History
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
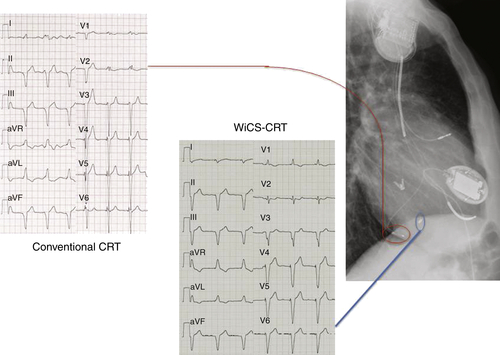
Electrocardiogram
Findings
Chest Radiograph
Findings
Echocardiogram
Findings
Focused Clinical Questions and Discussion Points
Question
Discussion
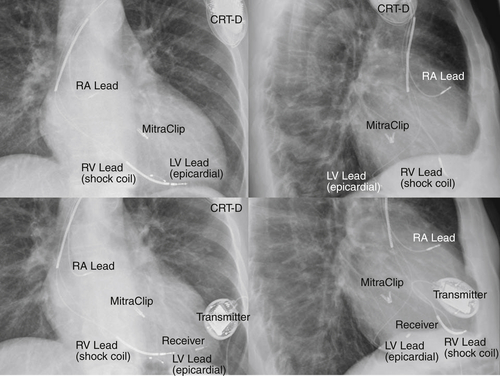
FIGURE 20-2
Question
Discussion
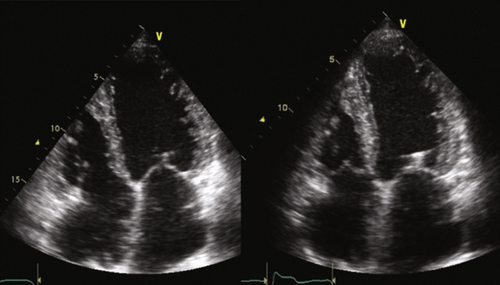
FIGURE 20-3 See expertconsult.com for video. ![]()
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
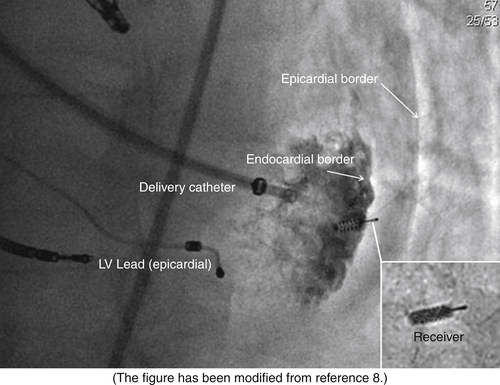
FIGURE 20-4
Outcome
Findings
Comments
Wireless Cardiac Stimulation Technology For Cardiac Resynchronization Therapy
Selected References
1. Van Deursen C., Van Geldrop I., Van Hunnik A. et al. Improved myocardial repolarization and left ventricular systolic and diastolic function during endocardial cardiac resynchronization. Heart Rhythm. 2008;5:S188.
2. Van Deursen C., van Geldorp I.E., Rademakers L.M. et al. Left ventricular endocardial pacing improves resynchronization therapy in canine left bundle-branch hearts. Circ Arrhythm Electrophysiol. 2009;2:580–587.
3. Strik M., Rademakers L.M., van Deursen C.J. et al. Circ Arrhythm Electrophysiol. 2012;5:191–200.
4. Garrigue S., Jaïs P., Espil G. et al. Comparison of chronic biventricular pacing between epicardial and endocardial left ventricular stimulation using Doppler tissue imaging in patients with heart failure. Am J Cardiol. 2001;88:858–862.
5. Spragg D.D., Dong J., Fetics B.J. et al. Effective LV endocardial pacing sites for cardiac resynchronization in patients with ischemic cardiomyopathy. Heart Rhythm. 2010;7:S75–S76.
6. Kutyifa V., Merkely B., Szilágyi S. et al. Usefulness of electroanatomical mapping during transseptal endocardial left ventricular lead implantation. Europace. 2012;14:599–604.
7. DeFaria Yeh D., Lonergan K.L. et al. Clinical factors and echocardiographic techniques related to the presence, size, and location of acoustic windows for leadless cardiac pacing. Europace. 2011;13:1760–1765.
8. Auricchio A., Delnoy P.P., Regoli F. et al. First-in-man implantation of leadless ultrasound-based cardiac stimulation pacing system: novel endocardial left ventricular resynchronization therapy in heart failure patients. Europace. 2013;15:1191–1197.