CHAPTER 21. Fluid, Electrolyte, and Acid-Base Balance
Kim A. Noble
OBJECTIVES
At the conclusion of this chapter, the learner will be able to:
1. Identify the three primary fluid compartments of the body and the volume and distribution of fluid in each.
2. Differentiate between the individual forms of crystalloid and colloid solutions and their indications for use.
3. Identify fluid and electrolyte imbalances and the nursing assessment and management for each.
4. Describe the primary mechanisms responsible for the regulation of fluid and electrolyte balance.
5. Identify the physiological origination of acid-base balance in the body and the potential implications of abnormalities in the perianesthetic patient.
6. Describe the components of arterial blood gas (ABG) results specific to acid-base interpretation and their physiological rationale for analysis.
7. Identify common abnormalities in acid-base balance and their application to the perianesthetic population.
I. FLUID AND ELECTROLYTE BALANCE OVERVIEW
A. Body cells function in a tightly regulated fluid- and electrolyte-filled environment.
1. Fluid and electrolyte homeostasis
a. Maintained via hormonal mechanisms
b. Maintained via neural mechanisms
2. Alterations in fluid and electrolyte homeostasis impact cellular function.
a. Change the electrical potential of excitable cells
b. Lead to intracompartmental fluid shifts
c. Directly impact organ system function
3. There is a constant flux or movement of water and solutes between the three primary body fluid compartments.
a. Extracellular fluid compartment
(1) Intravascular fluid
(2) Interstitial fluid
b. Intracellular compartment
c. Transcellular compartment
4. Fluid flux is constrained by:
a. Compartmental membranes
b. Solute and plasma protein concentrations
5. Sodium/potassium (Na +/K +) adenosine triphosphatase (ATPase) pump
a. Active (energy dependent) pump on cell membranes
b. Functions in the maintenance of solute concentration gradients across the cell membranes
B. Fluid balance requires both:
1. Normal volume of water
2. Normal concentrations of particles in solution (Table 21-1)
| ADH, Antidiuretic hormone; ATPase, adenosine triphosphatase; BP, blood pressure; CNS, central nervous system; DI, diabetes insipidus; DKA, diabetic ketoacidosis; ECF, extracellular fluid; ECG, electrocardiogram; GI, gastrointestinal; ICF, intracellular fluid; NSS, normal saline solution; N/V, nausea/vomiting; PTH, parathyroid hormone; PVCs, premature ventricular contractions; RR, respiratory rate; SIADH, syndrome of inappropriate secretion of ADH. | |||
| *Electrolytes found in high concentration in ECF are in low concentration in ICF; similarly, the primary electrolytes of the ICF are present, but in low concentrations, in the ECF. |
|||
| ECF Ion | Normal Serum Value | Indicators | |
|---|---|---|---|
| Deficit (Hypo-) | Excess (Hyper-) | ||
| Sodium (Na +)
Regulates ECF osmolality and vascular fluid volume
↓ICF content; ↑ECF content maintained by Na +/K + ATPase pump
|
135-145 mEq/L | Hyponatremia
<130 mEq/L, ↓serum osmolality
Salt diluted by excess retained water
Bladder irrigations, electrolyte-free IV infusions, ADH oversecretion (SIADH)
Outcomes: Weak muscles
Confusion
Nausea/vomiting
Hypotension
Seizure
Coma if <115 mEq/L
|
Hypernatremia
>145 mEq/L, ↑serum osmolality
Excess salt from water losses
Inadequate osmotic diuresis; poor fluid intake; lack of ADH (DI)
Outcomes: Thirst
Flushed skin
Hypotension
Oliguria
Seizures, coma if extreme
|
| Chloride (Cl −)
Preserve acid-base balance
Reciprocal: if Cl − depleted,
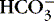 rises risesCombines with Na + to maintain osmolality
|
96-106 mEq/L | Hypochloremia
~ <98 mEq/L
Prolonged Cl − loss: gastric suction, diuresis
Metabolic Alkalosis Patient hypoventilates
|
Hyperchloremia
~ >108 mEq/L
Cl − gain; NSS resuscitation
Metabolic Acidosis Patient hyperventilates
|
| Bicarbonate (HCO 3−) | 22-28 mEq/L | Metabolic Alkalosis
pH >7.45
Acid loss/HCO 3− gain: N/V; ↑GI suction
Patient hypoventilates; compensatory ↓K +
|
Metabolic Acidosis
pH <7.35
Acid gain/HCO 3− loss: renal failure; DKA
Patient hyperventilates; compensatory ↑K +
|
| Osmolality (mOsm) | 280-300 Osm/kg | Dehydration
ECF concentrated (DI)
↑ Risk of thrombosis
|
Overhydration
ECF dilute (SIADH)
|
| Potassium (K +)
↓ECF content; ↑ICF content maintained by Na +/K + ATPase pump
Potent effect on cell and neuromuscular irritability
Acidosis, catabolism: move K + to serum
Insulin, glucose shift K + back to cell
↑ Concentration in ECF maintained by Na +/K + ATPase pump
|
3.5-5.0 mEq/L | Hypokalemia
~ <3.5 mEq/L
Reflects ECF loss: diuretics, diarrhea, N/V, digitalis, bowel preps
↓ECF K + → ↓ICF K +
Muscle weakness
Hypoventilation
Flaccid paralysis
Cardiac arrhythmias: more PVCs, U wave classic, conduction blocks
Slow KCI doses: 10 mEq/h peripheral; 20 mEq/h central line
|
Hyperkalemia
~ >5.0 mEq/L
↑ Serum K +: tissue lysis, acidosis (renal or DKA)
Malignant hyperthermia: LETHAL
Muscle weakness
Hypoventilation
Paralysis
Cardiac arrhythmias: peaked T waves; wide QRS; asystole
Stat insulin (glucose), bicarbonate and Ca + drives K + back into ICF
Dialyze renal patients
Stop any K + intake
|
| Magnesium (Mg +)
Promotes acetylcholine release at neuromuscular junction
Regulates K +
Opposes Ca ++
|
1.5-2.5 mEq/L | Hypomagnesemia
<1.5 mEq/L
Causes: Diarrhea
Mal-absorption
Long-term N/V
↑ Aldosterone
Signs: Neuromuscular irritability, seizures
Cardiac: long PR, wide QRS, flat T; torsades risk
Affects serum K +, Ca ++, and
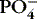 |
Hypermagnesemia
>2.5 mEq/L
Causes: MgSO 4 infusion (eclampsia)
Ketoacidosis
Chronic renal failure
Signs: CNS depression, sedation, muscle weakness, ↓ reflexes
↓BP; ↓heart rate
If Mg + >12 → ↓RR
|
| Phosphate (PO 4−)
Most stored in bone
Essential for energy and acid-base balance
Inverse relationship with calcium: if PO 4−↑, Ca ++↓
Need parathyroid hormone (PTH) to excrete
|
1-2 mEq/L (3-4.5 mg/dL) | Hypophosphatemia
<1.5 mg/dL
Causes: Aspirin overdose
Ketoacidosis
Steroids
Malabsorption
↑Ca ++
Outcome: Energy depletion: weak muscle, seizures, cardiorespiratory failure
|
Hyperphosphatemia
>4.5 mg/dL
Causes: Laxative excess
Supplement in diet
Trauma
Outcome: Cell death, renal failure, PTH decreases
|
| Calcium (Ca ++)
Critical for impulse conduction, contraction, and coagulation
Is stored in bone
Present in blood (ECF): ionized (50%), protein bound
Inverse relationship with
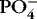 : when Ca ++↑, : when Ca ++↑, 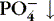 |
4.5-5.3 mEq/L (8.5-10.5 mg/dL) | Hypocalcemia
<4.5–5.3 mEq/L
Causes: Low albumin
Renal failure (chronic)
Hypoparathyroidism
Signs and symptoms: Tingling/weakness
Twitching/tetany
Low BP
ECG change
Postoperative laryngospasm
|
Hypercalcemia
>4.5 mEq/L
Causes: Immobility
Malignancy
Low
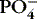 Hyperparathyroidism
Signs and symptoms: Lethargy
Short QT
|
II. BODY FLUID DISTRIBUTION
A. Body water accounts for:
1. Approximately 60% of adult total body weight
2. As much as 75% to 77% of infant total body weight
3. Average male (154 lb or 70 kg) has 42 L of total body water.
a. Extracellular fluid (ECF) accounts for approximately 14 L of fluid.
(1) Intravascular fluid: accounts for approximately one third of total ECF
(2) Interstitial fluid: accounts for approximately two thirds of total ECF
b. Intracellular fluid (ICF) accounts for approximately 28 L of fluid.
4. Percentage of water varies with percentage of body fat.
a. Muscle: high water content
b. Fat: low water content
c. Female body contains a higher proportion of fat than male.
B. Body fluid compartments
1. ECF compartment: accounts for one third of total body water.
a. Fluid circulating outside of cells
b. Volume: 33% to 40% of adult’s total body weight, nearly 75% of a young child’s body weight
c. Three subcomponents of ECF
(1) Intravascular fluid: fluid within the vascular system
(a) Crucial for cardiovascular function
(b) Accounts for one third of ECF volume or 8% of total body water
(2) Interstitial fluid: fluid between the cells
(a) Returns to circulation via lymphatics
(b) Controlled by capillary cell wall integrity, oncotic and hydrostatic pressures
(c) About two thirds of ECF volume (20% of adult total body water
(3) Transcellular fluid
(a) Includes:
(i) Synovial
(ii) Cerebrospinal
(iii) Intestinal, hepatic
(iv) Biliary
(v) Pancreatic
(vi) Sweat
(vii) Pleural
(viii) Pericardial
(ix) Peritoneal
(x) Intraocular fluids
(b) Accounts for about 1% of adult total body weight
d. Anesthetic medications dilate vasculature and expand ECF capacity.
(1) Ease fluid overload and improve diastolic filling in the heart.
(2) If ECF volume insufficient, significant hypotension results.
2. ICF compartment: accounts for two thirds of total body water
a. Volume accounts for 66% to 75% of total body water.
b. Fluid found within cells
C. Three processes that govern water movement
1. Osmosis
a. The movement of water from a dilute space with few particles across a semipermeable membrane to a more densely concentrated space; “salt sucks”
b. Osmosis seeks to establish equilibrium between ECF and ICF.
c. The unequal numbers and size of particles controls fluid movement between ECF and ICF.
(1) Glucose, urea, and protein are large molecules that normally cannot pass from blood (ECF) through selectively permeable cell walls.
(2) Because of large particles in the blood, ECF contains more particles, and is therefore more concentrated, than in cells (ICF).
(3) Water shift is constant.
(a) Net movement of water is towards the ECF.
(b) Prevents cells from becoming:
(i) Waterlogged
(ii) Edematous
(iii) Bursting
d. Factors influencing osmosis or the movement of water
(1) Cell wall permeability (integrity)
(2) Serum sodium levels
(3) Na +/K + ATPase pump
(a) Active pump found on cell membranes
(b) Functions in the maintenance of intracellular to extracellular ion concentration gradients
2. Oncotic pressure
a. Also called colloid osmotic pressure
b. Colloids are large particles, such as protein, that normally cannot cross cell membrane.
c. Plasma colloid osmotic pressure: primarily contained in serum and pulls fluid from interstitial space into capillaries across a pressure gradient
3. Hydrostatic pressure
a. Pump pressure exerted by blood against blood vessel (capillary) walls
(1) Elevated capillary hydrostatic pressure with rise in arterial pressure or vessel resistance
(2) Low capillary resistance or low arterial pressure reduces capillary hydrostatic pressure.
b. Principal force causing capillary filtration, or the movement of fluid out of the capillary into the interstitial space
c. Greater at arterial end of the capillary (32 mm Hg) than venous (15 mm Hg)
d. Opposes oncotic or osmotic pressure
III. PHYSIOLOGICAL PARTICLE (SOLUTE) DISTRIBUTION
A. Components (solute) distributed within body water
1. Electrolytes: electrically active ions with either a positive or negative charge when dissolved in solution (Box 21-1). NOTE: A measure of the serum (ECF) concentration of an electrolyte does not necessarily reflect the electrolyte content of intracellular electrolytes (ICF).
a. Primary extracellular (ECF) electrolytes
(1) Cation: positively charged ion
(a) Sodium (Na +)
(i) Reflects serum osmolality
(ii) Regulates fluid balance
(iii) The cation in highest concentration in the ECF
(b) Expect fluid imbalance if serum sodium increased or decreased.
(c) Inverse relationship with serum potassium
(i) If Na + rises, expect low K +.
(d) Na + concentration gradient (ICF: ECF) maintained by the activity of the Na +/K + ATPase pump
(2) Anion: negatively charged ion
(a) Chloride (Cl −) competes with bicarbonate ( 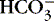 ) to combine with sodium.
) to combine with sodium.
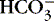 ) to combine with sodium.
) to combine with sodium.(b) Bicarbonate: immediately available acid-base buffer
b. Primary ICF electrolytes: cannot directly measure; reflected by ECF values. NOTE: Status of ICF electrolytes is not necessarily reflected by a laboratory measure of an electrolyte in the serum (ECF).
(1) Cations: positively charged ions, critical for cardiac function
(a) Potassium (K +): poorly stored, deficits occur quickly with loss or reduced intake; cation in highest concentration in ICF
(b) Magnesium (Mg +)
(c) Calcium (Ca ++); stored in ICF; released for cellular activity
(d) Replace all cations slowly.
(i) Always in diluted solution
(ii) Never intravenous (IV) push
(2) Anions: negatively charged ions
(a) Phosphorus (P), present in body fluid as phosphate (PO 4)
BOX 21-1
TONICITY OF REPLACEMENT IV SOLUTIONS
Normal Serum Osmolarity: 290 mOsm/L
Tonicity
Hypotonic: Osmolality <240 mOsm/L
ECF concentration < ICF
Causes water to move from serum into cells
Isotonic: Osmolality 240-340 mOsm/L
Concentration of dissolved particles in ECF = ICF
Hypertonic: Osmolality >340 mOsm/L
ECF concentration > ICF
Causes water to move from cell to serum
IV Solution Tonicity
Half normal saline (0.45 NS): 154 mOsm/L
5% dextrose in water (D 5W): 252 mOsm/L
2.5% dextrose in one-half NS (D 2.5 0.45NS): 265 mOsm/L
Lactated Ringer’s (LR): 5% dextrose in one-half normal saline: 310 mOsm/L
0.9 normal saline (NS): 308 mOsm/L
5% dextrose in one-fourth NS (D 5 0.225 NS): 326 mOsm/L
5% dextrose in one-half NS (D 5 0.45 NS): 406 mOsm/L
10% dextrose in water (D 10W): 505 mOsm
5% dextrose in LR (D 5LR): 524 mOsm/L
5% dextrose in NS (D 5 0.9 NS): 560 mOsm/L
ECF, Extracellular fluid; ICF, intracellular fluid.
2. Nonelectrolyte particles, undissolved
a. Large, osmotically active molecules
b. Influence movement of water across permeable cell membranes
c. Examples
(1) Sugar
(2) Urea
(3) Protein
3. Buffers: physiological controls to regulate acids and bases
a. Bicarbonate: immediate chemical buffer
(1) Present in ECF
(2) Regulate (buffer) pH by accepting or releasing acidic hydrogen ions (H +).
(3) Maintain serum’s chemical neutrality, specifically pH – 7.4: a mathematic representation of hydrogen ion in ECF.
(4) Maintain bicarbonate-to–carbonic acid ratio of 20:1.
b. Phosphate, hemoglobin, and protein: chemical buffers
(1) Present in all body fluids to help maintain acid-base balance and coagulation
(2) Proteins create colloid osmotic pressure to regulate fluid distribution.
(a) Low-protein conditions include:
(i) Hemorrhage (red blood cell loss)
(ii) Malnutrition
(iii) Severe infections
(iv) Fistulas
(v) Fluid imbalances
(b) Low-protein conditions allow fluid to leak from vascular space (ECF) to ICF because of loss of oncotic pressure.
(c) Need serum albumin level greater than 4 g/dL for adequate protein level
4. Salts: potassium chloride (KCl) is one example.
B. Osmolality is a measure of the amount of solute per volume of solution.
1. An index of the body’s hydration status
2. Normal value: 280 to 294 milliosmoles (mOsm)/kg
3. Total number of “osmotically active” particles in solution
a. Determined by total of electrolyte and nonelectrolyte particles
b. Creates osmotic pressure per liter of solution to maintain water in appropriate compartment
c. Serum sodium is the most important determinant.
(1) Water follows sodium to equalize concentration and establish equilibrium; “salt sucks.”
(2) When serum sodium elevated, water shifts into serum (ECF) by osmosis, diluting sodium and normalizing osmolality.
(3) When serum sodium low, water shifts from serum by osmosis to concentrate sodium and normalize osmolality.
4. Serum osmolarity monitored by the hypothalamus
a. Increased osmolarity (increased Na +; decreased water) causes thirst and the negative feedback release of antidiuretic hormone (ADH).
(1) ADH causes the kidney to:
(a) Reabsorb water from the distal tubule
(b) Expand the water in the ECF
(c) Normalize the osmolarity
(2) The normal osmolarity leads to the negative feedback (decreased) release of ADH.
b. Decreased osmolarity (decreased Na +; increased water) decreases the release of ADH.
(1) As ADH secretion decreases:
(a) The water reabsorbed from the distal tubule decreases, causing an increased urinary output.
(b) This leads to decreased water in the ECF and normalizes the osmolarity.
5. Osmolality, in the form of volume or pressure, is also sensed by baroreceptors in the right atrium, leading to the release of atrial natriuretic peptide (ANP).
a. Osmolality high
(1) Low volume and pressure
(2) ECF-concentrated (hypertonic) patient is dehydrated.
b. Osmolality low
(1) High volume and pressure
(2) ECF-dilute (hypotonic) patient is overhydrated.
c. Primarily adjusted by titrating the release of ADH
C. Mechanisms of solute transport
1. Passive or non–energy-expending transport
a. Diffusion: results in the movement of particles in solution across a selectively permeable cell membrane “down” the concentration gradient, or from an area of high solute concentration to an area of lower solute concentration
(1) Purpose: try to equalize concentration of particles between compartments
(2) Electrolytes are small; pass easily across cell walls
(3) Larger particles inhibited from crossing selectively permeable membrane
(4) Although individual ions move constantly, passively, and randomly between ECF and ICF mostly towards the dilute solution
(5) Particle concentration dissolved in ECF or ICF determines water movement (osmosis) and fluid balance.
(6) Solute concentration difference between areas is a concentration gradient.
b. Facilitated diffusion: a substance (for example, insulin) facilitates the diffusion of particles (e.g., glucose) across the semipermeable membrane.
c. Filtration: transfer of water and dissolved substances through the semipermeable gradient via a pressure gradient from higher to lower pressure (hydrostatic pressure).
(1) Pressure created by the weight of the solute-laden solution
(2) Glomerular filtration in kidney’s nephron is an example
(a) Arterial blood pressure is greater than intrarenal pressure.
(b) This pressure gradient forces blood into the glomerulus for filtration.
(3) A force opposing oncotic pressure
d. Osmotic pressure: pressure exerted within a compartment by osmotically active particles in solution
(1) Differences in particle concentration between two compartments create a concentration gradient.
(2) Pressure across this gradient moves (redirects) water across the gradient to equalize water between cells or fluid compartments.
(3) After water equilibrates:
(a) Concentrations of particles in solution equalize.
(b) Volume of water in the compartments may not be equal.
(4) Opposes interstitial fluid pressure
2. Active or energy-dependent solute transportation; primarily through the action of the Na +/K + ATPase pump
a. Metabolic energy in the form of adenosine triphosphate (ATP) is consumed to move substances against their concentration gradient(s) through semipermeable cell membranes.
b. Oxygen also required
(1) During cellular processes, Na + diffuses down concentration gradient, through cell wall and into ICF; K + moves passively in opposite fashion out of the cell.
(2) Na +/K + ATPase (active, energy-dependent pump) returns Na + (against concentration gradient; uphill) to ECF and K + (uphill) to ICF.
IV. HORMONAL REGULATORS OF BLOOD VOLUME
A. ADH: adjusts serum osmolality, concentrates electrolytes
1. Regulates reabsorption or elimination of water, but not Na +, in the distal renal tubules, thereby concentrating or diluting Na +
2. Released by the pituitary’s posterior (hypophysis) in response to a 1% to 2% increase or decrease in serum osmolality, as sensed by osmoreceptors in hypothalamus
3. Increased ADH secretion: response to increased serum osmolality
a. Prompts water reabsorption at kidney’s collecting ducts: urine concentrates and output decreases.
(1) Normal urine specific gravity: 1.010-1.025
(2) Specific gravity increases: more concentrated with dissolved solutes
b. Secretion stimulated by stress such as:
(1) Pain
(2) Trauma
(3) Surgery
(4) Hypovolemia
(5) Opioids
(6) Hypoxia
(7) Hypercapnia
4. Decreased ADH secretion: response to decreased serum osmolality
a. Promotes water elimination through collecting ducts
(1) Urine dilutes.
(2) Output increases.
b. Secretion halted by:
(1) Mechanical ventilation
(2) Pulmonary disease, such as pneumonia
(3) Central nervous system pathology, such as:
(a) Cranial trauma
(b) Tumors
(c) Surgery
(d) Infection
c. Diabetes insipidus may follow pituitary hypophysectomy
(1) Observe for dilute, unconcentrated urine (may be up to 1000 mL/h).
(2) Thirst
(3) Dehydration
d. Urine specific gravity decreases: fewer solutes dissolved in urine
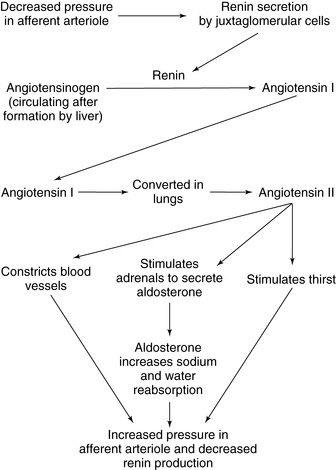
B. Renin-angiotensin-aldosterone: regulates circulating blood volume and peripheral vascular resistance to sustain blood pressure (Figure 21-1)
1. Secretion via feedback mechanism to the renal nephron’s distal tubule; senses blood flow changes (pressure or flow) at the glomerulus impacting glomerular filtration rate (GFR).
2. Renin, an enzyme, is released by the juxtaglomerular apparatus in the distal tubule of the nephron to raise blood pressure and GFR. Renin converts the plasma protein angiotensinogen to angiotensin I, an inactive substance.
3. Angiotensin I circulates in the plasma and is converted to angiotensin II primarily in the lung by angiotensin-converting enzyme.
4. Angiotensin II has two effects:
a. Vasoconstriction from contraction of arterial vascular smooth muscle
b. Release of aldosterone from the adrenal cortex, leading to increased Na + reabsorption by the distal tubule
c. Renin release increases pressure in the glomerulus by increasing peripheral vascular resistance, and increases volume through the action of aldosterone. This increased pressure and flow then decrease the release of renin (negative feedback).
 |
| FIGURE 21-1 ▪
The renin-angiotensin-aldosterone system.
(From Dennison RD: Pass CCRN! ed 3, St Louis, 2007, Mosby.)
|
C. Aldosterone
1. Primary mineralocorticoid hormone of adrenal cortex
2. Acts at the kidney’s distal renal tubule
3. Actively increases total body water by:
a. Regulating sodium reabsorption in response to:
(1) Serum osmolality
(2) Serum K +
(3) Renin secretion
4. Renal tubule excretes K + or H + into the urine in exchange for Na +.
5. Water migrates with Na +.
a. Water is retained.
b. Vascular volume increases.
6. Aldosterone regulation does not alter ECF sodium concentration.
a. Regulates only about 2% of total body sodium
b. Sufficiently prevents hypovolemia and hypotension
7. Decrease in aldosterone secretion leads to:
a. Excretion of Na + and water
b. Retention of K +
D. Atrial natriuretic peptide
1. Secreted by cardiac atrium when stretched by increased venous return (preload)
2. Leads to the excretion of Na +, followed by water excretion
V. FLUID AND ELECTROLYTE-RELATED PERIANESTHESIA ISSUES
A. Clinical status alters fluid status.
1. Cell function requires an exquisite yet dynamic fluid and solute balance.
2. Normal required daily fluid intake is approximately 2 L.
a. Altered normal fluid requirements by:
(1) Stress
(2) Food and fluid restrictions
(3) Preexisting chronic conditions
(4) Acute illness
(5) Trauma
(6) Surgically induced losses
(7) Medications
b. Extent of preoperative dehydration undervalued for patients with limited fluid reserves.
(1) Healthy ambulatory surgery patient is mildly dehydrated (by 5%), because of nothing by mouth (NPO) restrictions.
(2) Children can become significantly:
(a) Dehydrated
(b) Hypoglycemic
(3) Percentage of body water decreases with age
(a) Muscle decreases
(b) Fat increases
(c) Kidneys less able to conserve fluid (concentrate) and regulate Na +
(4) Percentage of body water less in obese patients: fat contains little water.
(5) Malnutrition alters protein intake and use, altering oncotic pressure and water balance.
(6) Preanesthesia NPO rules relaxed: clear liquids permitted 2 to 4 hours preprocedure
3. Preoperative deficit, surgical blood loss replaced with an isotonic crystalloid solution
a. Consider fluid spacing when managing fluid infusions.
(1) First spacing: normal distribution of body fluids ( 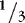 ECF;
ECF; 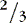 ICF).
ICF).
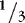 ECF;
ECF; 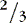 ICF).
ICF).(2) Second spacing: excess accumulation of interstitial fluid with edema, with puffy:
(a) Eyelids
(b) Fingers
(c) Ankles
(3) Third spacing: fluid migration from vascular space (ECF) to areas normally with minimal or no fluid; also known as the transcellular spaces
(a) Examples: ascites, or bowel after peritonitis, injury, or surgery
(b) Depletes vascular circulation: hypovolemia, ongoing hypotension
B. Before day of surgery, determine stable biochemical status, organ function.
1. Clinically relevant laboratory tests are within normal limits.
a. Selectively assess preoperative laboratory values only when warranted by a patient’s:
(1) Health needs
(2) Medications
(3) Coexisting disease
(4) Medical history
(5) Age
(6) Physical examination (Table 21-2)
| BUN, Blood urea nitrogen; INR, international normalized ratio; MVA, motor vehicle accident; NSAIDs, nonsteroidal anti-inflammatory drugs; PT, prothrombin time; PTT, partial thromboplastin time. | |
| *Some recommend potassium and glucose values be updated on day of surgery. |
|
| Obtain Preoperative Test | To Assess |
|---|---|
| POTASSIUM*IF | |
| Potassium-depleting diuretics; digoxin, especially with toxicity; corticosteroids; preoperative colon preparation, or laxative; acid-base disorders: alkalosis Chronic renal failure; acid-base disorders: acidosis; MVA or crushing injuries; acute tubular necrosis |
Hypokalemia: lethal cardiac tachydysrhythmia Hyperkalemia: lethal cardiac bradydysrhythmia; muscle weakness, including respiratory; metabolic dysfunction |
| ELECTROLYTE PANEL AND CHEMISTRIES IF | |
| Renal failure or renal insufficiency; diabetes; cardiopulmonary disease; chemotherapy | Hyperkalemia: acidosis; BUN and creatinine increases; dilutional hyponatremia |
| GLUCOSE*IF | |
| Metabolic syndrome, insulin resistance; diabetes mellitus (type 1 or type 2) | Baseline preoperative blood sugar; day of admission preoperative blood sugar (type 1) or elevation; monitor intraoperatively (type 1); postoperative blood sugar |
| Chronic corticosteroid use | Possible hyperglycemia, need for insulin; baseline preoperative blood sugar as indicated |
| HEMOGLOBIN, HEMATOCRIT | |
| Infants younger than 1 year | Normal physiological anemia |
| Anticoagulants | Unrecognized bleeding potential and determine baseline status |
| Malignancy, radiation/chemotherapy, use of nonsteroidal anti-inflammatories | Suppressed bone marrow function; potential for decreased red blood cell count, white blood cell count, and platelets, mild anemia |
| COAGULATION: PT/PTT/INR/PLATELETS | |
| Chronic anticoagulation | Great risk of excessive or prolonged bleeding |
| Warfarin stopped at least 3-7 days preoperatively | Increased bleeding risk for spinal/epidural anesthetic; risk of operative bleeding and puncture sites. Verify return to normal with PT/INR parameters. |
| Chronic aspirin | Altered platelet function for the life of the platelet |
| Anti-inflammatory drugs (NSAIDs) | Potential for prolonged postsurgical bleeding |
(7) If no new clinical events:
(a) Lab results acceptable for 3 to 6 weeks; or as per institution policy
(8) Preoperative renal assessment with blood urea nitrogen and creatinine:
(a) Indicated if elderly
(b) Systemic disease
(c) Uses nephrotoxic medications as per policy
(9) Verify stable fluid status, update specific tests (K +, glucose) on the day of surgery if indicated.
b. For ambulatory surgery, no extensive physiological fluid or electrolyte shifts
(1) No increased risk of perianesthetic crisis is foreseen.
(2) Aged, American Society of Anesthesiologists classification III and IV patients increasingly accepted or as per policy or physician order
(3) Anticipated need for blood transfusion is a debatable issue.
(a) Some surgeons transfuse autologous blood after liposuction.
(b) Large blood loss often results in unplanned hospital admission.
(4) For preterm infants younger than 60 weeks, hematocrit less than 30% increases risk of apnea.
c. “Routine” laboratory tests: need versus cost in ambulatory surgery
(1) A controversial, well-scrutinized issue
(2) No lab measures truly required for healthy, asymptomatic patients for either ambulatory or inpatient surgery
(3) A battery of “routine” laboratory tests costly, frequently medically unnecessary
(4) Studies demonstrate even new abnormal lab findings in asymptomatic patients rarely cause surgery to be canceled.
(5) False-positive abnormal results in healthy, asymptomatic patients create undue concern, increase costs, and/or cause surgical delays.
C. Postanesthetic hydration and chemical concerns
1. Postoperative nausea and vomiting (PONV). (See Chapter 25 for ASPAN’s PONV/PDNV guideline for patient risk stratification and treatment recommendations.)
a. Increases potential for hypovolemia
b. Significantly delays discharge to home and increases cost of care
c. Infants, children, and elderly patients dehydrate easily.
d. Unrelenting, protracted vomiting can result in clinically significant chemical imbalances and hospital readmission.
e. Highly associated with laparoscopy, strabismus correction, and ear surgery
2. Replace preoperative deficit and surgical blood loss with an isotonic crystalloid solution and colloids as required.
3. In perianesthesia care unit (PACU), measure and replace postoperative electrolytes, magnesium, calcium, particularly when intraoperative blood loss is high, fluid and colloid replacement is large (see Box 21-1).
VI. FLUID IMBALANCES (Box 21-2)
A. Fluid spacing may be:
1. Localized: migration to single area or organ, as with a sprained ankle or blister
2. Multisystem: postoperative migration to abdominal spaces after organ removal, repair of obstructions, or fluid leakage from sites of severe burns: third spacing
3. Caused by:
a. Decreased plasma proteins: insufficient to maintain oncotic osmotic pressures and ECF fluid volumes—renal protein losses
b. Increased capillary permeability: alteration from sepsis, allergic reaction, radiation, and trauma allows fluid leakage.
c. Lymphatic blockage: lymph system is an accessory route to return excess interstitial fluid and leaked proteins into vascular space.
4. Related to anesthetic issues
a. Anesthetic depth and medications, sepsis, or fever can mask fluid volume excess or deficit until the postoperative period.
b. Rewarming after intraoperative hypothermia may cause peripheral vasodilation, thereby expanding the vascular compartment (ECF); transient but significant hypotension results, requiring fluid volume expansion.
c. Spinal and epidural anesthetic techniques expand the ECF by blocking sympathetic tone and dilating peripheral vasculature.
(1) Vasopressors and fluid volume expansion are needed until anesthetic effects have resolved and normal vessel tone has returned, often in phase I PACU.
(2) Excess interstitial fluid and leaked proteins can flow back into vascular space.
5. Surgical shifts: third-space fluid loss
a. Shifts begin immediately after massive trauma or surgery.
(1) Capillary permeability increases.
(a) Protein leaks from cell into inflamed or traumatized areas.
(b) Fluid shifts through leaky cell walls from vascular to interstitial space.
(2) Ongoing hypotension common
b. Reabsorption phase: within 72 hours after injury or trauma
(1) Injured tissues heal.
(a) Capillaries repair; normal permeability restored
(b) Lymph blockage clears.
(c) Plasma proteins return to normal.
(d) Capillary pressures, filtration, reabsorption restored
(2) Fluid volume returns (shifts) to vascular compartment.
(a) Urine volume increases as excess fluid excreted
(b) Low urine specific gravity
(c) Fluid output exceeds intake.
(d) Water weight loss
(3) Monitor electrolyte homeostasis (see Box 21-1).
B. ECF volume deficit (hypovolemia): ECF shift to ICF, or total loss from body
1. Caused by:
a. Abrupt decrease in fluid intake such as NPO status
b. Acute loss: blood loss (hemorrhage), fluid shifts caused by altered capillary membrane permeability, diuretics, excess fistula drainage, burns, vomiting, diarrhea
c. Bowel preparation with large fluid losses through the gastrointestinal (GI) tract
d. Third spacing
2. Assess and monitor:
a. Dehydration and hemoconcentration
(1) Increased serum osmolality and sodium: thirst
(2) Impaired renal perfusion: oliguria (<15 mL/h)
(a) Low-volume concentrated urine, high specific gravity
(b) Acute tubular necrosis and renal failure if protracted
(3) Poor skin turgor: dry skin and mucous membranes
(4) Decreased cardiac output
(a) Hypotension and tachycardia
(b) Decreased central venous pressure (CVP), pulmonary artery pressure
(5) Clear lung fields
(6) Inadequate cerebral perfusion: confusion, lethargy
3. Intervene and evaluate (Tables 21-3 and 21-4)
a. Generous fluid replacement with isotonic solutions and/or colloid
b. Treat underlying cause
(1) Blood losses: return to operating room to reexplore, cauterize bleeding vessels, or repair anastomoses.
(2) Replace large urine losses hourly, caused by diabetes insipidus, for example, and monitor electrolytes.
(3) Replace large fluid and electrolyte losses via surgical wound drains, nasogastric tube, vomiting, and diarrhea hourly.
| BS, Blood sugar; CO 2, carbon dioxide; ECF, extracellular fluid. | |
| Symptom | Possible Clinical Significance |
|---|---|
| CARDIOVASCULAR | |
| Bounding pulse, neck vein distention | Fluid overload, increased ECF |
| Weak or thready pulse | Dehydration, decreased ECF volume |
| Increased heart rate | May reflect fever, acidosis, or ECF volume deficit |
| Irregular pulse | Cardiac dysrhythmia—may signal K + abnormality |
| Hypotension, orthostatic | ECF volume deficit |
| RESPIRATORY | |
| Increased rate and depth |
Anxiety with hyperventilation→respiratory alkalosis
Perhaps compensation for acidosis or ↑CO 2
|
| Decreased rate and depth |
Perhaps compensation for alkalosis and ↓CO 2
With somnolence, may signal oversedation
|
| “Crackles” and rales at lung bases | Overhydration, cardiac congestion or failure; atelectasis |
| NEUROLOGICAL | |
| Altered level of consciousness | Abnormal Na +, dehydration, acid-base imbalance; ↓BS |
| Vertigo | ECF volume deficit |
| Muscle weakness |
Severely elevated or low potassium, hypercalcemia, hypermagnesemia
May reflect volume losses
|
| Altered reflexes | Magnesium or calcium imbalance |
| Tingling | Hyperventilation with respiratory alkalosisSuspect calcium elevation |
| Excitability | Decreased calcium or magnesium |
| SKIN | |
| Turgor at sternum |
Dehydration if remains “tented” when pinched
Not reflective of fluid status in elderly patients
|
| Mucous membranes | Dryness may indicate ECF deficit |
| ECF, Extracellular fluid; ICP, intracranial pressure; INR, international normalized ratio; NPO, nothing by mouth; PTT, partial thromboplastin time. | |
| Solution | Considerations |
|---|---|
| COLLOID | Raise oncotic pressure in ECF |
| Synthetic protein replacement fluids | Blood products refused, contraindicated |
| Hydroxyethyl starch (Hetastarch) | Most Jehovah Witness beliefs allow use |
|
Variety of suspensions available (70/.05 to 450/0.7)
Effect on vascular volume medium to long depending on suspension
|
|
| Dextran (40 or 60) |
High antibody titers: cannot cross-match
Less expensive than blood products
Caution: Can interfere with clotting
Effect on VASCULAR volume medium too long depending on suspension
|
| Gelatins | Inexpensive; similar effectiveness to hetastarch |
| Albumin: 5%, 25% |
Expensive; short effect on vascular volume
Limited availability, replaces low albumin
|
| Blood products |
Exposure to blood-transmitted diseases
Need indicated by laboratory measures
Anemia: Hemoglobin, hematocrit decrease
Coagulopathy: Elevated INR, PTT
|
| CRYSTALLOID | |
| Hypertonic electrolyte solutions |
Rarely used; acute treatment for ↑ICP by reducing cerebral edema
Administration of concentrated Na +/Cl −
|
| Isotonic fluids |
Restore circulating fluid volume, electrolytes
Maintenance fluid: 100-200 mL/h
Operative: 1-2 L to replace NPO and replace surgical, insensible losses
Critical hypovolemia, massive burns: replace up to 8-10 L
|
| Hypotonic fluids |
Rehydrate cells
Hyperosmolar diabetes
|
C. ECF volume excess (hypervolemia): shift from ICF to ECF (serum) or second spacing
1. Caused by:
a. Fluid intake, either oral or parenteral, beyond physiological tolerance
(1) Renal failure: inability to excrete fluid
(2) Congestive heart failure: circulatory overload
(3) Remobilization of third-space fluid 48 to 72 hours postoperatively
b. Excess Na + intake
(1) Intravenous Na +
(2) Hyperaldosteronism: Na + retention
(3) Seawater ingestion
c. Sodium hemodilution: relative fluid excess
(1) Intraoperative absorption of fluid through vascular “beds” during transurethral resection of prostate (TURP):
(a) Confusion
(b) Hyponatremia
(c) Possibly seizures
2. Assess and monitor: overhydration, hemodilution, low osmolality
a. Circulatory overload: observe
(1) Increased CVP, pulmonary artery pressures
(2) Congestive heart failure
(a) Pulmonary congestion
(b) Respiratory compromise
(i) Rales
(ii) Dyspnea
(iii) S3 heart sound
(3) Peripheral edema—pitting edema at:
(a) Ankles
(b) Fingers
(c) Eyelids
(4) Jugular vein distention
(5) Pleural effusion
(6) Hypertension or hypotension, perhaps tachycardia
(7) Renal perfusion and urinary output
(8) Skin: plump, moist, and perhaps weeping through pores
(9) X-ray evidence of pulmonary congestion, enlarged cardiac silhouette
(10) Hypoxia, hypercapnia per ABGs, electrolyte measures
(11) Mental status
3. Intervene and evaluate: remove excess fluid, maintain electrolyte balance.
a. Treat underlying cause.
b. Diuretics
c. Fluid restriction
D. Volume replacement
1. Crystalloids: electrolytes in dextrose- or water-based solutions (see Box 21-1)
a. Advantages of crystalloids:
(1) Inexpensive
(2) Promote urinary output and restore third-space losses
(3) Good for maintenance IV fluid administration, to replace insensible fluid losses and for replacement of fluid and electrolyte losses
b. Disadvantages of crystalloids: can dilute plasma proteins and decrease oncotic pressure, leading to a net outward filtration of fluid from vascular space to interstitial space
2. Colloids: solutions containing natural or synthetic protein impermeable to the vascular membrane (see Table 21-4)
a. Advantages: restore vascular colloid pressure and ECF fluid balance between interstitial and intravascular spaces, and smaller amounts required for fluid replacement.
b. Disadvantages: expensive, protein basis may trigger coagulation abnormalities and anaphylaxis, and protein movement into interstitial space and increased edema.
3. Fluid replacement calculation: Adults
a. Replacement of NPO status: replace
b. Fluid maintenance dependent on the surgery type
4. Fluid replacement calculation: Pediatrics (Box 21-3)
a. Fluid maintenance is weight dependent.
BOX 21-3
FLUID REPLACEMENT FOR PEDIATRIC PATIENT
0-10 kilogram (kg): 4 mL/kg/hr for each kg body weight
10-20 kg: 40 mL bolus + 2 mL/kg/hr for each kg >10 kg
>20 kg: 60 mL bolus + 1 mL/kg/hr for each kg >20 kg
E. Blood loss replacement
1. Healthy patients
a. Blood loss replaced 3 mL crystalloid for each 1 mL blood loss
b. Blood loss replaced 1 mL colloid or blood solution for each 1 mL blood loss
BOX 21-2
REGULATORS OF FLUID AND ELECTROLYTE EQUILIBRIUM
Diffusion: Movement of particles such as potassium or calcium through a cell’s permeable wall from an area of high concentration to a lower concentration.
Osmosis: Movement of water from a dilute solution toward a more concentrated fluid.
Concentration gradient: Difference in concentration (osmolality) between two solutions that causes fluid or electrolyte movement.
Osmotic pressure: A physical force, determined by the number (or concentration) of particles in a solution, causing the movement of fluid (osmosis) toward the concentrated solution.
Oncotic pressure: Osmotic force produced in vascular spaces by molecules such as plasma proteins.
Antidiuretic hormone (ADH): Hypothalamic hormone released by the posterior pituitary gland in response to increased serum osmolality; ADH regulates sodium concentration and thereby the passive movement of water with sodium.
VII. ACID-BASE CONCEPTS: PHYSIOLOGY OF CHEMICAL BALANCE
A. Body cells: extremely sensitive to the chemical environment
1. Cell wall protects environment to maintain life-sustaining intracellular functions.
2. Minor changes in acidity or alkalinity alter cellular function, cause cell death.
3. Chemical imbalance affects:
a. Electrolyte charge
b. Changes ion concentrations in solution
4. Carbonic acid (H 2CO 3), the body’s dynamic chemical buffer system, compensates for moment-to-moment acid-base shifts to maintain acid-base “harmony” in a normal ratio of 20 base to 1 acid (Table 21-5).
| *Henderson-Hasselbach equation: describes a dynamic buffer of body fluids. Hydrogen ion is regulated by combining with or dissociating from bicarbonate. |
|
| Immediate response as directed by Henderson-Hasselbach equation*: | |
| Hydrogen + Bicarbonate → Carbonic Acid → Water + Carbon Dioxide | |
H + → 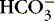 → H 2CO 3→ H 2O + CO 2 → H 2CO 3→ H 2O + CO 2 |
|
| ACIDIC CONDITIONS (PH <7.35):
Bicarbonate ion reabsorbed
Recombines with hydrogen ion
Forms more carbonic acid
|
ALKALOTIC CONDITIONS (PH >7.45):
Bicarbonate ion excreted
Relative excess of hydrogen ion
Less carbonic acid formed
|
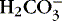 dissociates to water and CO 2. dissociates to water and CO 2.Respiratory rate and depth increase (↑)
CO 2 is exhaled (↓ P co2).
pH restored toward 7.4
(↑) |
Respiratory rate and depth decrease (↓)
CO 2 accumulates (↑ P co2).
pH restored toward 7.4 (↓)
|
5. Oxygen
a. Critical component of acid-base balance
b. Metabolism occurs even during oxygen lack—termed anaerobic metabolism.
c. Anaerobic metabolism leads to an acidic environment.
6. Adequate hemoglobin is necessary for effective oxygen transport to cells.
7. ECF
a. Accessible for measurement by serum analysis
b. Means for treatment for acid-base disharmony
c. Semipermeable cell walls allow some equilibration of ions—ICF affected.
8. Carbon dioxide (CO 2) more soluble in cool temperature
a. Hypothermia
b. ↑CO 2
c. Acidity increases
B. Perianesthesia concerns
1. Acid-base disruption relatively common among preoperative and postoperative perianesthesia patients due to:
a. Ability to sustain acid-base balance is disturbed
(1) Trauma
(2) Acute or chronic illness
(3) Surgical fluid shifts
(4) Anesthetic effects
b. The body of a healthy patient “automatically” compensates to sustain normal acid-base parameters.
c. The perianesthesia nurse must:
(1) Anticipate conditions that disrupt a patient’s acid-base balance
(2) Analyze ABG results
(3) Quickly initiate nursing and medical treatment
d. Acidosis is likely:
(1) With hypothermia
(2) With hypoxia
(3) Corrected by active rewarming techniques
(4) Corrected with delivery of supplemental oxygenation
e. Anesthetic agents can cause myocardial depression → ↓ tissue perfusion
(1) ↓ Oxygen (O 2) supply → anaerobic metabolism → lactic acidosis
(2) ↑CO 2 levels → respiratory acidosis
C. Definitions
1. Acid: a hydrogen ion (H +) donates a hydrogen ion when in solution.
a. Binds with a base to form an inert compound
b. The body’s primary acid is hydrogen ion (H +).
2. Base: a hydrogen ion (H +) acceptor when a compound is in solution
a. Synonymous with the term alkali
b. The body’s primary base is bicarbonate ( 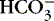 ).
).
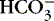 ).
).3. Acidosis: abnormal increase of acid content within body fluids from:
a. Accumulation of acid (H +)
b. Loss of base ( 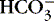 )
)
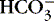 )
)c. May arise from respiratory or metabolic causes
d. Acidemia refers to an acid condition in the blood.
4. Alkalosis: abnormal decrease in acid content within body fluids from:
a. Loss of acid (H +)
b. Accumulation of base ( 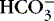 )
)
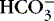 )
)c. Alkalemia refers to an alkaline status of blood.
D. Determinants of acid-base homeostasis
1. pH: a mathematical calculation reflecting the concentration of H + in solution
a. An abbreviation for “potential hydrogen”
b. Negative algorithm of the amount of H + in a solution
c. Describes the relative balance between acids and bases in solution
d. pH of a neutral, neither acid nor alkaline, solution is 7.0.
(1) Increasing a solution’s acidity (adding acid [H +] or decreasing the base [ 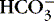 ]) decreases pH to <7.0.
]) decreases pH to <7.0.
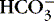 ]) decreases pH to <7.0.
]) decreases pH to <7.0.(2) Decreasing a solution’s acidity (adding base [ 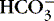 ] or losing acid [H +]) increases pH to >7.0.
] or losing acid [H +]) increases pH to >7.0.
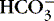 ] or losing acid [H +]) increases pH to >7.0.
] or losing acid [H +]) increases pH to >7.0.e. Body’s buffer systems normally maintain the pH of blood in a range of 7.35 to 7.45.
f. Initiate treatment pH
(1) Decreased (7.30-7.35)
(2) Increased (7.45-7.50)
g. Definitive therapy indicated when pH <7.15 or >7.60
h. Death is imminent if no intervention
(1) pH <6.90
(2) pH >7.90
2. Partial pressure of carbon dioxide in arterial blood (P co 2): respiratory component
a. Represents the amount of CO 2 dissolved in arterial blood
b. CO 2 dissolves in plasma and reversible
(1) Binds with water (H 2O) to create carbonic acid (H 2CO 3)
(2) Considered a nonfixed or volatile acid
c. Carbonic acid forms in tissue capillaries to transport CO 2 to the lungs for excretion.
d. Regulated by breathing to exhale CO 2 (acid) from body
(1) Encourage a groggy perianesthesia patient with a purely respiratory acidosis (elevated Pa co 2) to deep breathe.
(2) Correct this acid-base disturbance by exhaling excess CO 2.
(3) Add supplemental oxygen.
e. Unresponsiveness may render the patient unable to follow the request to deep breathe due to:
(1) Residual muscle relaxant
(2) Sedation
f. To excrete CO 2 it may be necessary for:
(1) Reintubation
(2) Positive pressure ventilation
(3) Manually/mechanical ventilator
g. A patient with severe metabolic acidosis will “automatically” compensate by:
(1) Increasing respirations to exhale excess acid in the form of CO 2
(2) An example is Kussmaul respiration, the deep, blowing respiratory compensation seen in diabetic ketoacidosis.
3. Bicarbonate ( 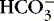 ): metabolic component
): metabolic component
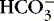 ): metabolic component
): metabolic component
a. Represents amount of 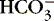 available to buffer acids
available to buffer acids
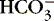 available to buffer acids
available to buffer acidsb. Regulated at kidney: excreted or reabsorbed at the collecting tubule
c. Influenced by amount of fixed or nonvolatile acid
(1) Infuse sodium bicarbonate to increase 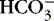 levels and buffering capacity.
levels and buffering capacity.
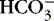 levels and buffering capacity.
levels and buffering capacity.(2) Correct other electrolyte disturbances.
4. Anion gap: expressed as base excess
a. Calculated difference between serum cations and anions
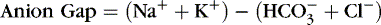
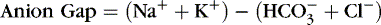
b. Used to determine the potential cause of metabolic acidosis
c. Formula: Serum sodium value minus sum of bicarbonate and chloride
d. Normal anion gap = 10 to 12 mOsm/L
e. Increased anion gap: associated with metabolic acidosis with H + gain
(1) Ketoacidosis: diabetic, alcoholic, starvation
(2) Lactic acidosis: hypoxia (anaerobic metabolism), shock, sepsis
(3) Rhabdomyolysis: acute, massive tissue destruction
(4) Acute renal failure: acute tubular necrosis, shock
f. Normal anion gap: associated with metabolic acidosis due to 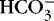 losses with retention of Cl − to maintain ionic balance
losses with retention of Cl − to maintain ionic balance
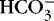 losses with retention of Cl − to maintain ionic balance
losses with retention of Cl − to maintain ionic balance
(1) Diarrhea, intestinal or biliary fistulas
(2) Excessive sodium chloride (NaCl) intake
5. Temperature: pH decreases (produces acidosis) as temperature decreases.
a. P co 2 decreases by 4.5% per degree Celsius.
b. Hemoglobin, one of the body’s acid-base buffers, accepts more H + in cool temperatures, so pH increases.
6. Oxygenation: P o 2, percent saturation, and hemoglobin
a. Pa o 2: measure of partial pressure of dissolved oxygen in arterial blood
b. Oxygen loosely bound to hemoglobin (saturation) or dissolved in blood (Pa o 2)
c. Oxyhemoglobin dissociation: relationship between P o 2 and saturation
(1) P o 2 >70 mm Hg is the critical point: at >70 mm Hg, hemoglobin saturation is nearly 100%.
(2) As P o 2 dips <70 mm Hg, small decrease in Pa o 2 correlates with large decrease in oxygen saturation as hemoglobin quickly releases oxygen tissues.
(a) When P o 2 ≥40 mm Hg, hemoglobin approximately 70% saturated
(b) Temperature, pH, and P co 2 are indicators of metabolism and affect oxygen binding to hemoglobin and the release of oxygen from hemoglobin.
(i) ↑Temperature; ↓ pH; ↑ P co 2 indicate ↑ metabolism and ↓ affinity or strength of oxyhemoglobin bond and ↑ release of O 2 from hemoglobin to tissues (e.g., fever with hypermetabolism).
(ii) ↓ Temperature; ↑ pH; ↓ P co 2 indicate ↓ metabolism and ↑ affinity or strength of bond and ↓ release of O 2 from hemoglobin to tissues (e.g., hypothermia with hypometabolism).
VIII. PRIMARY ACID-BASE IMBALANCE
A. Acidosis
1. Respiratory: Pa co 2 >45 mm Hg and pH <7.35
a. Results from alveolar hypoventilation: failure to excrete carbonic acid (CO 2)
b. Metabolic state normal
c. Clinical causes
(1) Depression of central respiratory centers
(a) Effects of residual anesthetic agents, such as muscle relaxants that render the patient unable to breathe effectively
(b) Consider pseudocholinesterase deficiency if respiratory effort ineffective after succinylcholine.
(c) Sedation from narcotics or hypnotic (intentional or as part of conscious sedation) or caused by overmedication
(d) Compression of medullary centers from increases in intracranial pressure
(i) Edema from surgical intervention or trauma
(ii) Intracranial masses caused by lesions
(iii) Increased P co 2, a potent intracerebral vasodilator
(e) Hypothermia: slows metabolism of depressant medications
(f) Exhaustion from ineffective respiratory effort
(2) Interference with muscles of respiration
(a) Residual effects of neuromuscular blocking agents
(b) Pain causes splinting and limited chest expansion; more pronounced after thoracic and abdominal surgery
(c) Physical limitation of chest expansion from:
(i) Tight chest binders
(ii) Chest tubes
(iii) Dressings
(iv) From burn eschar
(v) Kyphosis
(d) Obesity: lung expansion especially hampered in supine position
(e) Neuromuscular diseases: myasthenia gravis, poliomyelitis
(f) Inadequate mechanical ventilation: rate or tidal volume too low to exhale CO 2
(3) Airway obstruction
(a) Oropharynx
(i) Secretions
(ii) Relaxed tongue
(iii) Pharyngeal edema, tracheal or subglottic stenosis
(b) Laryngospasm or bronchospasm
(c) Pulmonary aspiration
(d) Endotracheal tube (ETT)
(i) Malpositioned resulting in single-lung ventilation
(ii) Blocked ETT by secretions or kinks
(4) Pulmonary disease
(a) Chronic obstructive pulmonary disease
(b) Pulmonary fibrosis
(c) Atelectasis and pneumonia
(d) Bronchospasm or asthma
d. Therapeutic interventions to correct alveolar hypoventilation
(1) Stimulate! Stir up! Remind patient to breathe.
(2) Ensure airway patency.
(a) Jaw lift
(b) Head reposition
(c) Suction
(d) Insert oral or nasal airway.
(e) Intubation if stimulation ineffective
(f) Mechanical ventilation as needed
(3) Provide oxygen.
(4) Reverse muscle relaxants and/or sedatives or narcotics as appropriate.
(5) Rewarming measures if patient is hypothermic
2. Metabolic: 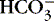 <22 mEq/L and pH <7.35
<22 mEq/L and pH <7.35
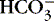 <22 mEq/L and pH <7.35
<22 mEq/L and pH <7.35
a. Results from accumulated ionized acid (H +) or depletion of base
b. Respiratory status normal, except as in compensation
c. Clinical causes
(1) Acid overproduction: promotes K + release from cells
(a) Ketoacidosis: type 1 diabetes or starvation with protein catabolism
(b) Anaerobic metabolism: lactate production (acidosis)
(c) Renal failure, acute and chronic
(d) Muscle destruction: rhabdomyolysis
(e) Overdose: salicylic acid (aspirin) or ferrous sulfate (iron)
(i) Salicylate metabolites increase fixed acids.
(ii) Directly stimulates respiratory chemoreceptors to cause hyperventilation
(iii) Respiratory alkalosis predominates in adults.
(iv) Metabolic acidosis predominates in infants, young children.
(f) Fevers caused by infection
(2) Severe bicarbonate loss
(a) GI: diarrhea, small bowel or pancreatic fistulas
(b) Excessive doses: acetazolamide (Diamox) or ammonium chloride
d. Therapeutic interventions to correct
(1) Encourage deep breathing (↑ respiratory rate and depth) so CO 2 is exhaled.
(2) Administer sodium bicarbonate, usually 1 mEq/kg.
(3) Remonitor ABGs, K + retreat as needed: aim for slow resolution.
(4) Give insulin (+ dextrose) to return potassium to cells as acidosis resolves.
(5) Monitor cardiac rhythm (electrocardiogram) for dysrhythmia, peaked T waves.
(6) Frequently monitor vital signs, neurologic and respiratory status.
B. Alkalosis
1. Respiratory: P co 2 <35 mm Hg and pH >7.45
a. Results from alveolar hyperventilation: ↑ excretion of CO 2
b. Respirations increased, metabolic status normal
c. Clinical causes
(1) Psychogenic causes: pain, anxiety, and panic
(2) Respiratory center overstimulation: tumors at level of medulla or pons; surgical manipulation of brainstem
(3) Overzealous mechanical ventilation: rate, tidal volume too high.
(4) Normal finding in pregnancy
d. Patient reports headache, dizziness, tingling, paresthesias.
e. Therapeutic interventions to correct
(1) Sedate or provide analgesia.
(2) Coach breathing: slow, regular, moderate depth.
(3) Emotional support and calming reassurance
(4) Adjust mechanical ventilator settings to reduce rate, tidal volume.
(5) Monitor ABGs, labs, clinical status.
2. Metabolic: 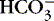 >26 mEq/L and pH >7.45
>26 mEq/L and pH >7.45
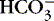 >26 mEq/L and pH >7.45
>26 mEq/L and pH >7.45
a. Results from excessive loss of acid (H +) or accumulation of bases
b. Respirations normal, though may be shallow as compensatory means
c. Clinical causes
(1) Excessive loss of gastric acid from upper GI tract, or insufficient replacement
(a) Protracted vomiting
(b) Gastric suction
(c) Gastric lavage
(2) Excessive circulating 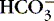
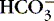
(a) Chemical response relative to chloride loss
(b) Overcorrection of acidosis with bicarbonate
(c) Overingestion of antacid or baking soda
(d) Overinfusion of lactated solution
(3) Overretention of base ions
(a) Diuretics
(i) Furosemide (Lasix)
(ii) Thiazides
(b) Excessive administration of corticosteroids
(4) Systemic diseases: Cushing’s syndrome, aldosteronism
d. Therapeutic interventions to correct
(1) Treat or eliminate cause.
(2) Monitor lab values, particularly hypokalemia as K + moves to cell.
(3) Observe clinical status, reporting confusion, muscle cramps, twitching, tingling.
IX. MIXED ACID-BASE IMBALANCES
A. Inadequate compensation: several concurrent acid-base disorders
1. For example, if pH <7.35 (acidosis), Pa co 2 − 55 mm Hg (respiratory acidosis), and 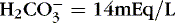 (metabolic acidosis), then have a mixed acidosis
(metabolic acidosis), then have a mixed acidosis
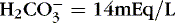 (metabolic acidosis), then have a mixed acidosis
(metabolic acidosis), then have a mixed acidosis
a. Could occur in patient with chronic lung disease (chronic respiratory acidosis, usually compensated) who develops diarrhea with large 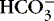 losses
losses
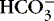 losses
lossesB. pH change is dramatic with mixed acidosis or mixed alkalosis disorders.
C. pH change less severe if mixed acidosis-alkalosis: opposing disorders balance.
X. PHYSIOLOGICAL COMPENSATION OF ACID-BASE IMBALANCES
A. The body’s natural effort to restore acid-to-base ratio toward 1:20 and pH toward 7.40
1. Compensation occurs when pH is within normal range.
2. Partial compensation results when Pa co 2 or HCO 3− changes but pH changes minimally.
3. Rarely overcompensates
B. Compensation via three mechanisms
1. Cellular acid-base compensation
a. Compensation begins immediately with the accumulation of acid (H +).
(1) H + moves into the cell and intracellular K + moves out of cell; any acidotic state will be accompanied by hyperkalemia.
(2) H + is buffered by intercellular protein.
b. Effective but limited compensation
2. Pulmonary acid-base compensation
a. Compensation begins within minutes with the accumulation of acid (H +).
(1) ↓ pH is monitored by respiratory center in the medulla.
(2) Causes an ↑ in the rate and ↑ depth of ventilation; ↓ CO 2 → normalizing pH
b. Effective but limited compensation
3. Renal acid-base compensation
a. Compensation begins within days with the accumulation of acid (H +).
b. Single mechanism has two effects.
(1) H + excreted into the urine
(2) In the same mechanism causes reabsorption of HCO 3−
c. Effective long-term compensation
C. Compensation for common acid-base derangements
1. Respiratory acidosis
a. [Acute] Immediate rise in serum K + (K +/H + exchange)
b. [Chronic] Occurs slowly in kidneys over days
(1) Excretion of H +: acidic urine results
(2) Reabsorption of HCO 3−
2. Respiratory alkalosis
a. Immediate decline in serum K + as K + enters cell in exchange for H + (K +/H + exchange)
b. [Chronic; as seen in pregnancy] Occurs slowly in kidneys over days
(1) ↓ in renal excretion of H +: ↓ acidity of urine
(2) ↓ in the reabsorption of HCO 3−
3. Metabolic acidosis
a. Immediate rise in serum K + (K +/H + exchange)
b. Within minutes, ↑ rate and ↑ depth of breathing (hyperventilation) to eliminate CO 2
c. Occurs slowly in kidneys over days
(1) Excretion of H +: acidic urine results
(2) Reabsorption of HCO 3−
4. Metabolic alkalosis
a. Immediate decline in serum K + as K + enters cell in exchange for H + (K +/H + exchange)
b. Within minutes, ↓ rate and ↓ depth of breathing (hypoventilation) to retain CO 2
c. Occurs slowly in kidneys over days
(1) excretion of H +: alkalotic urine results
(2) ↓ reabsorption of HCO 3−
XI. INTERPRETING ABGs
A. Purpose for measuring ABGs
1. Determine status of alveolar ventilation and arterial oxygenation.
a. Determine acid-base status of patient.
b. Guide respiratory and metabolic interventions.
c. Must interpret in the context of the patient’s clinical status.
B. Systematic ABG analysis: name the disorder.
1. Consider pH, the acidosis/alkalosis component—normal: 7.35 to 7.45.
a. If <7.35 (low), condition is acidosis.
b. If >7.45 (high), condition is alkalosis.
c. If in normal range, condition is “normal pH,” and patient either has normal acid-base balance or acidosis or alkalosis is compensated.
2. Next, consider P co 2, the respiratory component—normal: 35 to 45 mm Hg.
a. If <35 mm Hg (↓) and pH ↑, condition is respiratory alkalosis.
b. If >45 mm Hg (↑) and pH ↓, condition is respiratory acidosis.
c. If normal, move on to consider 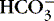 as cause of high or low pH.
as cause of high or low pH.
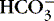 as cause of high or low pH.
as cause of high or low pH.3. Then, consider HCO 3−, the metabolic component—normal: 22 to 26 mEq/L.
a. If <22 mEq/L (↓) and pH ↓, condition is metabolic acidosis.
b. If >26 mEq/L (↑) and pH ↑, condition is metabolic alkalosis.
4. Consider P o 2: Is patient hypoxic? Normal: 80 to 100 mm Hg
a. If <80 mm Hg: Stimulate patient to increase respiratory effort, treat airway obstructon, pulmonary congestion, obstruction or bronchospasm, or measure hemoglobin level.
b. If >100 mm Hg: Monitor status.
c. If >150 mm Hg: Adjust oxygen delivery.
d. Is percent saturation >95%? Verify respiratory quality and adequacy of circulating hemoglobin to transport oxygen.
e. Remember that hypoxemia contributes to acidosis.
5. Determine abnormality and determine whether acute (primary abnormality) or compensated.
a. Assess pH to identify the trend.
b. If pH within normal range but not exactly 7.40
(1) If pH is 7.35 to 7.39, leans toward acidosis
(2) If pH is 7.41 to 7.45, leans toward alkalosis
c. Determine processes P co 2 and 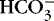 as in steps 2 and 3 above.
as in steps 2 and 3 above.
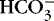 as in steps 2 and 3 above.
as in steps 2 and 3 above.
(1) Primary process: signified by component that supports leaning tendency of pH
(2) Compensation: signified by component that supports opposite tendency before treatment is initiated
d. Now state your decision based on the ABG facts.
(1) Does decision mesh with the patient’s history or clinical status?
(a) Respiratory acidosis or metabolic acidosis?
(b) Respiratory alkalosis or metabolic alkalosis?
(2) Report ABG results to physician; plan interventions.
BIBLIOGRAPHY
1. Agodoa, L., Acute renal failure in the PACU, J Perianesth Nurs 17 (2002) 377–383.
2. Ard, J.L.; Prough, D.S., Perioperative electrolyte and acid-base abnormalities, In: (Editors: Benumof, J.L.; Saidman, L.G.) Anesthesia and perioperative complicationsed 2 ( 1999)Mosby, St Louis, pp. 503–535.
3. Berry, B.E.; Pinard, A.E., Assessing tissue oxygenation, Crit Care Nurse 22 (2002) 22–40.
4. Boldt, J.; Priebe, H.J., Intravascular volume replacement therapy with synthetic colloids: Is there an influence on renal function?Anesth Analg 96 (2003) 376–382.
5. Chernecky, C.; Butler, S.W.; Graham, P.; et al., Real-world nursing survival guide: Fluids and electrolytes. ( 2002)WB Saunders, Philadelphia.
6. Chernecky, C.; Macklin, D.; Murphy-Ende, K., Saunders nursing survival guide: Fluids and electrolytes. ( 2006)Saunders, St. Louis.
7. Cowling, G.E.; Haas, R.E., Hypotension in the PACU: An algorithmic approach, J Perianesth Nurs 17 (2002) 159–163.
8. Czekaj, L.A., Promoting acid-base balance, In: (Editors: Kinney, M.R.; Brooks-Brunn, J.A.; Molter, N.; et al.) AACN clinical reference for critical care nursinged 4 ( 1998)Mosby, St Louis, pp. 135–145.
9. Drain, C.B.; Odom-Forren, J., Perianesthesia nursing: A critical care approach. ed 5 ( 2009)Saunders, St Louis.
10. Ferrara-Love, R., Immediate postanesthesia care, In: (Editors: Burden, N.; DeFazio Quinn, D.M.; O’Brien, D.) Ambulatory surgical nursinged 2 ( 2000)WB Saunders, Philadelphia, pp. 442–448.
11. Golembiewski, J.A.; O’Brien, D., A systematic approach to the management of postoperative nausea and vomiting, J Perianesth Nurs 17 (2002) 364–376.
12. Goskowicz, R., Complications of blood transfusions, In: (Editors: Benumof, J.L.; Saidman, L.G.) Anesthesia and perioperative complicationsed 2 ( 1999)Mosby, St Louis, pp. 536–574.
13. Grocott, M.P.; Mythen, M.G.; Gan, T.J., Perioperative fluid management and clinical outcomes in adults, Anesth Analg 100 (2005) 1093–1106.
14. Heitz, U.E.; Horne, M.M.; Webber, K.S.; et al., Guide to fluid, electrolyte and acid-base balance. ( 2001)Mosby, St Louis.
15. Josephson, D., Intravenous infusion therapy for nurses: Principles & practice. ed 2 ( 2003)Thomson Delmar Learning, Clifton Park, NY.
16. Matthias, J.; Chappel, D.; Rehm, M., Clinical update: Perioperative fluid management, Lancet 369 (2007) 1984–1986.
17. Nisanevich, V.; Felsenstein, I.; Almogy, G.; et al., Effect of intraoperative fluid management on outcome after intraabdominal surgery, Anesthesiology 103 (2005) 25–32.
18. O’Brien, D., Acute postoperative delirium: Definitions, incidence, recognition, and interventions, J Perianesth Nurs 17 (2002) 384–392.
19. O’Flaherty, J.E.; Berry, F.A., Anesthesia complications occurring primarily in the very young, In: (Editors: Benumof, J.L.; Saidman, L.G.) Anesthesia and perioperative complicationsed 2 ( 1999)Mosby, St Louis, pp. 606–625.
20. Roth, S.; Gillesberg, I., Injury to the visual system and other sensory organs, In: (Editors: Benumof, J.L.; Saidman, L.G.) Anesthesia and perioperative complicationsed 2 ( 1999)Mosby, St Louis, pp. 377–408.
21. Simpson, P.J.; Popat, M., Understanding anaesthesia. ed 4 ( 2002)Butterworth/Heinemann, Boston.