms, commonly experience disturbed sleep; in addition, those with insomnia, sleep apnea, narcolepsy, and other sleep disorders often have psychiatric symptoms. Field trials have found that “insomnia due to another mental disorder” is the most common sleep disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria.1 Sleep loss may also precipitate affective dysregulation (e.g., mania).2,3 Insomnia may predict the development of depression4 and when present may worsen depression outcomes.5 Patients with depression have physiological sleep abnormalities. These include reduced amounts of deep (delta) sleep; disturbances in the overall continuity and maintenance of sleep; and alterations in the timing, amount, and composition of rapid eye movement (REM) sleep.6
THE HISTORY OF SLEEP RELATED TO PSYCHIATRY
Our modern understanding of the pathophysiology of sleep began in the early part of the twentieth century, with von Economo’s autopsy observation of damage to the junction between the rostral brainstem and the basal forebrain in patients with encephalitis lethargica. He suggested that these areas were necessary for the maintenance of wakefulness and sleep. Since then, our understanding of the wakefulness centers in the posterior hypothalamus, of the “sleep center” in the anterior hypothalamus (the ventrolateral preoptic area [VLPO]), and of their interdependence has advanced dramatically.7
In 1956 Burwell8 noted abnormalities of sleep during respiration in obese patients and in 1965 Gastaut and colleagues9 in France (and Jung and Kuhlo10 in Germany) outlined the syndrome of sleep apnea. Our understanding of the prevalence and significance of this important disorder continues to evolve. New disorders, such as REM sleep behavior disorder and nocturnal eating disorder, have been recognized within the past 15 years, underscoring the fact that sleep disorders is a relatively young and growing field of study.
Diagnostic classification systems are an important part of any medical specialty, especially one that is emerging. Psychiatrists, psychologists, and other mental health professionals have played key roles in the development of the diagnostic and classification systems used for sleep disorders. The first, Diagnostic Classification of Sleep and Arousal Disorders, was published in 1979, with Howard Rofwarg, a psychiatrist, as chair of its classification committee. The second edition of the International Classification of Sleep Disorders (ICSD) was published in 2005; Peter Hauri, a psychologist, chaired the committee to revise ICSD-1, and Michael Sateia, a psychiatrist, was the editor of ICSD-2.
SLEEP PHYSIOLOGY
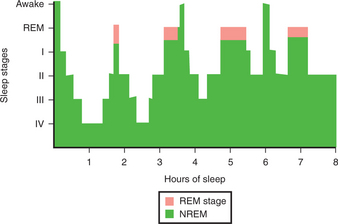
The all-night sleep EEG reveals an architecture or typical pattern of sleep that in normal humans involves an approximately 90-minute cycling between non–rapid eye movement (NREM) sleep and REM sleep (Figure 22-1). NREM sleep is divided into stages I through IV on the basis of the EEG, an electromyogram (EMG), and eye movements. Stage I sleep is the transition between wakefulness and sleep; normally individuals awaken frequently during stage I and often deny being asleep during this stage. Subtle respiratory instability occurring in stage I may interfere with sleep initiation. Stages III and IV are often termed delta sleep because of the presence of slow, high-voltage delta waves on the EEG. Delta sleep tends to predominate during the first part of the night, and diminishes with successive cycles of REM and NREM sleep through the night. It is associated with slow respiratory and heart rates, lowered blood pressure (BP) and muscle tone and temperature, growth hormone (GH) secretion, and increased arousal thresholds; it may be a time of deep rest and tissue restoration. In contrast, REM sleep is a time of physiological activation. The EEG during this stage resembles the waking EEG, which is why REM was once called “paradoxical sleep.” Pulse and BP are increased and variable during REM sleep. Muscle tone is suppressed by the activation of cholinergic cells in the brainstem, producing inhibition of spinal motor neurons, which prevents the sleeper from acting out REM-related mental activity.
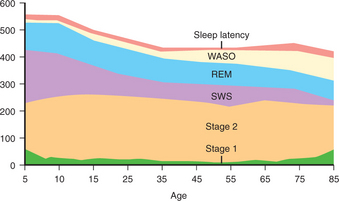
Sleep architecture changes throughout the normal life cycle (Figure 22-2).11 Normal elders have somewhat less delta sleep, slightly less REM sleep, and lower sleep efficiency (i.e., more nocturnal awakenings and arousals) than normal middle-aged people. The elderly also tend to go to sleep earlier and to wake early (a “lark” versus “night-owl” pattern), are more vulnerable to sleep disruption, and have a physiologically driven tendency toward voluntary and involuntary daytime sleep periods often felt to be compensatory for the nocturnal sleep changes.
The prevalence of sleep disorders of all types is increased in people over age 65 years. Complaints of insomnia (i.e., an inability to fall asleep or to stay asleep) are seen in up to 30% of the elderly, especially in women. Rates of restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) also are increased, as are rates of sleep-disordered breathing.12,13 While age-related changes in sleep architecture may be related to loss of cells in VLPO, it is important to recognize that aging per se does not produce unsatisfactory sleep. Clinicians should look carefully for the presence of primary sleep disorders and the sleep disturbances associated with medical conditions in older patients, and not dismiss their complaints as the consequence of “getting old.”
REM and NREM sleep cycle at roughly 90-minute intervals during a night’s sleep. Circadian rhythms are regulated by the suprachiasmatic nuclei (SCN), small (50,000 cells) areas in the anterior hypothalamus, dorsal to the optic chiasm and lateral to the third ventricle. The SCN generate their own rhythms that vary slightly from the 24-hour day based on a transcription-translation feedback system that involves a set of nine genes.14 The SCN also integrate photic and nonphotic stimuli to keep the wake-sleep cycle properly and flexibly entrained with the demands of our environment. Photic stimuli arrive at the SCN via the retinohypothalamic tract; light-sensitive renal cells include melanopsin neurons, as well as rod and cones. The SCN has outputs to the thalamus and hypothalamus, which in turn drive cycling throughout the brain. In this way the SCN are critical to the regulation of melatonin synthesis and corticosteroid secretion. Melatonin production is greatest at night, during the dark, and may be suppressed by exposure to light. Melatonin may be more associated with the absence of light than with sleep per se, as it may be elevated during the night even in nocturnal mammals. The primary function of melatonin appears to be the suppression of the alerting output of the SCN, but the role of melatonin in the regulation of sleep, and an understanding of the use of exogenous melatonin or melatonin receptor agonists as therapeutic agents, continues to evolve.
The control of sleep-wake function has historically been viewed as the result of two distinct processes: a homeostatic process and a circadian process.15,16 In this model homeostatic sleep drive accumulates across periods of wakefulness. The pressure to sleep produced by the homeostatic drive is directly proportional to duration of wakefulness (time since last sleep) and the duration of this prior sleep. Slow-wave activity has been thought to be a marker of the degree of homeostatic drive. Although the neurobiological substrate of sleep drive is not fully understood, adenosine, an inhibitory neurotransmitter in the CNS, probably plays a role in the accumulation of sleep drive. Circadian factors may offset or augment the homeostatic drive, depending on clock time. During the daylight hours, and most powerfully in the early morning, the SCN put out an alerting pulse that opposes the drive for sleep. This normally allows for waking in the morning and maintenance of wakefulness across the day. Lesions to the SCN may produce increased sleep time over the 24-hour period, as well as the inability to sustain prolonged periods of wakefulness. Timing mismatches between the homeostatic drive to sleep and the alerting pulse of the circadian processes may produce difficulty sleeping during the day for people who work at night, and are probably related to the pathogenesis of shift-work sleep disorder,17–19 as well as some of the sleep disturbances experienced by the blind.20,21
EXAMINATION OF SLEEP AND SLEEP-RELATED COMPLAINTS
Patient-Completed Rating Scales
A number of patient- and clinician-rated scales have been used to measure sleepiness and the symptoms of insomnia. One of the most popular of these is the Epworth Sleepiness Scale (ESS) (Table 22-1). While the ESS and other scales can help clinicians obtain a more detailed and accurate view of a patient’s sleep-related complaints and sleep habits, no scale by itself can establish or rule out any diagnosis.
| How likely are you to doze off or fall asleep in the following situations, in contrast to just feeling tired? This refers to your usual way of life in recent times. Even if you have not done some of these things recently, try to work out how they would have affected you. Use the following scale to choose the most appropriate number for each situation: |
| 1 = low chance of dozing |
| 2 = moderate chance of dozing |
| 3 = high chance of dozing |
| Situations | Score |
|---|---|
CLASSIFICATION OF SLEEP DISORDERS
An outline of both the DSM-IV and ICSD-2 classification of sleep disorders is provided in Tables 22-2 and 22-3. One of the differences in these systems is that the ICSD system moves away from classifying insomnia as primary or secondary. Clinicians who care for patients with sleep disorders may find the ICSD-2 useful as a reference, with its outline of current knowledge regarding the symptoms, pathophysiology, epidemiology, and work-up of various sleep disorders. The other main difference between the two classifications is that the DSM-IV tends to lump conditions whereas the ICSD-2 tends toward splitting them. This chapter uses material from both classification systems; it groups conditions into insomnia, hypersomnia and excessive sleepiness, parasomnias, and others.
Table 22-2 Outline of Sleep Disorders as classified by DSM-IV
NOS, Not otherwise specified.
Table 22-3 ICSD-2 Classification of Sleep Disorders
| I. INSOMNIA |
| Insomnia (acute insomnia) Psychophysiological insomnia Paradoxical insomnia Idiopathic insomnia Insomnia due to a mental disorder Inadequate sleep hygiene Behavioral insomnia of childhood Insomnia due to a drug or substance Insomnia due to a medical condition Insomnia not due to a substance or known physiological condition, unspecified (nonorganic insomnia NOS) Physiological (organic) insomnia, unspecified |
| II. SLEEP-RELATED BREATHING DISORDERS |
| Central sleep apnea syndromes Primary central sleep apnea Central sleep apnea due to high-altitude periodic breathing Central sleep apnea due to a medical condition (not Cheyne-Stokes) Central sleep apnea due to a drug or substance Primary sleep apnea of infancy (formerly primary sleep apnea of newborn) Obstructive sleep apnea syndromes Obstructive sleep apnea, adult Obstructive sleep apnea, pediatric |
| III. SLEEP-RELATED HYPOVENTILATION/HYPOXEMIC SYNDROMES |
| Sleep-related nonobstructive alveolar hypoventilation, idiopathic Congenital central alveolar hypoventilation syndrome Sleep-related hypoventilation/hypoxemia due to a medical condition Sleep-related hypoventilation/hypoxemia due to pulmonary parenchymal or vascular pathology Sleep-related hypoventilation/hypoxemia due to lower airway obstruction Sleep-related hypoventilation/hypoxemia due to neuromuscular and chest wall disorders Other sleep-related breathing disorder Sleep apnea/sleep-related breathing disorder, unspecified |
| IV. HYPERSOMNIAS OF CENTRAL ORIGIN NOT DUE TO A CIRCADIAN RHYTHM SLEEP DISORDER, SLEEP-RELATED BREATHING DISORDER, OR OTHER CAUSE OF DISTURBED NOCTURNAL SLEEP |
| Narcolepsy with cataplexy Narcolepsy without cataplexy Narcolepsy due to a medical condition Narcolepsy, unspecified Recurrent hypersomnia Kleine-Levin syndrome Menstrual-related hypersomnia Idiopathic hypersomnia with long sleep time Idiopathic hypersomnia without long sleep time Behaviorally induced insufficient sleep syndrome Hypersomnia due to a medical condition Hypersomnia due to a drug or substance Hypersomnia not due to a substance or known physiological condition (nonorganic hypersomnia NOS) Physiological (organic) hypersomnia, unspecified (organic hypersomnia NOS) |
| V. CIRCADIAN RHYTHM SLEEP DISORDERS |
| Circadian rhythm sleep disorder, delayed sleep phase type (delayed sleep phase disorder) Circadian rhythm sleep disorder, advanced sleep phase type (advanced sleep phase disorder) Circadian rhythm sleep disorder, irregular sleep-wake type (irregular sleep-wake rhythm) Circadian rhythm sleep disorder, free-running type (nonentrained type) Circadian rhythm sleep disorder, jet lag type (jet lag disorder) Circadian rhythm sleep disorder, shift-work type (shift work disorder) Circadian rhythm sleep disorder due to a medical condition Other circadian rhythm sleep disorder (circadian rhythm disorder NOS) Other circadian rhythm sleep disorder due to a drug or substance |
| VI. PARASOMNIAS |
| Disorders of arousal (from NREM sleep) Confusional arousals Sleepwalking Sleep terrors Parasomnias usually associated with REM sleep REM sleep behavior disorder (including parasomnia overlap disorder and status dissociatus) Recurrent isolated sleep paralysis Nightmare disorder Other parasomnias Sleep-related dissociative disorders Sleep-related groaning (catathrenia) Exploding head syndrome Sleep-related hallucinations Sleep-related eating disorder Parasomnia, unspecified Parasomnia due to a drug or substance Parasomnia due to a medical condition |
| VII. SLEEP-RELATED MOVEMENT DISORDERS |
| Restless legs syndrome Periodic limb movement disorder Sleep-related leg cramps Sleep-related bruxism Sleep-related rhythm movement disorder Sleep-related movement disorder, unspecified Sleep-related movement disorder due to a drug or substance Sleep-related movement disorder due to a medical condition |
| VIII. OTHER SLEEP DISORDERS |
| Other physiological (organic) sleep disorder Other sleep disorder not due to a substance or known physiological condition Environmental sleep disorder |
| IX. SLEEP DISORDERS ASSOCIATED WITH CONDITIONS CLASSIFIABLE ELSEWHERE |
| Fatal familial insomnia Fibromyalgia Sleep-related epilepsy Sleep-related headaches Sleep-related gastroesophageal reflux disease Sleep-related coronary artery ischemia Sleep-related abnormal swallowing, choking, and laryngospasm |
NOS, Not otherwise specified; NREM, non–rapid eye movement; REM, rapid eye movement.
INSOMNIA
Surveys find that 10% to 15% of the general population has chronic insomnia. The prevalence of insomnia is higher for patients seen in general medical and psychiatric settings, where approximately 25% to 35% of patients have at least transient insomnia.22 These figures may represent an underreporting of the problem.23
The pathophysiological basis of chronic insomnia remains incompletely defined. Polysomnography and other objective measures of sleep disruption often fail to correlate with the sleep complaints of those with complaints of insomnia.24 However, data from other sources suggest that patients with insomnia suffer excessive levels of arousal or the drive to wakefulness in sleep as well as during wakefulness. It may be this arousal that produces the perception of not sleeping. Evidence of excessive arousal has been found on analysis of EEG spectra,25 on positron emission tomography (PET) scans,26 and on markers of metabolic rate.27 Excess physiological arousal may have cognitive correlates and consequences for patients with insomnia, leading to a maladaptive and selective focus on internal and external markers of sleeplessness, and the development of avoidance.28
Until recently most insomnia was regarded as a symptom arising from an underlying, primary disorder.29 The goals of treatment were to diagnose and to manage the underlying disorder, which would then resolve the complaint of insomnia. Currently, insomnia is viewed as a significant clinical problem in its own right (i.e., a disorder). Even though approximately 90% of patients with insomnia have co-morbid problems (including psychiatric disorders [such as depression or anxiety] or medical disorders [often those producing pain or dyspnea]), the course of insomnia is often independent of the co-morbid problem, and treatment is targeted toward resolution of insomnia, along with treatment targeted at the co-morbid disorder. Indeed, the course of each disorder typically affects the other.
Insomnia is associated with significant health care costs. It has been estimated that $12 billion is spent each year in the United States for health care services, and $2 billion is spent on medications to treat insomnia.30,31 Insomnia also has a significant impact on quality of life (QOL); patients with insomnia have as much dysfunction as do patients with major depression or congestive heart failure (CHF).32,33 Finally, patients with insomnia may be at increased risk of sustaining a motor vehicle accident or some other kind of accident.34
A strong bidirectional relationship exists between insomnia and depression. Insomnia is one of the core symptoms of depression listed in DSM-IV, and patients with depression commonly experience one or more symptoms of insomnia. Depressed outpatients experience trouble falling asleep and staying asleep with approximately equal difficulty.1 Insomnia may be an early symptom or perhaps even a predictor of insomnia for some patients.35 Breslau and colleagues36 found that normal young adults with insomnia at baseline were four times more likely to develop depression than those without insomnia. Ford and Kamerow37 had similar findings, noting that while subjects with insomnia at baseline had high rates of depression 1 year later, subjects who had continued insomnia had the highest rates of depression, whereas those with resolved insomnia had lower rates of depression.
In addition to preceding or predicting depression, insomnia may have an impact on depression-related outcomes. This is why the relationship between insomnia and depression is regarded as bidirectional. Depressed patients with insomnia tend to have worse outcomes for depression, higher rates of recurrence of depression, and higher rates of suicide than those without insomnia.4,5,38 Insomnia may persist when the symptoms of depression remit or fully resolve39,40; the presence of insomnia despite treatment for depression may predict return of the depression.41
Insomnia (see Tables 22-2 and 22-3 for a classification) is typically associated with complaints of mood disturbance (e.g., irritability, dysphoria, and excess reactivity to stress); cognitive inefficiency (trouble concentrating on and completing tasks, trouble with complex or abstract thinking, or memory disturbance); and fatigue (since some patients with insomnia are activated, they report fatigue rather than excessive sleepiness).42
As noted in Table 22-3, ICSD-2 includes several subtypes of insomnia. Adjustment insomnia, or acute insomnia, was called short-term insomnia because it lasted a few days to a few weeks and resolved on its own (though it may recur); adjustment insomnia occurs in response to a known stressor.43 In contrast, idiopathic insomnia, also known as chronic or primary insomnia, often begins in childhood, without a known stressor, and continues throughout adulthood. Psychopathology is absent or limited, and this condition is not diagnosed when the insomnia is secondary to an Axis I or II mental disorder. Some data suggest that there is an association between this type of insomnia and childhood attention-deficit/hyperactivity disorder (ADHD), but this remains to be confirmed. Polysomnography may reveal reduced total sleep time with increased sleep latency and frequent awakenings. The percentage of delta sleep may be reduced. However, some patients may have normal or relatively normal PSGs, and there are no known biological markers of this disorder.44,45
Paradoxical insomnia, formerly known as sleep state misperception, or subjective complaint of sleep initiation and maintenance without objective findings, is diagnosed when patients complain of severe difficulty falling or staying asleep, despite normal or relatively normal polysomnography, MSLT, bed partner report, and relatively normal daytime performance. This is not an uncommon problem. These patients may be hypervigilant, and may worry excessively about the impact of perceived loss of sleep on their longevity. Many patients with insomnia overestimate their degree of actual sleep disturbance. The reasons for this and the etiology of formal paradoxical insomnia are incompletely understood.46,47
Psychophysiological insomnia, formerly known as learned or conditioned insomnia, is associated with excessive focus on, and anxiety about, falling asleep, which produces arousal, and becomes associated with sleep initiation. Patients report that they sleep well when on vacation or away from their bedroom, but become stirred up when they try to fall asleep in their usual sleep setting.48
Medications for Insomnia
A wide variety of medications are available for the management of insomnia. Over-the-counter (OTC) sleep aids, which usually contain a sedating antihistamine as the active component, are widely used by members of the general population.49 While many members of the public, and some clinicians, view OTC agents as safe and effective, some evidence available suggests that sedating antihistamines may not reduce sleep latency, increase sleep duration, or improve daytime function, in comparison to placebo or to other hypnotic agents.50 They may also produce delirium and disturbances of gait and memory, especially in the elderly. The use of these agents remains controversial.
Table 22-451 shows the relative estimated frequency of agents available by prescription for the treatment of insomnia. Trazodone appears to be the agent most frequently used, but the dose-related safety and efficacy of trazodone as a hypnotic has not been formally established.52 Walsh and associates52 showed that 50 mg of trazodone is as effective as 10 mg of zolpidem (compared to placebo) during the first, but not the second, week of use for a group of patients with insomnia. Trazodone may be considered in patients at risk for abuse of other hypnotics. Further study of trazodone for the treatment of insomnia is needed to establish recommendations about its use.
Table 22-4 Relative Frequency of Agents Available by Prescription for the Treatment of Insomnia
| Drug | Occurrences (Millions) |
|---|---|
| Trazodone | 2.730 |
| Zolpidem | 2.074 |
| Amitriptyline | 0.774 |
| Mirtazapine | 0.662 |
| Temazepam | 0.558 |
| Quetiapine | 0.459 |
| Zaleplon | 0.405 |
| Clonazepam | 0.394 |
| Hydroxyzine | 0.293 |
| Alprazolam | 0.287 |
| Lorazepam | 0.277 |
| Olanzapine | 0.216 |
| Flurazepam | 0.205 |
| Doxepin | 0.199 |
| Cyclobenzaprine | 0.195 |
| Diphenhydramine | 0.192 |
From Walsh JK: Drugs used to treat insomnia in 2002: regulatory-based rather than evidence-based medicine, Sleep 27:1441-1442, 2004.
Tricyclic antidepressants (TCAs) are also frequently used for insomnia. A limited amount of data suggest that nortriptyline, doxepin, low doses of mirtazapine, and other TCAs may improve sleep continuity, reduce sleep latency, increase total sleep time, and improve daytime function.53–55 Data on the optimal dosing for TCAs for insomnia are also limited. TCAs (e.g., doxepin and amitriptyline) are toxic in overdose and have potent anticholinergic and antihistaminic effects; they may produce delirium, as well as cause problems with gait and cognition, especially in the elderly. Therefore, TCAs should be used with caution in the treatment of insomnia.
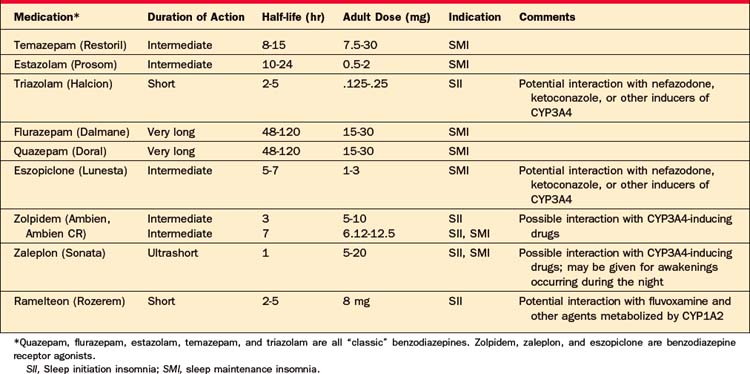
Table 22-5 lists the agents approved by the Food and Drug Administration (FDA) for the treatment of insomnia. Among these, the older agents (triazolam, quazepam, temazepam, and flurazepam) are benzodiazepines. The newer agents (zolpidem, eszopiclone, and zaleplon) work via their effect at the benzodiazepine receptor, and they are often called benzodiazepine receptor agonists (BZRAs). The newer agents may stimulate only a selected subset of benzodiazepine receptors (e.g., the benzodiazepine alpha1 receptor), whereas the older agents may be less selective at these sites.56 The clinical significance of the stimulation of a subset of receptors remains undetermined. The main difference between the two groups (older and newer) is that the newer agents have been shown to be safe and effective for long-term (i.e., 6 months) use in insomnia.57,58 The labeling of these agents does not specifically state that these agents are approved only for short-term use. Further, the BZRAs appear to have a very low potential for abuse and dependence, and like the typical benzodiazepines are labeled as Class IV agents by the FDA.59–61 Most important, all of the classic benzodiazepines (with the exception of triazolam) have long or relatively long elimination half-lives, and may thus produce daytime sedation, and in some cases daytime impairment. The newer medications for insomnia have shorter half-lives, and are essentially devoid of residual next-day effects (according to an NIH Consensus Conference Statement).29
Melatonin itself has been used as a treatment for insomnia, but it produces mixed results,62–64 and therefore it should not be recommended as a first-line treatment for insomnia at this time. Tryptophan and valerian root are so-called “natural” remedies for insomnia, and are available as dietary supplements. These agents also produce variable results, and their use has not been established as safe.
ADVERSE EFFECTS OF HYPNOTICS
Rebound Insomnia
Rebound insomnia is a worsening of sleep beyond the level of sleep disturbance seen previous to treatment. Rebound insomnia occurs with virtually all short- and intermediate-acting sleep agents and is highly dependent on dose.65 Long-acting medications do not produce rebound as their blood level gradually declines across several nights. The potential for rebound can be reduced when short-acting agents are tapered, and when they are used on alternative nights, rather than being abruptly discontinued.66 It is important to differentiate rebound from a return to pretreatment status.
Falls
Some studies suggest that the BZRAs may increase the risk of falls, especially in the elderly.67 However, recent work by Avidan and associates68 suggests that insomnia itself, rather than the drugs used to treat it, may elevate the risk of falls in the elderly. Given the morbidity rates, mortality rates, and economic cost of falls, more work on this subject is required.
Cognitive and Performance Disturbances
All BZRAs interfere with cognitive performance and impair motor performance when they are at their peak concentrations.69 Patients may be at risk for falls if they awaken and ambulate while these agents are at peak levels. Some of the older hypnotics with long elimination half-lives produce impaired cognitive performance the day following their use. There are reports of patients who cannot recall events in the hours or day following the use of BZRAs (especially the high-potency short-duration agents), even though their behavior may appear normal; there are also reports of patients who have periods of abnormal behavior (such as nocturnal binge eating) following the use of hypnotics, but the frequency of this problem has not been well established.
Addiction and Dependence
Controversy exists about the frequency with which BZRAs become drugs of abuse or produce dependence. Barbiturates are commonly linked with abuse, which is one of the reasons these agents are no longer recommended for treatment of insomnia. Several studies indicate that benzodiazepines and the newer non-BZRAs have a low propensity for abuse or dependence when prescribed by physicians for an appropriate indication, to a patient without a history of substance use or abuse.70 Caution is required when providing BZRA hypnotics. Careful monitoring of dose and refill patterns is important. These agents should rarely if ever be provided to patients with a history of substance abuse. However, since insomnia has a significant impact on QOL and on morbidity, it should be treated.
Insomnia may be produced by a wide range of medications and substances (Table 22-6) and medical problems (Table 22-7). Treatment aimed specifically at insomnia, used in conjunction with treatment for co-morbid medical problems, has been associated with significantly improved QOL, and may lead to better outcomes for both medical and psychiatric disorders.71
| Substance Type | Notes |
|---|---|
| Stimulants | Used as medications and as substances of abuse (including caffeine found in coffee, tea, chocolate, and cola) |
| Antihypertensives | Including alpha- and beta-blockers, calcium channel blockers, methyldopa, and reserpine |
| Asthma agents and bronchodilators | Including theophylline and albuterol |
| Corticosteroids | |
| Decongestants | Including pseudoephedrine, phenylpropanolamine, and phenylephrine |
| Antidepressants | Including fluoxetine, bupropion, venlafaxine, phenelzine, and parnate |
| Tobacco/nicotine | In cigarettes, cigars, and pipes |
| Alcohol | |
Table 22-7 Medical Problems That Produce Insomnia
| Medical Problem | Examples |
|---|---|
| Pain, acute or chronic | Arthritis, low back pain, cancer pain of any type, headache, fibromyalgia, burns, facial or dental pain, neuropathies |
| Cardiac/vascular disorders | Paroxysmal nocturnal dyspnea, peripheral vascular disease with cramps in extremities, congestive heart failure with orthopnea |
| Endocrine disorders | Menopause (with hot flashes and mood changes), hyperthyroidism or hypothyroidism (especially if associated with sleep apnea); diabetes (if associated with polyuria, nocturnal hypoglycemia or hyperglycemia and associated autonomic changes, neuropathic pain) |
| Gastrointestinal disorders | Gastroesophageal reflux disease of any cause, ulcers |
| Neurological disorders | Dementia of any type; strokes, Parkinson’s and other neuromuscular degenerative diseases |
| Pulmonary disorders | Chronic obstructive pulmonary disease, nocturnal asthma, sleep-related laryngospasm, and rhinitis/sinusitis |
| Urological disorders | Uremia, nocturia, and dysuria |
Behavioral Management
Pharmacological treatment can provide immediate relief of insomnia, but alone may be insufficient to change the habits, beliefs, and behaviors developed by patients, especially those with chronic insomnia. Behavioral approaches may address these issues and may provide effective, long-lasting relief from insomnia for many patients. Behavioral approaches can also provide relief for insomnia (Table 22-8).
| Approach | Explanation |
|---|---|
| Cognitive-behavioral therapy (CBT) | Can be used to identify and change maladaptive beliefs, behaviors, and affects around sleep. |
| Progressive muscle relaxation | Can help reduce some of the excess stimulation experience by some patients with insomnia. |
| Stimulus-control therapy | Can help break the association between bed and sleeplessness, and its associated frustrations that can develop in patients with insomnia. |
| Sleep-restriction therapy | Helps patients limit the time spent in bed to the time they actually sleep. |
| Attention to good sleep hygiene | Helps patients wind down, find a suitable sleep environment, get reasonable amounts of well-timed exercise, and avoid substances (such as caffeine) that may interfere with sleep. |
SLEEP APNEA
Sleep apnea is a syndrome of sleep-disordered breathing, associated with impaired daytime function, most commonly associated with excessive daytime sleepiness (EDS). Apneas may be obstructive, central, or mixed. An obstructive apnea is due to the complete cessation of airflow at the nose and mouth despite respiratory effort (related to collapse of the upper airway lasting for longer than 10 seconds associated with oxygen desaturation or arousal from sleep). An incomplete apneic episode, called hypopnea, is a reduction of airflow (usually greater than 30%) despite respiratory effort, associated with partial collapse of the airway, oxygen desaturation, and disruption of sleep continuity.72 Hypopneas are called partial obstruction when accompanied by a snore or snort. Most sleep laboratories will consider partial airflow reduction and a drop in O2 saturation of greater than 4% as hypopnea, whether or not signs of arousal are present. In some patients, hypopneas are often more frequent than are apneas.
Other symptoms of OSA include morning headaches and impotence. Some patients with OSA are depressed and become cognitively impaired, but the relationship among OSA, depression, and cognitive status is incompletely understood.73,74
EDS is a common consequence of OSA. However, some patients with severe OSA do not complain of EDS, and EDS may occur without OSA.75
Hypertension is an important correlate of OSA76 and may predispose patients to developing CHF. Levels of C-reactive protein, and other markers of inflammation, may be elevated in patients with OSA; this may contribute to the development, or lead to the worsening, of cardiovascular disease in patients with OSA.77
Obesity is another important correlate of OSA. An increase of 10% in body weight for patients who already suffer from mild OSA is associated with a sixfold increase in the development of moderate to severe OSA.78 This may be mediated by a critical increase in the soft tissues of the neck and throat that narrows the airway space and predisposes to airway collapse. Indeed, males with a neck diameter of 17 inches or above are at high risk for OSA.79 Finally, patients with OSA may have increased propensity for adult-onset diabetes mellitus (AODM), due to apnea-mediated insulin resistance, and leptin response. Insulin resistance worsens with increasing weight, but it may be moderated by CPAP.80,81
OSA is diagnosed by polysomnography. The PSG usually generates a measure called the Apnea-Hypopnea Index (AHI) (which is the average number of apneas and hypopneas observed per hour of sleep). An AHI of 5 to 15 is regarded as reflective of mild OSA, a score of 15 to 30 reflects moderate OSA, and a score greater than 30 indicates severe OSA.82,83 The AHI does not take into account the severity of oxygen desaturation, the presence of cardiac arrhythmias, the position and stage of sleep associated with apnea, the amount of EDS, or the degree of disruption of sleep architecture (arousals), so it cannot by itself define the severity of OSA.
| Weight loss helps but may not cure. Some patients sustain significant weight loss that may obviate the need for continuous positive airway pressure (CPAP). CPAP helps patients with hypersomnolence. Surgical approaches include uvuloplasty and related procedures, though these may lose their benefit over time. Stimulatory agents (e.g., modafinil) help those with EDS (even when the symptom persists after treatment with CPAP). Sedatives should be used with caution, as they may reduce arousal thresholds, thereby delaying arousal and lengthening apneic spells; this may worsen oxygen desaturation and the other negative consequences of apnea. Excessive alcohol is known to decrease upper airway muscle tone, so it may worsen both snoring and sleep apnea.84 |
NARCOLEPSY
The core feature of narcolepsy is an inability to maintain wakefulness during the day and to maintain sleep at night. The intrusion of sleep into wakefulness produces EDS and sleep attacks, the hallmark symptoms of narcolepsy. Left untreated, those with narcolepsy tend to fall asleep several times each day. The tendency to fall asleep may be highest during periods of quiet or boredom, but may occur even during activity (such as driving, walking, or working), when falling asleep is highly inappropriate, even life-threatening. The need to sleep may be irresistible. The sudden onset of irresistible sleep produces a sleep attack. Sleep attacks are usually brief (less than 1 hour), and patients typically awaken feeling refreshed. Their sleepiness, however, recurs within several hours, so planned napping may be helpful; however, by itself it is not a sufficient treatment.85
Narcoleptics tend to have a normal amount of sleep time during each 24-hour day.86 Improvement of their disturbed nocturnal sleep may reduce, but not completely resolve, excess daytime sleepiness.87
Narcolepsy occurs with cataplexy (a sudden, brief decrease in or loss of muscle tone triggered by emotion) in approximately 50% to 80% of narcoleptics. Patients experience the change and may try to resist. Tone may be lost in selected muscles (producing, for example, only the droop of an eyelid or jaw), or it may be widely distributed (producing collapse). The diaphragm is unaffected, so patients continue to breathe during even a severe cataplectic attack. Muscle tone begins to return after a few seconds to a few minutes, and recovery is complete and rapid. Prolonged cataplexy and status cataplecticus may occur, if agents (such as antidepressants) used to manage cataplexy are suddenly withdrawn, but this is uncommon. If an episode of cataplexy is prolonged, a REM-onset sleep episode may arise. The frequency of cataplexy is variable, and may decrease with age.88 Cataplexy may occur with, or several years after, the onset of the other symptoms of narcolepsy. The emotional triggers of cataplectic attacks often involve “negative” emotions (such as anger or sadness), but attacks may also be triggered by humor and other “positive” emotions.89
Narcoleptics have a tendency toward elevated body mass index (BMI), and many become formally obese.90 Disorders that are related to obesity, such as sleep apnea and diabetes, thus appear with a higher frequency in narcoleptics than in the general population.
An understanding of the neurophysiological basis for the difficulty of maintaining wakefulness and sleepiness in narcolepsy has begun to emerge over the past several years.91 Patients who have narcolepsy with cataplexy have been shown to have low or undetectable levels of orexin, also called hypocretin, in their cerebrospinal fluid (CSF). Orexin is a neuropeptide produced by cells in the posterior hypothalamus, and the reduced level of orexin is believed to be correlated with loss of the cells in the posterior and lateral hypothalamus that produce it.92
The reason why orexin-producing cells are lost in patients with narcolepsy plus cataplexy remains unknown; genetic factors may play a role. There is a twentyfold to fortyfold increase in the rate of narcolepsy with cataplexy in the family members of affected patients,93 but only 25% of monozygotic twins are affected. Many but not all Caucasian and Japanese narcoleptics test positive for the HLA marker DQB1*0602, but genetic testing is of uncertain value because 99% of people with DQB1*0602 do not have narcolepsy. Thus, the role of genetic factors remains incompletely understood. Autoimmune factors, producing damage to the orexin cells, have also been suspected. Further, factors beyond orexin loss alone may be involved, as patients with narcolepsy without cataplexy may have reduced or normal levels of CSF orexin. Most cases of narcolepsy without cataplexy have normal CSF Hcrt-1 levels, and there is no association with HLA-DQB1*0602.
TREATMENT
Excessive Daytime Sleepiness
Gamma hydroxybutyrate (GHB) (sodium oxybate) is a metabolite of GABA that occurs naturally in the brain. The mechanism of action is unknown, but it may improve the disturbed nocturnal sleep and EDS for patients with narcolepsy.
Cataplexy
Idiopathic hypersomnia may overlap with narcolepsy without cataplexy. Patients with this condition may routinely sleep more than 10 hours per night, but remain excessively sleepy. They may experience extreme difficulty waking up with external stimuli, such as an alarm, and have periods of “sleep drunkenness” on arousal. They may have long, nonrestorative daytime naps. MSLT confirms objective sleepiness, but afflicted patients do not have two or more SOREMs. Patients often have symptoms associated with dysfunction of the autonomic nervous system (such as orthostatic hypotension with syncope, and peripheral vascular dysfunction producing cold hands and feet). The etiology of this disorder is unknown; the response to stimulants and wake-promoting agents is often disappointing.94
Secondary narcolepsy, usually due to focal lesions of the posterior hypothalamus, has been reported, but such cases are unusual.85
INSUFFICIENT SLEEP
The most common cause of EDS in the United States is the failure to get enough sleep.95 Many people in our society use an alarm to awaken in the morning, rather than waiting for the operation of the natural arousal mechanisms that would awaken them when their sleep has reached a sufficient duration. Such people often feel sleepy during the day, frequently use stimulants such as caffeine, and try to “catch up on sleep” during weekends or breaks. A careful sleep history, including the use of a sleep log as needed, and showing that daytime sleepiness remits when sleep duration is lengthened, can help prevent confusion of insufficient sleep with the other causes of excess sleepiness. Sustained lifestyle change, permitting sufficient sleep, is difficult or sometimes impossible for patients, but the use of stimulants or wake-promoting agents for people with insufficient sleep remains controversial.
RESTLESS LEGS SYNDROME
RLS appears in approximately 10% of the general population and in up to 24% of primary care patients; women are more commonly affected than are men. Primary, or idiopathic, RLS is often familial.96 Secondary RLS appears when medical, neurological, or metabolic conditions produce iron deficiency. While the complex role of iron is incompletely understood, it probably is advisable to measure serum ferritin levels97 and to obtain a complete blood count (CBC) looking for the signs of iron deficiency anemia in patients with RLS, especially if the patients have renal disease or are pregnant.
RLS is often co-morbid with PLMS, which may contribute to further sleep disruption and to EDS.98 Patients with RLS have an increased incidence of depression,99 and many antidepressant medications, in particular selective serotonin reuptake inhibitors (SSRIs), can produce RLS and PLMS. Table 22-10 shows the diagnostic criteria of RLS.100
| Criteria Type | Description |
|---|---|
| Essential criteria | Urge to move the legs, usually accompanied by uncomfortable or unpleasant sensations in legs. Arms and other body parts may be involved. Cognitively impaired elderly may rub legs, pace, fidget, kick, tap, or toss and turn in bed. |
| Urge to move or unpleasant sensation begins or worsens during rest or inactivity. | |
| Urge to move or unpleasant sensation is relieved by movement while movement continues. | |
| Urge to move or unpleasant sensation is worse in the evening or at night than during the day, or only during evening or at night. In very severe cases, worsening at night may not be noticeable but must have been previously present. | |
| Supportive clinical features | Positive family history |
| Response to dopaminergic treatment | |
| PLMS (during wakefulness or sleep) | |
| Associated features | Natural clinical course |
| Sleep disturbance | |
| Generally normal medical and physical examination |
PLMS, Periodic limb movements of sleep.
From Allen RP, Picchietti D, Hening WA, et al: Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health, Sleep Med 4:101-119, 2003.100
Treatment
Iron replacement can help when RLS is secondary to iron deficiency. Avoidance of alcohol (particularly wine) and caffeine and the performance of moderate exercise can also help. When RLS is caused by antidepressants or by the taper of opiates, switching to another antidepressant or revising a tapering schedule of opiates can be useful. Acupuncture may help some patients.101
When medication is required, long-acting dopamine agonists are the first choice. Pramipexole, starting at 0.125 mg orally (PO) every day at bedtime (qHS), moving up to 1.5 mg/day in divided doses, can be effective.102 Ropinirole, starting at 0.25 mg PO qHS and moving up to 4 mg/day in divided doses, is an effective alternative.103 A risk of sudden sleep attacks has been reported with patients taking pramipexole or ropinirole, so patients should be cautioned about driving. Gabapentin can be effective with doses starting at 300 mg PO qHS; dosing up to 2,400 mg PO qHS may be effective and tolerable for some patients. Clonazepam, starting at 0.25 mg PO qHS and moving up to 2 mg PO qHS, can help.104
SHIFT-WORK SLEEP DISORDER
Shift-work sleep disorder (SWSD) occurs when the circadian rhythms that regulate sleep-wake behavior are misaligned by night-shift work to the degree that patients experience clinically significant excessive sleepiness during night work or insomnia when trying to sleep the following day.105 While the majority of chronic night-shift workers complain of some initial sleep difficulty, many are able to compensate for this temporal sleep-wake dysregulation; however, between 5% and 10% of night-shift workers (nearly 6 million Americans) cannot adapt their natural circadian rhythms and their drive for sleep and, as a result, experience levels of excessive sleepiness and daytime insomnia that meet the criteria for diagnosis of SWSD.106 While night-shift workers have traditionally been shown to be at increased risk for a variety of negative health-related and social outcomes, the burden of illness associate with SWSD is substantial, with profound impact on a patient’s health (increased rate of ulcers and depression), job (impaired work performance and absenteeism), and safety (increased risk for accidents on the job and during commute).106–108 Additionally, the excessive sleepiness related to SWSD is associated with impaired social relationships, marital disharmony, and irritability.105 The treatment of SWSD entails both the management of daytime sleep and nighttime alertness. Clearly a first step is to maximize nocturnal sleep with behavioral and pharmacological approaches outlined in the section on insomnia management. In terms of the sleepiness during work and especially during the commute home, many workers self-medicate with high doses of caffeine. Recently, modafinil was shown to enhance alertness on the job, as well as to decrease accidents on the commute home.109 An alternative approach to SWSD is to move the circadian rhythms closer to that of the work schedule. The appropriate exposure to light and the use of melatonin have been shown to be effective in moving the clock. However, the intrusion of light during the day limits the utility of these approaches.
PARASOMNIAS
Nightmare Disorder, or Dream Anxiety Disorder
Between 50% and 85% of the population has occasional nightmares; 2% to 8% of the adult population has recurrent nightmares. Nightmares are common after traumatic events; nightmares continuing for more than 4 weeks after the trauma may predict the appearance of posttraumatic stress disorder (PTSD). Nightmares usually start in children ages 3 to 6 years, and are most frequent in children ages 6 to 10 years. While nightmares usually abate with adolescence, they may persist in some patients into adulthood. Nightmares may appear in adults or children following trauma.
1 Buysse DJ, Reynolds DCIII, Hauri PJ, et al. Diagnostic concordance for DSM-IV sleep disorders: a report from the APA/NIMH DSV-IV field trial. Am J Psychiatry. 1994;151:1351-1360.
2 Wehr TA. Sleep loss: a preventable cause of mania and other excited states. J Clin Psychiatry. 1989;50(suppl):8-16.
3 Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord. 2003;74:209-217.
4 Perlis ML, Giles DE, Buysse DJ, et al. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42:209-212.
5 Moos RH, Cronkite RC. Symptom-based predictors of a 10-year chronic course of treated depression. J Nerv Ment Dis. 1999;187:360-368.
6 Benca RM, Obermeyer WH, Thisted RA, et al. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry. 1992;49:651-668.
7 Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2004;437:1257-1263.
8 Burwell T. Pickwickian syndrome, obesity and hypoventilation. Am J Med. 1965;21:811-818.
9 Gastaut H, Tassinari C, Duron B. Etude polygraphique des manifestations episodiques (hypniques et respiratoires) du syndrome de Pickwick. Rev Neurol. 1965;112:568-579.
10 Jung R, Kuhlo W. Neurophysiological studies of abnormal night sleep and the pickwickan syndrome. Prog Brain Res. 1965;18:140-159.
11 Ohayon M, Carskadon MA, Guilleminault C, et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255-1273.
12 Redline S, Kirchner HL, Quan SF, et al. The effects of age, sex, ethnicity and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406-418.
13 Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med. 2002;162(8):893-900.
14 King DP, Taksahashi JS. Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci. 2000;23:713-743.
15 Achermann P, Borbely AA. Combining different models of sleep regulation. J Sleep Res. 1992;1:144-147.
16 Borbely AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14:557-568.
17 Cziesler CA, Johnson PI, Duffy JF, et al. Exposure to bright light and darkness to treat physiologic maladaptation to night work. N Engl J Med. 1990;322:1253-1259.
18 Akerstedt T. Shift work and disturbed sleep/wakefulness. Occup Med. 2003;53:89-94.
19 Spitzer RL, Terman M, Williams IBW, et al. Jet lag: clinical features, validations of a new syndrome-specific scale, and lack of response to melatonin in a randomized double-blind trial. Am J Psychiatry. 1999;156:1392-1396.
20 Sack R, Lewy A, Blood M, et al. Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab. 1992;75:127-134.
21 Tabandeh H, Lockley SW, Buttery R, et al. Disturbances of sleep in blindness. Am J Opthalmol. 1998;126:707-712.
22 Roth T. New developments for treating sleep disorders. J Clin Psychiatry. 2001;62(suppl 10):3-4.
23 Benca RM. Diagnosis and treatment of chronic insomnia: a review. Psych Services. 2005;56:332-343.
24 Erman MK. Sleep architecture and its relationship to insomnia. J Clin Psychiatry. 2001;62(suppl 10):9-17.
25 Krystal AD, Edinger JD, Wohlgemuth WK, et al. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630-640.
26 Nofzinger EA, Buysse DJ, Germain A, et al. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126-2128.
27 Bonnet MH, Arand DL. Insomnia, metabolic rate, and sleep restoration. J Intern Med. 2003;254:23-31.
28 Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869-893.
29 Consensus conference on insomnia. Available at http://consensus.nih.gov.
30 Walsh JK, Englehart CL. The direct economic costs of insomnia in the United States in 1995. Sleep. 1999;22(suppl 2):S386-S393.
31 Simon GE, Von Korff M. Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry. 1997;154:1417-1428.
32 Zammit GK, Weiner J, Damato N, et al. Quality of life in people with insomnia. Sleep. 1999;22(suppl 2):S354-S358.
33 Katz DA, McHorney CA. The relationship between insomnia and health related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229-235.
34 Powell NB, Schechtman KB, Riely RW, et al. Sleepy driving: accidents and injury. Otolaryngol Head Neck Surg. 2002;126:217-227.
35 Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255-259.
36 Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411-418.
37 Ford ED, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA. 1989;262:1479-1484.
38 Agargun MY, Kara H, Solmaz M. Subjective sleep quality and suicidality in patients with major depression. J Psychiatr Res. 1997;31:377-381.
39 Fava GA, Fabbri S, Sonino N. Residual symptoms in depression: an emerging therapeutic target. Progr Neuropsychopharmacol Biol Psychiatry. 2002;26:1019-1027.
40 Nierenberg AA, Keefe BR, Leslie VC, et al. Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry. 1999;60:221-225.
41 Reynolds CFIII, Frank E, Houck FE, et al. Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am J Psychiatry. 1997;154:958-962.
42 Moul DE, Nofzinger, Pilkonis PA, et al. Symptom reports in severe chronic insomnia. Sleep. 2002;25:553-563.
43 Morin CM, Rodrique S, Ivers H. Role of stress, arousal and coping skills in primary insomnia. Psychosom Med. 2003;65:259-267.
44 Bastien CH, Morin CM. Familial incidence of insomnia. J Sleep Res. 2000;9:49-54.
45 Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97-111.
46 Krystal A, Edinger JD, Wholgemuth WE, et al. Non-REM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002;25:630-640.
47 Salin-Pascual RJ, Roehrs TA, Merlotti LA, et al. Long-term study of the sleep of insomnia patients with sleep state mispercep-tion and other insomnia patients. Am J Psychiatry. 1992;149:904-908.
48 Ohayon MM, Roberts RE. Comparability of sleep disorders diagnoses using DSM IV and ICSD classifications with adolescents. Sleep. 2001;24:920-925.
49 Basu R, Dodge H, Stoehr GP, et al. Sedative-hypnotic use of diphenhydramine in a rural, older adult, community-based cohort: effects on cognition. Am J Geriatr Psychiatry. 2003;11:205-213.
50 Richardson GS, Roehrs TA, Rosenthal L, et al. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol. 2002;22:511-515.
51 Walsh JK. Drugs used to treat insomnia in 2002: regulatory-based rather than evidence-based medicine. Sleep. 2004;27:1441-1442.
52 Walsh JK, Erman M, Erwin CW, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol. 1998;13:191-198.
53 Hajak G, Rodenhbeck A, Vodernholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled double blind polysomnographic study. J Clin Psychiatry. 2001;62:453-463.
54 Caspar RC, Katz MM, Bodwen CL, et al. The pattern of psychical symptom changes in major depression disorder following treatment with amitriptyline or imipramine. J Affect Disord. 1994;31:151-165.
55 Winokur A, DeMartinis NAIII, McNally DOP, et al. Comparative effectives of mirtazapine and fluoxetine on sleep physiology measures in patients with major depression and insomnia. J Clin Psychiatry. 2003;64:1224-1229.
56 Bateson AN. The benzodiazepine site of the GABA a receptor: an old target with new potential? Sleep Med. 2004;1(5 suppl):S9-S15.
57 Krystal AD, Walsh JK, Laska E, et al. Sustained efficacy of eszopliclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study of adults with chronic insomnia. Sleep. 2003;26:793-799.
58 Perlis ML, McCall WV, Krystal AD, et al. Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry. 2004;65:1128-1137.
59 Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use, and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-14.
60 Schenck CH, Mahowald MW. Long term nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996;100:333-337.
61 Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States. Sleep. 1999;22(suppl 2):S354-S358.
62 Sack RL, Hughes RJ, Edgar DM, et al. Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20:908-915.
63 Baskett JJ, Broad JB, Wood PC, et al. Does melatonin improve sleep in older people? Age Aging. 2003;32:164-170.
64 Mendelson WB. A critical evaluation of the hypnotic efficacy of melatonin. Sleep. 1997;20:916-919.
65 Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J Clin Psychiatry. 2005;66(suppl 9):10-13.
66 Roehrs T, Merlotti L, Zorick F, et al. Rebound insomnia in normals and in patients with insomnia after abrupt and tapered discontinuation. Psychopharmacology. 1992;108:67-71.
67 Allain H, Bentue-Ferrer D, Pollard E, et al. Postural instability and consequent falls and hip fractures associated with use of hypnotics in the elderly. Drugs Aging. 2005;22(9):749-765.
68 Avidan YY, Fries BE, James ML, et al. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005;53:955-962.
69 Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8:45-58.
70 Mendelson WB, Roth T, Cassella J, et al. The treatment of chronic insomnia: drug indications, chronic use and abuse liability. Summary of a 2001 new clinical drug evaluation unit meeting symposium. Sleep Med Rev. 2004;8:7-17.
71 Thase ME. Correlates and consequences of chronic insomnia. Gen Hosp Psychiatry. 2005;27:100-112.
72 Strollo PJJr, Rogers RM. Obstructive sleep apnea. N Engl J Med. 1996;334:99-104.
73 Sateia MJ. Neuropsychological impairment and quality of life in obstructive sleep apnea. Clin Chest Med. 2003;24:249-259.
74 Engleman HM, Douglas NJ. Sleep: sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnea syndrome. Thorax. 2004;59:618-622.
75 Gottlieb DJ, Whitney CW, Bonekat WH, et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am J Respir Crit Care Med. 1999;159:502-507.
76 Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
77 Mier-Ewert HK, Ridker PM, Rifani N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678-683.
78 Peppard PE, Young T, Palta M, et al. Longitudinal study of moderate weight changes and sleep-disordered breathing among middle-aged adults. JAMA. 2000;284:3015-3021.
79 Davies RJ, Ali NJ, Stradling JR. Neck circumference and other clinical features in the diagnosis of the obstructive sleep apnea syndrome. Thorax. 1992;47:101-105.
80 Harsch IA, Schahin SP, Radespiel-Troger M, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156-162.
81 Brooks B, Cisutlli PA, Borkman M, et al. OSA in obese non-insulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79:1681-1685.
82 American Academy of Sleep Medicine. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine task force. Sleep. 1999;22:667-689.
83 Sharhar E, Whitney CS, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Study. Am J Respir Crit Care Med. 2001;163:19-25.
84 Nerfeldt P, Graf P, Borg S, et al. Prevalence of high alcohol and benzodiazepine consumption in sleep apnea patients studied in blood and urine tests. Acta Otolaryngol. 2004;124:1187-1190.
85 Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53:154-166.
86 Broughton R, Dunham W, Newman J, et al. Ambulatory 24 hour sleep-wake monitoring in narcolepsy-cataplexy compared to matched controls. Electroencephalogr Clin Neurophsysiol. 1988;70:472-481.
87 Harsh J, Peszka J, Hartwig G, et al. Night-time sleep and daytime sleepiness in narcolepsy. J Sleep Res. 2000;9:309-316.
88 Guilleminault C, Gelb M. Clinical aspects and features of cataplexy. Adv Neurol. 1995;67:65-77.
89 Overeem S, Lammers GJ, van Dijk DR. Weak with laughter. Lancet. 1999;354:838.
90 Schuld A, Hebebrand J, Geller F, et al. Increased body mass index in patients with narcolepsy. Lancet. 2000;355:1274-1275.
91 Saper CB. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257-1263.
92 Thannickal T, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469-474.
93 Guilleminault C, Mingot E, Grumet FC. Familial patterns of narcolepsy. Lancet. 1989;2:1276-1379.
94 Bassetti C, Aldrich MS. Idiopathic hypersomnia: a series of 42 patients. Brain. 1997;120:1423-1435.
95 Liebowitz SM, Brooks SN, Black JE. Excessive daytime sleepiness: considerations for the psychiatrist. Psychiatr Clin North Am. 2006;29:921-945.
96 Winkelmann J, Wetter TC, Collado-Seidael V, et al. Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep. 2000;23:597-600.
97 Earley CJ, Connor JR, Beard JL, et al. Ferritin levels in the cerebrospinal fluid with restless leg syndrome. Sleep. 2005;28(9):1069-1075.
98 Rothdach AJ, Trenkwalder C, Haberstock J, et al. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Neurology. 2000;54:1064-1068.
99 Ufberg J, Nystrom B, Carter N, et al. Prevalence of restless legs syndrome among men aged 18 to 64 years: an association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16:1159-1163.
100 Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101-119.
101 Early CJ. Clinical practice: restless legs syndrome. N Engl J Med. 2003;348:2103-2109.
102 Silber MH, Girish M, Isureita R. Pramipexole in the management of restless legs syndrome: an extended study. Sleep. 2003;26:819-821.
103 Adler CH, Hauser RA, Sethi K, et al. Ropinrole for restless legs syndrome: a placebo-controlled crossover trial. Neurology. 2004;62:1405-1407.
104 Thorpy MJ. New paradigms in the treatment of restless leg syndrome. Neurology. 2005;64(suppl 3):S28-S33.
105 American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual, ed 2. Westchester, IL: American Academy of Sleep Medicine, 2005.
106 Drake CL, Roehrs T, Richardson G, et al. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27:1453-1462.
107 Knutsson A. Health disorders of shift workers. Occup Med. 2003;52:103-108.
108 Akerstedt T. Shift work and sleep disorders. Sleep. 2005;28:9-11.
109 Czeisler CA, Walsh JK, Roth T, et al. Modafinil for excessive sleepiness associated with shift-work sleep disorder. N Engl J Med. 2005;353:476-486.