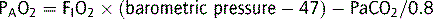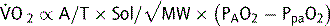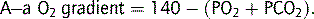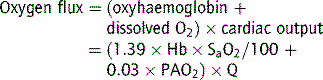Section 2 Critical Care
2.1 Airway and ventilation management
Introduction
Assessment and management of the airway is the first step in the resuscitation of a critically ill patient in the emergency department (ED). Evaluation of the airway commences with a ‘look, listen, feel’ approach to detect partial or complete airway obstruction. If airway compromise is suspected, initial basic airway manoeuvres include the jaw thrust, chin lift and head tilt (providing there is no suspicion of cervical spine injury), and placement of an oropharyngeal airway such as the Guedel (see Chapter 1.1 on Basic Life Support).
Non-invasive ventilation
Many patients in respiratory failure with hypoxaemia and/or hypercapnia may benefit from a trial of non-invasive ventilation.1 The use of NIV involves administration of a controlled mixture of oxygen and air delivered at a set positive pressure via a tightly sealed face mask. The pressure is maintained between 5 and 10 cmH2O during both inspiration and expiration. This continuous positive airways pressure (CPAP) recruits lung alveoli that were previously closed, improving the ventilation/perfusion ratio and helping to correct hypoxaemia. There is also a reduction in the work of breathing as a result of an increase in pulmonary compliance. More recently, NIV machines have become available that administer positive pressure (i.e. 5–20 cmH2O) above the elevated baseline pressure during inspiration, known as bilevel NIV. This additional inspiratory support is thought to further reduce the work of breathing.
Clinical indications for non-invasive ventilation in the ED
Patients who present with severe acute pulmonary oedema (APO) should receive CPAP to improve cardiac and pulmonary function while medical therapy with nitrates and diuretics is initiated.2 However, the use of bilevel NIV in patients with APO gives no additional benefit and may increase the rate of myocardial infarction. On the other hand, patients who present with an exacerbation of chronic obstructive pulmonary disease (COPD) do benefit from bilevel NIV rather than CPAP alone.3
There is also some evidence to support the use of NIV in patients with respiratory failure due to other common ED conditions, such as community-acquired pneumonia4 or asthma.5 Thus it is common ED practice to now administer a trial of NIV in many patients with respiratory failure, prior to instituting ETI and mechanical ventilation. Contraindications to NIV include comatose or combative patients, poor tolerance of a tight-fitting face mask, and the lack of familiarity or lack of trained medical staff to institute and monitor the NIV.
Endotracheal intubation
Rapid sequence induction (RSI) intubation
Intubation process
The conscious patient should receive explanation and reassurance. Pre-oxygenate with 100% oxygen to prevent oxygen desaturation during the procedure. Ideally, administer NIV with 100% oxygen for a 3-minute period.6 If this is not possible, then breathing through a tight-fitting oxygen mask circuit using 15 L/min oxygen flow is an alternative way to pre-oxygenate the patient. Position the patient in the ‘sniffing the morning air’ position with the neck flexed and the head extended, using a pillow under the head. If the patient has suspected spinal column injury, immobilize the neck in the anatomically neutral position. Ensure there is reliable intravenous access, as well as equipment for suctioning the airway and a tipping trolley.
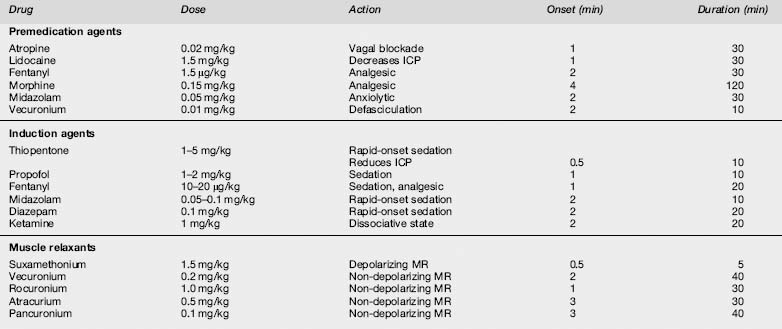
Drugs used in RSI
The drugs required will depend on physician preference and the clinical situation. Common choices for induction include propofol at 1–2 mg/kg,7 a narcotic such as morphine 0.15 mg/kg with a benzodiazepine such as midazolam 0.05–0.1 mg/kg, followed by a rapid-onset depolarizing neuromuscular blocking drug such as suxamethonium 1.5 mg/kg (Table 2.1.1). An alternative when suxamethonium is contraindicated is the rapid acting non-depolarizing drug rocuronium 1 mg/kg. Contraindications to suxamethonium include known allergy, hyperkalaemia or risk of from burns, spinal cord injury or crush injury (not in the acute setting), and a history of malignant hyperthermia (rare).8 Details of the indications, dosages and side effects of all the commonly used drugs for RSI intubation are shown in Table 2.1.1.
Preparation of equipment and personnel prior to RSI
All drugs must be drawn up and checked in advance, and the syringes clearly labelled. A spare laryngoscope must be available in case of failure of the first, and the appropriate size of endotracheal tube (ETT) opened, lubricated and the cuff checked. Another ETT (one size smaller) should be immediately available. Finally, an introducer and a gum-elastic or plastic bougie must be ready to hand.
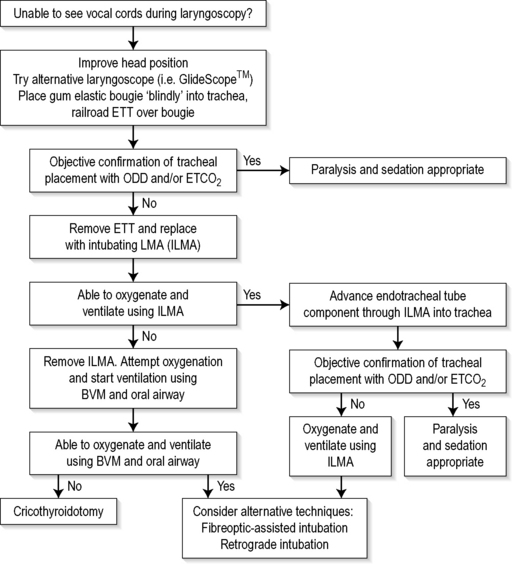
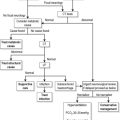
At least two assistants will be required, one to assist the operator with the drugs and equipment, and another to provide cricoid pressure following the induction of sedation and muscle relaxation.9 An additional person is required to provide in-line manual immobilization in the case of RSI for the trauma patient with possible spinal column injury. Additional equipment in case of difficult or failed intubation should be readily available, ideally kept together in a ‘Difficult Airway Kit’ containing the items necessary for a failed intubation protocol (Fig. 2.1.1).
Endotracheal tube insertion
When all preparations are complete, including pre-oxygenation, give the sedative drugs and, as consciousness is lost, the muscle relaxant (usually suxamethonium), with gentle cricoid pressure applied via the cricoid ring cartilage. Following fasciculations and the loss of muscle tone, firm cricoid pressure is applied and the laryngoscopy performed. If the larynx is sighted, the endotracheal tube is placed through the vocal cords into the trachea, the cuff inflated and the ETT secured with tapes. Cricoid pressure must be maintained until the position of the tube is checked and secured, and the intubator indicates that he or she is happy with this.
Ensuring optimal tracheal position
Clinical methods of ensuring optimal tracheal position include sighting the passage of the ETT through the vocal cords, misting of the ETT during exhalation, and auscultation of breath sounds in the lung fields. However, when visualization of the vocal cords has been difficult, these clinical tests may be misleading and confirmatory tests are essential. The characteristic appearance of waveform capnography is regarded as the gold standard for confirmation of tracheal placement in patients with a palpable pulse.10 However, during cardiac arrest there may be inadequate delivery of carbon dioxide to the lungs and hence a false negative reading. In this setting, the use of an oesophageal detector device (ODD) may be more appropriate.11
Complications of RSI intubation
The following additional measures need to be considered during intubation in patients with severe head injury. An assistant must hold the head in the neutral position as there is the possibility of cervical spine instability, which increases the difficulty of visualizing the larynx. Also, laryngoscopy may raise intracranial pressure, although the benefit of pretreatment with lignocaine (lidocaine) 1.5 mg/kg is uncertain.12 Thiopentone or propofol must be used cautiously in patients with shock, or with severe head injury and possible hypovolaemia, as precipitate and prolonged hypotension may occur. Doses as small as one-tenth of normal may be necessary, e.g thiopentone 0.5 mg/kg or propofol 0.2 mg/kg.
The difficult intubation
Endotracheal intubation under direct vision may be easy or difficult, depending on the view of the larynx during laryngoscopy. This view has been classified by Cormack and Lehane13 into grades 1–4.
Cormack and Lehane laryngoscopy view
Difficult intubation may be anticipated in the presence of pathological facial and upper airway disorders that may be congenital or acquired, such as maxillofacial and airway trauma, airway tumours and abscesses, or cervical spine immobility. There may also be anatomical reasons for a Cormack and Lehane grade 3–4 laryngoscopy, such as micrognathia or microstomia, poor mouth opening and/or a large tongue. A range of clinical tests have been proposed that may predict difficulty in visualization of the larynx, including relative size of the tongue to the pharynx, atlanto-occipital joint mobility, and a thyromental distance <6 cm. However, these are not always clinically useful in the emergency setting.14
Failed intubation drill
Attempts at blind placement of the ETT down the trachea when the larynx is not visualized are unlikely to be successful, and repeated attempts may result in direct pharyngeal or laryngeal trauma (making the situation even more difficult) and hypoxaemia. In this situation a failed intubation drill must be initiated.15 A failed intubation algorithm suitable for use in the ED is shown in Figure 2.1.1. Depending on local hospital staffing and resources, an urgent call for assistance from another physician with additional experience should also be made.
Simple initial manoeuvres to improve visualization of the larynx include adding a second pillow to further flex the neck (unless cervical spine injury is suspected), the use of a straight Mackintosh laryngoscope blade, and ‘backward/upward/rightward external pressure’ (BURP) on the thyroid cartilage. A new approach to laryngoscopy using the GlideScope Video Laryngoscope (GVL) (Verathon, Bothell, WA, USA) provides a real-time view of the larynx on a colour monitor, which has been shown in a large case series to convert a Cormack and Lehane grade 3–4 view to a grade 1–2 view 77% of the time.16 In the absence of a GlideScope, and if the larynx still cannot be visualized, blind placement of a gum-elastic bougie and subsequent insertion of the ETT by railroading it over the bougie should be attempted as the preferred next manoeuvre.17 Rotating the ETT through 90° in an anticlockwise direction may be helpful if resistance to its passage occurs at the larynx.
If these initial steps are unsuccessful, adequate oxygenation must be maintained using a bag/mask with an oral airway at all times. Alternative equipment suitable for use in the ED should be prepared.18 A summary of these devices for a failed intubation drill is given below (see Fig. 2.1.1). However, if oxygenation can not be maintained during the attempted use of these devices, immediate cricothyroidotomy is indicated. Make sure additional help has also been summoned.
Laryngeal mask airway
The laryngeal mask airway (LMA) is now used routinely for airway management during elective general anaesthesia. During a failed intubation drill, the LMA may be superior to a bag/mask and oral airway for oxygenation and ventilation.15,17 However, the LMA has had a limited role in the ED, for two reasons. First, if pulmonary compliance is low or airway resistance is high, there will be a leak around the cuff of the LMA when peak inspiratory airway pressures exceed 20–30 mmHg. Second, there is the potential risk of aspiration pneumonitis as the airway remains unprotected. The LMA ProSeal (Vitaid Ltd, Toronto, Ontario, Canada) modification of the standard LMA minimizes this risk, and includes a double cuff to improve the seal and a distal drainage tube to provide access for suctioning the upper oesophagus. The LMA may also be used to assist in orotracheal intubation, using either a 6 mm ETT passed blindly through the LMA, or an ETT placed over a fibreoptic bronchoscope which is then passed through the LMA into the trachea.
Intubating laryngeal mask airway
The ‘intubating LMA’ (ILMA) is a modification of the standard LMA that incorporates a rigid, anatomically curved airway tube with handle, and a special modified endotracheal tube and extender specifically made to pass blindly through the ILMA into the trachea. This appears to have a high success rate even with inexperienced operators, both in managing patients with a difficult airway in hospital19 and in the pre-hospital setting.20 The ILMA is placed and ventilation commenced, and once oxygenation is assured, the ETT component is passed through the ILMA. Once sited, the ETT cuff is inflated and the position confirmed using capnography and then chest X-rays.
Retrograde intubation
When other techniques fail the technique of retrograde intubation may occasionally be used in the ED if time permits.21 The cricothyroid membrane is punctured by a needle/cannula and a guide-wire is passed through the cannula, directed cephalad. The wire is then brought out through the mouth using Magill’s forceps. There are a number of techniques used to then guide the ETT over the wire and back into the larynx, such as a proprietary device (Cook, Cook Medical Inc, Bloomington, IN, USA), or the introducer of a Minitrach II kit (Portex Ltd, Hythe, Kent, UK).22 Alternatively, the wire may be passed inside the end of the ETT and then out through the ‘Murphy eye’. Resistance may be felt when the ETT reaches the larynx, and some anticlockwise rotation may be required to facilitate passage into the larynx. When the level of the cricothyroid is reached, the guide-wire is removed and the ETT passed further down the trachea. The technique of retrograde intubation takes time and experience to perform and is usually unsuitable in a critical airway emergency.
Blind nasotracheal intubation
The head may need to be flexed, extended or rotated to facilitate entry into the larynx, the ETT rotated clockwise through 90°, and/or a suction catheter used to guide the ETT. When the tube passes into the trachea, louder spontaneous respirations heard from the ETT, or the onset of coughing down the tube, confirm successful placement. However, there are significant complications with BNTI, including epistaxis,23 injuries to the turbinates, perforation of the posterior pharynx, laryngospasm and injury to the larynx. In addition, an already jeopardized airway may be made worse, leaving the situation impossible to then control.
Cricothyroidotomy
Techniques for emergency cricothyroidotomy
Surgical cricothyroidotomy
Alternatively, perform a surgical cricothyroidotomy by making a small vertical incision over the cricothyroid membrane. Use artery forceps for blunt dissection to the cricothyroid membrane, which is incised and the cricothyroid membrane opened horizontally with artery forceps. Pass a size 6 mm cuffed ETT or tracheostomy tube through the opening into the trachea, inflate the cuff and commence bag/valve ventilation. This technique is usually faster to perform than a guide-wire technique, although physicians with limited surgical experience may prefer the Seldinger approach.24
Mechanical ventilation
Recommendations for optimal mechanical ventilation
A tidal volume of 10 mL/kg and a respiratory rate of 10–14 breaths per minute are considered safe for most patients. However, patients with acute lung injury may have reduced pulmonary compliance and hence elevated peak inspiratory pressures. These patients should receive a ‘protective lung strategy’.25 This involves limiting the tidal volume to 6 mL/kg, with the respiratory rate setting increased to 16–20 breaths per minute to prevent excessive hypercapnia. Deliberate hyperventilation using a respiratory rate of 16–20 breaths per minute may also be indicated to provide hypocapnia in other situations, such as in patients with severe metabolic acidosis, and in patients with raised intracranial pressure, in whom transient hypocapnia of 30–35 mmHg (4.0–4.7 kPa) may temporarily reduce intracranial pressure while other treatments for intracranial hypertension are being implemented.
Conversely, patients with severe airways obstruction such as asthma or COPD should receive a standard tidal volume of 10 mL/kg, but a decreased respiratory rate from 4 to 8 breaths per minute to allow sufficient time for adequate passive exhalation.26 This reduces the risk of pulmonary hyperinflation, with the development of auto-PEEP leading to hypotension, even electromechanical dissociation. Thus when ventilating a critical asthmatic the PaCO2 level will rise (known as ‘permissive hypercapnia’), with the aim being to initially concentrate only on oxygenation.
Extubation in the emergency department
Prediction of successful extubation
Prediction of successful extubation is problematic in the ICU,27 and there are even fewer published data to guide successful elective ED extubation. In general, patients should be awake, able to follow commands and cough, pass a trial of spontaneous breathing with the ventilator set to a continuous positive airways pressure (CPAP) of 5 cmH2O, with minimal pressure support of 5–10 cmH2O, and who require only modest supplemental oxygen, that is, < 50% inspired oxygen (FiO2 < 0.5). Ideally, the stomach should be emptied via an orogastric or nasogastric tube prior to extubation.
1 Hill NS, Brennan J, Garpestad E, Nava S. Noninvasive ventilation in acute respiratory failure. Critical Care Medicine. 2007;35:2402-2407.
2 Peter JV, Moran JL, Phillips-Hughes J, et al. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367:1155-1163.
3 Ram FS, Lightowler JV, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Systematic Review. 1, 2004. CD004104
4 Keenan SP, Sinuff T, Cook DJ, et al. Does non-invasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic review. Critical Care Medicine. 2004;32:2516-2523.
5 Ram FS, Wellington S, Rowe B, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Systematic Review. 3, 2005. CD004360
6 Baillard C, Fosse JP, Sebbane M, et al. Noninvasive ventilation improves preoxygenation before intubation of hypoxic patients. American Journal of Respiratory and Critical Care Medicine. 2006;174:171-177.
7 Wilbur K, Zed PJ. Is propofol an optimal agent for procedural sedation and rapid sequence intubation in the emergency department? Canadian Journal of Emergency Medicine. 2001;3:302-310.
8 Sluga M, Ummenhofer W, Studer W, et al. Rocuronium versus succinylcholine for rapid sequence induction of anesthesia and endotracheal intubation: a prospective, randomized trial in emergent cases. Anesthesia and Analgesia. 2005;101:1356-1361.
9 Ellis DY, Harris T, Zideman D. Cricoid. Pressure in emergency department rapid sequence tracheal intubations: a risk-benefit analysis. Annals of Emergency Medicine. 2007;50:653-665.
10 Deiorio NM. Continuous end-tidal carbon dioxide monitoring for confirmation of endotracheal tube placement is neither widely available nor consistently applied by emergency physicians. Emergency Medicine Journal. 2005;22:490-493.
11 Schaller RJ, Huff JS, Zahn A. Comparison of a colorimetric end-tidal CO2 detector and an esophageal aspiration device for verifying endotracheal tube placement in the prehospital setting: a six-month experience. Prehospital and Disaster Medicine. 1997;12:57-63.
12 Robinson N, Clancy M. In patients with head injury undergoing rapid sequence intubation, does pretreatment with intravenous lignocaine/lidocaine lead to an improved neurological outcome? A review of the literature. Emergency Medicine Journal. 2001;18:453-457.
13 Cormack RS, Lehane J. Difficult intubation in obstetrics. Anaesthesia. 1984;39:1105-1111.
14 Shiga T, Wajima Z, Inoue T, Sakamoto A. Predicting difficult intubation in apparently normal patients: a meta-analysis of bedside screening test performance. Anesthesiology. 2005;103:429-437.
15 Henderson JJ, Popat MT, Latto IP, et al. Difficult Airway Society. Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anaesthesia. 2004;59:675-694.
16 Cooper RM, Pacey JA, Bishop MJ, McCluskey SA. Early clinical experience with a new videolaryngoscope (GlideScope) in 728 patients. Canadian Journal of Anaesthesia. 2005;52:191-198.
17 Jabre P, Combes X, Leroux B, et al. Use of gum elastic bougie for prehospital difficult intubation. American Journal of Emergency Medicine. 2005;23:552-555.
18 Bair AE, Filbin MR, Kulkarni RG, et al. The failed intubation attempt in the emergency department: analysis of prevalence, rescue techniques, and personnel. Journal of Emergency Medicine. 2002;23:131-140.
19 Ferson DZ, Rosenblatt WH, Johansen MJ, et al. Use of the intubating LMA-Fastrach in 254 patients with difficult-to-manage airways. Anesthesiology. 2001;95:1175-1181.
20 Timmermann A, Russo SG, Rosenblatt WH, et al. Intubating laryngeal mask airway for difficult out-of-hospital airway management: a prospective evaluation. British Journal of Anaesthesia. 2007;99:286-291.
21 Weksler N, Klein M, Weksler D, et al. Retrograde tracheal intubation: beyond fibreoptic endotracheal intubation. Acta Anaesthesiologica Scandinavica. 2004;48:412-416.
22 Slots P, Vegger PB, Bettger H, et al. Retrograde intubation with a Mini-Trach II kit. Acta Anaesthesiologica Scandinavica. 2003;47:274-277.
23 Piepho T, Thierbach A, Werner C. Nasotracheal intubation: look before you leap. British Journal of Anaesthesia. 2005;94:859-860.
24 Sulaiman L, Tighe SQ, Nelson RA. Surgical vs wire-guided cricothyroidotomy: a randomised crossover study of cuffed and uncuffed tracheal tube insertion. Anaesthesia. 2006;61:565-570.
25 Girard TD, Bernard GR. Mechanical ventilation in ARDS: a state-of-the-art review. Chest. 2007;131:921-929.
26 Shapiro JM. Management of respiratory failure in status asthmaticus. American Journal of Respiratory and Critical Care Medicine. 2002;1:409-416.
27 Meade M, Guyatt G, Cook D, et al. Predicting success in weaning from mechanical ventilation. Chest. 2001;120:400S-4424S.
2.2 Oxygen therapy
Introduction
Physiology of oxygen
Oxygen transport chain
Pulmonary gas exchange
Oxygen diffuses across the alveoli and into pulmonary capillaries, and carbon dioxide diffuses in the opposite direction. The process is passive, occurring down concentration gradients. Fick’s law summarizes the process of diffusion of gases through tissues:
where 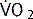 = rate of gas (oxygen) transfer, ∝ = proportional to, A = area of tissue, T = tissue thickness, Sol = solubility of the gas, MW = molecular weight, PA = alveolar partial pressure, and Ppa = pulmonary artery partial pressure.
= rate of gas (oxygen) transfer, ∝ = proportional to, A = area of tissue, T = tissue thickness, Sol = solubility of the gas, MW = molecular weight, PA = alveolar partial pressure, and Ppa = pulmonary artery partial pressure.
Oxygen carriage in the blood
Three steps are required to deliver oxygen to the periphery:
The haemoglobin–oxygen (Hb–O2) dissociation curve
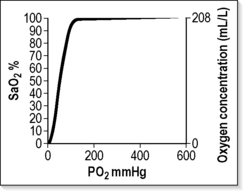
The haemoglobin-oxygen (Hb–O2) dissociation curve is depicted in Figure 2.2.1, which also summarizes the factors that influence the position of the curve. If the curve is shifted to the left, this favours the affinity of haemoglobin for oxygen. These conditions are encountered when deoxygenated blood returns to the lung. A shift of the curve to the right favours unloading of oxygen and subsequent delivery to the tissues.
A number of advantages are conferred by the shape of the Hb–O2 dissociation curve that favour uptake of oxygen in the lung and delivery to the tissues:1
Oxygen flux
The total amount of oxygen delivered to the body per minute is known as oxygen flux.1
where Hb = haemoglobin concentration g/L; SaO2 = arterial oxygen saturation (percentage); PaO2 = partial pressure of arterial oxygen (mmHg); Q = cardiac output (L/min).
A healthy individual breathing air transports approximately 1000 mL oxygen per minute to the tissues, with a cardiac output of 5 L/min; 30% or 300 mL/min of this oxygen is not available, because at least 2.7 kPa (20 mmHg) driving pressure is required to allow oxygen to enter the mitochondria, and therefore approximately 700 mL/minute are available for use by peripheral tissues. This provides a considerable reserve above the 250 mL/min consumed by a healthy resting adult.
General principles
The oxygen delivery systems available for use in emergency medicine are broadly summarized in Table 2.2.1. They can be further subdivided according to economy of oxygen use and whether or not the system can be used to ventilate the patient manually. Figures depicting the various systems have been published elsewhere.3
Variable performance systems
The FIO2 delivered by these systems is summarized in Table 2.2.2. Options available for use in emergency medicine include:
| Apparatus | Oxygen flow (L/min) | Oxygen concentration (%) |
|---|---|---|
| Nasal catheters | 1–4 | 24–40 |
| Semi-rigid mask | 6–15 | 35–60 |
| Semi-rigid mask + double O2 supply | 15–30 | Up to 80 |
| Semi-rigid mask + reservoir bag | 12–15 | 60–90 |
Nasal cannulae
The system must be used at flow rates 4 L/min or less to avoid painful drying of the nasal mucosa, although a flow rate of 2 L/min or less is insufficient to create a nasopharyngeal reservoir during the expiration pause, for inspiration with the next breath.
The inspired oxygen concentration is a function of the patient’s inspiratory flow rate, and is usually in the vicinity of 22–28%. At flow rates of 2–4 L/min the nasopharynx acts as a partial reservoir during the expiratory pause, resulting in an increased FIO2. The delivered FIO2 is then influenced by the pattern of breathing (mouth or nose) and the positioning of the nasal cannula.4
Face masks (e.g. Hudson, Edinburgh, Medishield)
Using a source oxygen supply of 15 L/min, the maximum FIO2 delivered via a Hudson mask to a quietly breathing adult is 0.6.3 By attaching another source of oxygen using a ‘T’ piece or ‘Y’ connector, the resultant flow rate of 30 L/min can deliver an FIO2 up to 0.8. With even greater flow rates the mask may be converted into a fixed-performance system delivering an FIO2 of almost 1.0. Then the ability to deliver 100% oxygen is limited by the mask’s ‘fit’.
Fixed-performance systems
Two systems are available for use in emergency departments:
High-flow Venturi mask
Oxygen flow through a Venturi system results in air entrainment with delivery of a fixed concentration of oxygen to the patient. The masks deliver FIO2 values from 0.24, 0.28, 0.35, 0.40 and 0.50 to 0.60, using different colour-coded adaptors, or by varying the position of a dial on the mask. Many studies have assessed their accuracy.5–8 It is generally considered that the patient receives the stated FIO2 provided the total flow rate exceeds 60 L/min or is 30% higher than the patient’s PIFR.9,10 As the patient’s PIFR increases, the system’s performance becomes variable.
In supplying an FIO2 of 0.24 using 6 L/min flow rate, the total flow rate delivered to the patient is 120 L/min. This falls to 30 L/min total flow for FIO2 = 0.6 using 15 L/min oxygen supply.3 This is just equal to the PIFR of a quietly breathing adult, and unlikely to be sufficient to provide consistent performance in delivery of the stated FIO2. In severe dyspnoea these masks may not deliver the stated FIO2.6 Increasing the oxygen flow rate above the manufacturer’s recommendations will increase the total gas flow to the mask, while maintaining the stipulated FIO2.8 At very high flow rates, however, turbulence is likely to reduce the performance of the system.
Venturi masks provide the best means of managing patients with chronic obstructive airways disease in the ED, because they provide a predictable FIO2 and the air entrained is more humid than fresh oxygen (see below). The entrained gas mixture can be further heated and humidified to assist with sputum clearance. High gas flows minimize rebreathing of CO2 and claustrophobia, but cause problems with sleeping due to noise.
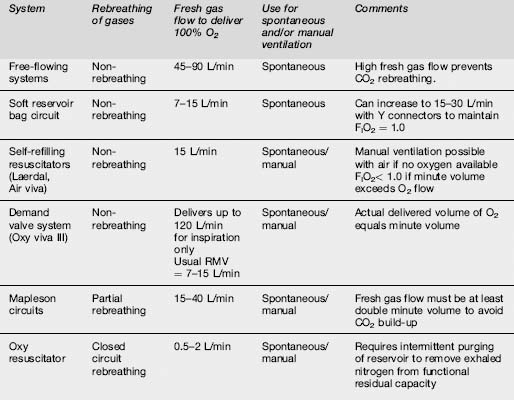
100% oxygen delivery systems
These systems vary in their economy of oxygen use, and are summarized in Table 2.2.3. The least economical is the free-flowing system, because it can only deliver 100% oxygen if the flow rate exceeds the patient’s PIFR. Incorporating a reservoir and unidirectional valves into the circuit enables greater economy of oxygen use by storing oxygen during expiration ready for the inspiratory phase.
Classification
One hundred per cent oxygen-delivery systems available for use in emergency medicine are summarized in Table 2.2.3.
Self-refilling, non-rebreathing resuscitators (Air viva and Laerdal systems)
Most Australasian emergency departments possess at least one type of these self-refilling systems. They can be used to ventilate patients manually as well as allowing spontaneous ventilation. The Laerdal system has three sizes for adults, children and infants, whereas the Air viva system has one size for adults only (Table 2.2.4).
| Self-refilling bag volume (mL) | Reservoir bag volume | |
|---|---|---|
| Air viva | 1700 | 2300 |
| Laerdal (Adult) | 1600 | 2600 |
| Laerdal (Child) | 500 | 2600 |
| Laerdal (Infant) | 240 | 600 |
Advantages
Oxygen-powered resuscitators
Advantages
Disadvantages
Mapleson circuits
A detailed description of these circuits has already been published.11 Partial rebreathing of gases occurs with all of the circuits, and CO2 retention can be avoided if fresh gas flow exceeds minute volume by a ratio of 2–2.5:1.
Advantages of the Mapleson B circuit
Closed-circuit systems
Advantages
Helium and oxygen mixtures
Helium (He2, MW = 4) is much lighter than nitrogen and therefore significantly lowers the density of the gas mix when combined with oxygen in the range of FIO2 = 0.2–0.4. This advantage is lost when FIO2 > 0.4. Despite lower density, the viscosity of Heliox is not significantly lower than that of air. Its main theoretical advantage is if there is turbulent gas flow that is density dependent. This may occur with COPD, where there is a combination of small and medium airways disease. Early studies also suggested that Heliox may enhance nebulizer particles in the lung; however, greater flow rates may be required to drive the nebulizer.12 Despite the potential advantages, the clinical evidence for use in COPD is not strong.
Two Cochrane reviews of the topic concluded that there is insufficient evidence to support the routine use of Heliox to treat COPD exacerbations, or exacerbations of asthma.13,14 However, the review of adults and children with asthma did conclude that Heliox may improve pulmonary function when there is more severe obstruction. Most of the studies of Heliox for asthma have assessed it as a driver of nebulizer therapy, rather than for continuous administration. Of the two studies of Heliox therapy for COPD assessed in the Cochrane review, only one study included acutely decompensated patients in the ED.15 This study failed to show a benefit from Heliox when it was used to drive nebulized β-agonist therapy. There is a need for further randomized studies using Heliox in asthma and COPD, both continuously and as a driver for nebulizer therapy, with hard endpoints such as physiological parameters, response to nebulized β agonists, need for non-invasive ventilation or intubation, and admission rates.
Measurement of oxygenation
Clinical assessment of oxygenation is unreliable, and the time-honoured sign of cyanosis varies with the level of haemoglobin, skin pigmentation, perfusion and external light.1,16,17 Arterial blood gases and pulse oximetry provide an objective measurement of oxygenation and enable precise titration of oxygen therapy to the clinical situation.
Pulse oximetry
Pulse oximetry has become the most frequently used indicator of oxygenation in emergency medicine, as it is non-invasive.18,19 It is colloquially known as the ‘fifth vital sign’, and provides continuous real-time assessment of a patient’s oxygenation and response to therapy. It has a proven role in emergency medicine and is an excellent clinical tool, provided the limitations are understood. The principles behind pulse oximetry have been described elsewhere.20,21
A detailed knowledge of the haemoglobin–oxygen dissociation curve is required to interpret pulse oximetry, as well as the factors that influence readings obtained by this equipment. These factors are summarized in Table 2.2.5.
Table 2.2.5 Factors that influence pulse oximetry readings16,17,20,21
| Factor | Cause |
|---|---|
| Signal interference | High-intensity external light source |
| Diathermy | |
| Shivering/movement of digit | |
| Reduced light transmission | Dark coloured nail polish |
| Dirt | |
| (NB: melanin pigment/jaundice have no effect) | |
| Reduction in plethysmographic volume | Peripheral vasoconstriction (shock, hypothermia) |
| Inaccurate readings due to abnormal haemoglobin | COHb causes over-estimation as is not distinguished from O2Hb |
| Methaemoglobin > 10% causes oximeter to read 85% saturation, regardless of O2 saturation | |
| Profound anaemia – insufficient haemoglobin for accurate signal | |
| Falsely low readings | Intravenous dyes with absorption spectra near 660 nm, e.g. methylene blue |
| Stagnation of blood flow |
Paediatric considerations in oxygen therapy
Body size
Peak inspiratory flow rate and respiratory minute volume are lower; hence a given oxygen supply flow rate will produce a higher FIO2 in a child than in an adult. A Hudson mask at 8 L/min may supply a FIO2 of 0.8 in a young child.2 Reservoir bags are not required to deliver FIO2 values near 1.0 to children weighing less than 15 kg because available supply flow rates (maximum 15 L/min) exceed the child’s PIFR.
A smaller Mapleson circuit, the Jackson-Rees (Mapleson F) circuit, is available to ventilate children. It can be used for both spontaneous and manual ventilation. Rebreathing of carbon dioxide does not occur provided fresh gas flow is 2–3 times minute volume, and the bag is separated from the patient by a tube of internal volume greater than the patient’s tidal volume. The overall relationship between fresh gas flow, minute volume and PaCO2 is complex.11 The principal advantages over the Laerdal system are that the operator can observe bag movement in spontaneous respiration, and has a better ‘feel’ for airway obstruction in manual ventilation. However, considerable skill and experience are required to use the system safely.
Transfer of patients on oxygen therapy
Supplemental oxygen therapy is a vital part of transporting the ill patient, and is especially important for air travel, where lower ambient PIO2 may exacerbate hypoxia already present as a result of the patient’s disease process. The partial pressures of oxygen at various altitudes have been summarized elsewhere.22 Patients with decompression illness or arterial gas embolism should not be transported at cabin pressures lower than 101.3 kPa (1 atmosphere absolute, ATA) because lower ambient pressure exacerbates their disease process by increasing bubble size. A number of factors must be considered for successful oxygen therapy during transport of a patient.23
The sizes of oxygen cylinders available in Australasia, their filling pressures and approximate endurances are summarized in Table 2.2.6. The most economical circuit for prolonged transport with FIO2 = 1.0 is a closed circuit with a CO2 absorber, and the least economical is a free-flowing circuit.
Oxygen therapy in specific circumstances
Asthma
Mechanical ventilation in asthma
Mechanical ventilation requires an FIO2 = 1.0, high inspiratory flow rate (100 L/min), low tidal volume (6–8 mL/kg), a prolonged I:E ratio of at least 1:3 and a low ventilation rate (6–10 breaths/min or less), to reduce the risks of progressive dynamic hyperinflation and the development of auto-PEEP (iPEEP) reducing venous return and hence preload, and of barotrauma with the development of a pneumothorax. Permissive hypercapnia is accepted with mechanical ventilation.
Chronic obstructive pulmonary disease
COPD groups
In terms of management, patients with COPD fall into two groups, although this classification is still a source of debate.24–6
Controlled titration of oxygen dose in COPD
One of the foundations of successful management of the cooperative patient with COPD is controlled titration of oxygen dose. Variable-performance oxygen masks do not have a role in the emergency management of COPD. It is reasonable to use a consistent initial approach to oxygen therapy for conscious patients with advanced COPD because at the time of presentation their ventilatory response to CO2 is unknown. In most patients the administration of 24–28% oxygen by Venturi mask will improve their oxygenation, with a target SaO2 of about 88–92%.27
Below 90% saturation the Hb–O2 dissociation curve is steep, and unless a pulmonary shunt is present even these small increments in oxygen will make a positive difference.1 The patient’s response to initial oxygen therapy (FIO2 = 0.24–0.28) will direct further oxygen dose changes and identify any patients not already known to be suffering chronic hypercapnia. A repeat ABG sample should be taken after 10 minutes of breathing FIO2 = 0.24–0.28. The PaCO2 may rise slightly because of the ‘Haldane effect’.1,25 If this rise is excessive (>1–1.3 kPa [8–10 mmHg]), it is consistent with an impaired ventilatory response to CO2. The FIO2 should then be adjusted downwards in steps to achieve a satisfactory pulse oximetry reading that is compatible with acceptable CO2 levels.
Arterial blood gas sampling in COPD
Arterial blood gas (ABG) samples taken during the initial assessment of these patients (breathing air or controlled oxygen) will assist management. If the bicarbonate level is >30 mmol/L or is elevated by more than 4 mmol/L for each 1.3 kPa (10 mmHg) rise in PaCO2 above normal (5.3 kPa, 40 mmHg), this provides strong evidence of chronic hypercapnia, provided there is no other cause of metabolic alkalosis.28
Management considerations in COPD
Non-invasive positive-pressure ventilation is indicated if the patient remains hypoxic, or becomes progressively more hypoxic and the elevation of PaCO2 persists or worsens, or their conscious state deteriorates.27 Intubation and ventilation may be required, but this should be regarded as a last resort. Supplemental oxygen should never be abruptly withdrawn from patients with COPD because a catastrophic fall in PaO2 will occur. All reductions in controlled oxygen dose should be in a stepwise manner.
A pilot study29 showed that the administration of bronchodilators using oxygen-driven nebulizers in the acute management of chronically hypercapnic patients may be safe. Caution is advised, however, because a recent Australian study30 suggested that COPD is still poorly managed in the emergency department with respect to oxygen dose. Interestingly, the authors of that paper offered only limited practical advice on the titrated use of oxygen in the acute management of COPD, and did not differentiate between COPD patients with an acute elevation of CO2 and those with chronic elevation.
Complications of oxygen therapy
These can be classified into three categories:
Carbon-dioxide narcosis
This can be prevented by controlled oxygen therapy titrating the FIO2 against SaO2, arterial blood gases and conscious state (see above). Unconscious patients should be intubated and manually ventilated using high FIO2, preferably 100% oxygen. Patients with deteriorating conscious state and respiration due to CO2 narcosis need to be vigorously stimulated and encouraged to breathe, whilst FIO2 is reduced in a stepwise manner. Oxygen should never be suddenly withdrawn, because this precipitates severe hypoxia. Reversible causes of respiratory failure should be treated, and non-invasive ventilation instituted.27,30
Oxygen toxicity
Oxygen is toxic in high doses and this is a function of PIO2 and duration of exposure. The toxicity is thought to occur by the formation of free radicals and toxic lipid peroxides, inhibition of enzyme systems, and direct toxic effects on cerebral metabolism.31 Toxicity is mainly restricted to the respiratory system and central nervous system (CNS), although it may affect other regions such as the eye. Premature infants develop retrolental fibroplasia after prolonged exposure to high FIO2. CNS oxygen toxicity manifested by neuromuscular irritability and seizures (Paul Bert effect) is restricted to hyperbaric exposures.
Pulmonary oxygen toxicity (Lorraine–Smith effect) is of the greatest relevance to emergency medicine, although exposures of 0.6–1 ATA for more than 24 hours are required to produce it.31 Acute changes such as pulmonary oedema, haemorrhage and proteinaceous exudates are reversible on withdrawal of oxygen. Longer durations of high PIO2 may lead to permanent pulmonary fibrosis and emphysema. Physicians should be alert to acute symptoms of cough, dyspnoea and retrosternal pain, although these are non-specific symptoms of oxygen toxicity. A progressive reduction in vital capacity may be demonstrated. As with all drugs, oxygen dose should be monitored and carefully titrated against SaO2 and clinical effect. However, oxygen therapy should never be withheld acutely because of fear of toxicity.
Special delivery systems
Oxygen humidification
Additional heat is required to provide effective humidification by vaporization of water. Various systems are available to humidify inspired gas, and ideally they should be able to deliver inspired gas to the trachea at 32–36 °C with low resistance and at greater than 90% humidity. These devices should be simple to use, and able to maintain temperature and humidity at varying gas flows and FIO2. There should also be safety alarms monitoring temperature and humidity.28
Continuous positive airways pressure
This topic has been reviewed in detail in the literature.32 Continuous positive airways pressure (CPAP) has a role in the management of pulmonary oedema, pneumonia, bronchiolitis, respiratory tract burns and acute respiratory failure.33–7 Benefit to the patient is achieved as a result of increasing functional residual capacity and reduced pulmonary compliance. Hypoxaemia is reversed by reduction in intrapulmonary shunting, and the work of breathing is reduced.32
Circuit designs for CPAP
Circuit designs usually consist of a reservoir based on the Mapleson D circuit, or a high-flow turbine system.33 Humidification can be added to the system, and is considered essential for long-term use (>6 hours). Use of an oxygen blender enables variable FIO2 to be administered. CPAP has a proven role in the emergency department in the acute management of cardiogenic pulmonary oedema. Reduced requirements for endotracheal intubation have been demonstrated when CPAP is used for severely ill patients.33 Complications of CPAP include aspiration and pulmonary barotrauma. It may elevate intracranial pressure, and precipitate hypotension by reducing venous return to the thorax.
Hyperbaric oxygen treatment
Uses of hyperbaric oxygen
The increased PO2 also creates a greater driving pressure of oxygen into ischaemic tissues in problem wounds, and reduces swelling by vasoconstriction in crush injuries. HBO treatment has a number of benefits in treating gas embolism and decompression illness (DCI). It provides extra oxygen to tissues rendered ischaemic by nitrogen bubbles, and the increased pressure reduces bubble size and enhances nitrogen removal from the body. HBO treatment is also of benefit in anaerobic infections by being bacteriostatic to anaerobes, inhibiting clostridial α toxin, and stimulating host defences via granulocyte function. Recognized indications for acute referral to a hyperbaric facility for HBO treatment are summarized in Table 2.2.731 (see also Chapter 28.3).
Table 2.2.7 Indications for acute treatment with hyperbaric oxygen31
1 West JB. Respiratory physiology – the essentials, 6th edn. Baltimore: Lippincott, Williams & Wilkins, 1999.
2 Oh TE, Duncan AW. Oxygen therapy. Medical Journal of Australia. 1988;149:141-146.
3 Smart DR, Mark PD. Oxygen therapy in emergency medicine. Part 1. Physiology and delivery systems. Emergency Medicine (Fremantle). 1992;4:163-178.
4 Bethune DW, Collins JM. An evaluation of oxygen therapy equipment. Thorax. 1967;22:221-225.
5 Campbell EJM. A method of controlled oxygen administration which reduces the risk of carbon dioxide retention. Lancet. 1960;2:10-11.
6 Hill SL, Barnes PK, Hollway T, Tennant R. Fixed performance oxygen masks: an evaluation. British Medical Journal. 1984;288:1261-1263.
7 Fracchia G, Torda TA. Performance of Venturi oxygen delivery devices. Anaesthesia and Intensive Care. 1980;8:426-430.
8 Friedman SA, Weber B, Briscoe WA, et al. Oxygen therapy. Evaluation of various air-entraining masks. Journal of the American Medical Association. 1974;228:474-478.
9 Goldstein RS, Young J, Rebuck AS. Effect of breathing pattern on oxygen concentration received from standard face masks. Lancet. 1982;27:1188-1190.
10 Woolner DF, Larkin J. An analysis of the performance of a variable Venturi-type oxygen mask. Anaesthesia and Intensive Care. 1980;8:44-51.
11 Dorsch JA, Dorsch SE. The breathing system. II. The Mapleson systems. In Dorsch JA, Dorsch SE, editors: Understanding anaesthesia equipment. Construction, care and complications, 2nd edn, Baltimore: Williams & Wilkins, 1984.
12 Hess DR. Heliox and non-invasive positive-pressure ventilations: a role for Heliox in exacerbations of chronic obstructive pulmonary disease? Respiratory Care. 1999;51:640-650.
13 Rodrigo G, Pollack C, Rodrigo C, et al. Heliox for nonintubated acute asthma patients. Cochrane Database of Systematic Reviews. (Issue 4):2006. Art No.: CD002884. DOI: 10.1002/14651858. CD002884.pub2
14 Rodrigo G, Pollack C, Rodrigo C, et al. Heliox for treatment of exacerbations of chronic pulmonary disease. Cochrane Database of Systematic Reviews. (Issue 1):2007. Art No.: CD003571. DOI: 10.1002/14651858. CD003571
15 deBoisblanc BP, DeBleiux P, Resweber S, et al. Randomized trial of the use of heliox as a driving gas for updraft nebulization of bronchodilators in the emergency treatment of acute exacerbations of chronic obstructive pulmonary disease. Critical Care Medicine. 2000;28:3177-3180.
16 Morgan-Hughes JO. Lighting and cyanosis. British Journal of Anaesthesia. 1968;40:503-507.
17 Hanning CD. “He looks a little blue down this end”. Monitoring oxygenation during anaesthesia. British Journal of Anaesthesia. 1985;57:359-360.
18 Jones J, Heiselman D, Cannon L, Gradisek R. Continuous emergency department monitoring of arterial saturation in adult patients with respiratory distress. Annals of Emergency Medicine. 1988;17:463-468.
19 Lambert MA, Crinnon J. The role of pulse oximetry in the Accident and Emergency Department. Archives of Emergency Medicine. 1989;6:211-215.
20 Adams AP. Capnography and pulse oximetry. In: Atkinson RS, Adams AP, editors. Recent advances in anaesthesia and analgesia. Edinburgh: Churchill Livingstone; 1989:155-175.
21 Phillips GD, Runciman WB, Ilsley AH. Monitoring in Emergency Medicine. Resuscitation. 1989;18:21-35.
22 Hackett PH, Roach RC, Sutton JR. High altitude medicine. In: Auerbach PS, Geehr EC, editors. Management of wilderness and environmental emergencies. 2nd edn. Missouri: CV Mosby; 1989:1-34.
23 Saunders CE. Aeromedical transport. In: Auerbach PS, Geehr EC, editors. Management of wilderness and environmental emergencies. 2nd edn. Missouri: CV Mosby; 1989:359-388.
24 Stradling JR. Hypercapnia during oxygen therapy in airways obstruction: a reappraisal. Thorax. 1986;41:897-902.
25 Aubier M, Murciano D, Milic-Emili J, et al. Effects of the administration of oxygen on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. American Review of Respiratory Disease. 1980;122:747-754.
26 Sassoon CSH, Hassell KT, Mahutte CK. Hyperoxic induced hypercapnia in stable chronic obstructive pulmonary disease. American Review of Respiratory Disease. 1987;135:907-911.
27 McKenzie DK, Frith PA, Burdon JGW, et al. The COPDX Plan: Australian and New Zealand Guidelines for the management of chronic obstructive pulmonary disease 2003. Medical Journal of Australia. 2003;178:S1-S39.
28 Bersten A, Soni M. Oh’s intensive care manual, 5th edn. Oxford: Butterworth–Heinemann, 2003.
29 Cameron P, Coleridge J, Epstein J, Teichtahl H. The safety of oxygen driven nebulisers in patients with chronic hypoxaemia and hypercapnia. Emergency Medicine (Fremantle). 1992;4:159-162.
30 Joosten SA, Xiaoning Bu, Smallwood D, et al. The effects of oxygen therapy in patients presenting to an emergency department with exacerbation of chronic obstructive pulmonary disease. Medical Journal of Australia. 2007;186:235-238.
31 Feldmeier JJ. Indications and results. In The Hyperbaric Oxygen Therapy Committee Report. Kensington: Maryland USA Undersea and Hyperbaric Medicine Society; 2003.
32 Duncan AW, Oh TE, Hillman DR. PEEP and CPAP. Anaesthesia and Intensive Care. 1986;14:236-250.
33 Bersten AD, Holt AW, Vedig AE, et al. Treatment of severe cardiogenic pulmonary oedema with continuous positive airway pressure delivered by face mask. New England Journal of Medicine. 1991;325:1825-1830.
34 Taylor GJ, Brenner W, Summer WR. Severe viral pneumonia in young adults. Therapy with continuous airway pressure. Chest. 1976;69:722-728.
35 Beasley JM, Jones SEF. Continuous positive airways pressure in bronchiolitis. British Medical Journal. 1981;283:1506-1508.
36 Venus B, Matsuda T, Copiozo JB, et al. Prophylactic intubation and continuous positive airways pressure in the management of inhalation injury in burn victims. Critical Care Medicine. 1981;9:519-523.
37 Katz JA, Marks JD. Inspiratory work with and without continuous positive airway pressure in patients with acute respiratory failure. Anaesthesiology. 1985;63:598-607.
2.3 Haemodynamic monitoring
Introduction
Haemodynamics is concerned with the physiology of blood flow and the forces involved within the circulation.1Haemodynamic monitoring involves studying this complex physiology using various forms of technology to understand these forces and put them into a clinical context that can be used to direct therapy.2 The utility of basic monitoring is universally accepted. However, the maxim that ‘not everything that counts can be counted and not everything that can be counted counts’ (Albert Einstein, 1879–1955) should be borne in mind.3 This is particularly salient in the emergency department (ED), where the pressure of work and the diversity of patients do not allow the unlimited use of complex and expensive monitoring systems.
Historical background
Intensive care as a medical/nursing specialty evolved in tandem with the electronic revolution of the 1960s.4 At the same time, increasingly sophisticated haemodynamic and laboratory techniques vastly improved diagnosis, and provided a way to further evaluate therapy. Despite these major advances in the ability to monitor multiple physiological variables, there is little evidence to suggest that they have resulted in tangible improvements in patient outcome.5
The practical use of any monitoring device must be appropriate to the individual clinical environment. Thus, it may be reasonable to insert a pulmonary artery catheter in the intensive care unit (ICU) where the necessary time can be taken, yet impractical and potentially unsafe in a busy ED.2 Another consideration is that haemodynamic monitoring should only be used when the clinical outcome can be influenced and potentially improved. Once irreversible cellular damage has occurred, current evidence suggests that no benefit will occur no matter how far therapy is maximized.6 Further, haemodynamic monitoring may not improve patient outcome unless linked to a clinical protocol or ‘goal-directed therapy’.7- Improvements in morbidity and mortality have been shown when such protocols are utilized.6,10,11
Clinicians should only introduce monitoring equipment that will have a direct influence on their choice of therapy, as the use of invasive monitoring carries potential risks of harm to the patient. The injudicious use of physiologically based treatment protocols has been shown to cause harm and lead to worse outcomes.12 All monitored variables must be evaluated and applied in a manner proven to lead to benefit, in terms of both the diagnosis and the management.13
Overview of cardiovascular physiology
The circulatory model
Haemodynamic data are traditionally considered in the context of a circulatory model. This model varies, but usually consists of a non-pulsatile pump, and a hydraulic circuit with discrete sites of flow resistance, alongside the Frank–Starling mechanism with its concepts of preload, contractility and afterload.14
Cardiac output
Cardiac output (CO) is the volume of blood pumped by the heart per unit of time, usually expressed in litres per minute (L/min).15 The heart operates as a pump and ejects a bolus of blood known as the stroke volume (SV) with each cardiac cycle. CO is the product of SV and heart rate (HR).
A complex set of interrelated physiological variables determines the magnitude of CO, including the volume of blood in the heart (preload), the downstream resistance to the ejection of this blood (afterload) and the contractility of the heart muscle.16 However, it is the metabolic requirements of the body that are the most potent determinant of cardiac output.4
The regulation of CO is therefore complex. A single measurement represents the interaction of many interacting physiological processes. Basal CO is related to body size and varies from 4 to 7 L/min in adults.16 The value can be divided by the body surface area to enable comparison between patients with different body sizes, giving the cardiac index (CI).
Although CO can be measured, this does not mean it should be done routinely. Indeed, misuse of CO data may worsen outcomes.18 The International Consensus Conference on Haemodynamic Monitoring in Shock (2007)17 suggested that monitoring of CO is only of value if it guides therapies to improve outcome.
Role of haemodynamic monitoring in the emergency department
The role of haemodynamic monitoring in the ED is even less well defined. Given the plethora of devices but the lack of a ‘gold standard’, there are insufficient data to recommend any one method over another.17
Recent advances in the management of sepsis include haemodynamic optimization with early goal-directed therapy (EGDT) during the pre-intensive care period, especially in the ED.19 The Surviving Sepsis Campaign guidelines published in 200420 emphasized that resuscitation of a patient with severe sepsis should begin as soon as the diagnosis is made, and should not be delayed until ICU admission. The use of such an approach based on strict treatment protocols has been shown to reduce morbidity and mortality6 (see Chapter 2.5).
Although widely accepted, the application of this strategy in clinical practice is far from common. Obstacles include a lack of skills to perform the initial procedures, and difficulties in providing the required higher level of care due to ED overcrowding.19 However, with a potential stay in the ED of >24 hours,21 and approximately 15% of critical care being provided in this setting,22 it is necessary to address this issue in education terms and by improved use of available haemodynamic monitoring. This will improve the recognition of therapeutic opportunities in the ED that may be missed.19
Clinical assessment
Current guidelines on haemodynamic monitoring recommend frequent measurement of blood pressure and physical examination variables, including signs of hypoperfusion such as reduced urine output and abnormal mental status.17 Clinical examination is ‘low risk’ yet may yield much important information, but the sensitivity and specificity are low, even when individual elements are interpreted in isolation. Also, clinical assessment of the circulatory state can be misleading.23
Clinical assessment still has an important role in the initial assessment of a critically ill patient,24 particularly as the use of some invasive methods leads to poorer outcomes.13
Paradoxically, the development of haemodynamic measuring devices was driven by the poor ability to assess the critically ill patient clinically, 25,26 yet those patients managed simply by clinical assessment may do better than those managed with invasive, complex devices.13
Clinical markers of cardiac output
The underlying issue may not be what a patient’s CO is, but rather whether this CO is effective for that particular patient.27 Trends are more important than specific, single-point values in guiding therapy. An effective CO should need no compensation, and therefore a patient should have warm toes simultaneously with a normal blood pressure and heart rate.28,29 One of the advantages of clinical endpoints is that they remain the same whatever the phase of the illness.27
Clinical endpoints that are important in the management of septic shock were set out by the American College of Critical Care Medicine (ACCM) in 1999,30 and again in 2007 by an International Consensus Conference.17 These are essentially markers of perfusion and include skin temperature, urine output and cerebral function.
Physiological measurements and clinical endpoints should be viewed as complementary. Physiological measurements combined with clinical examination may provide a numeric target for a management strategy.27 Measurements also provide a universal language for information exchange.
Sound clinical evaluation in the ED in terms of markers of effective CO aid the early diagnosis and implementation of EGDT.19 Abnormal findings also suggest the need for more invasive haemodynamic monitoring, and the need to involve the ICU team early in the patient’s management.
Blood pressure monitoring
The pressure under which blood flows is related to the force generated by the heart and the resistance to flow in the arteries.32 Measurement of mean arterial pressure (MAP) is a more reliable measure of blood pressure than either the systolic or diastolic pressures. It is least dependent on the site or method of measurement, least affected by measurement damping, and it determines the actual tissue blood flow.14
Traditionally, low blood pressure was used to reflect shock and haemodynamic instability. This approach is being challenged as more reliance is placed on concepts of global tissue hypoxia, and the measurement of CO and its adequacy.19
Non-invasive blood pressure measurement
Non-invasive blood pressure (NIBP) measurements using a sphygmomanometer and palpation were first proposed in the late 1800s before Korotkoff introduced the auscultatory method in 1905.32 Originally, routine blood pressure measurements were not a regular part of clinical patient assessment. Today, non-invasive or indirect blood pressure measurement is the most common method used in the initial assessment of cardiovascular status.31 Although there are significant differences between direct (i.e. invasive) and indirect measurements,33 non-invasive measurements should rightly form part of every patient’s assessment and management in the ED.17
Non-invasive blood pressure devices
The 95% confidence limits in the normotensive range are ±15 mmHg, but in states of hypotension and hypertension oscillometry tends to respectively over- and underestimate the pressures.14 Complications are unusual, although repeated measurements could cause skin bruising, oedema and even ulceration.
Invasive blood pressure measurement
Arterial cannulation allows continuous blood pressure measurement, beat-to-beat waveform display and repeated blood sampling.14 A cannula inserted into an artery is connected via fluid-filled, non-compliant tubing <1 m in length to a linearly responsive pressure transducer. The system is then zeroed with reference to the phlebostatic axis (the midaxillary line in the fourth intercostal space). Modern transducers are precalibrated, and therefore no further calibration is needed.34
Sites and safety of arterial cannulation
The most common site for cannulation is the radial artery,35 as this is easy to access during placement and subsequent manipulations, the wrist has a dual arterial supply,31 and there is a low complication rate.35–8 Temporary occlusion of the artery may occur, and in a small number of cases this may be permanent.35 Other complications include haematoma formation,39 bleeding,40 cellulitis,41 and those associated with the catheter itself.42 Alternative arterial cannulation sites are femoral, axillary and brachial, but all have similar complications. Arterial cannulation is a safe procedure if the optimal site for insertion is selected carefully for each patient.35 The preference in the ED is for the radial and femoral sites.
Use of invasive blood pressure monitoring
Invasive blood pressure monitoring should be used in all haemodynamically unstable patients and when vasopressor or vasodilator therapy is used.17 Relying on external NIBP monitoring to guide therapy and diagnosis does not provide sufficient diagnostic data, especially in sepsis.6 Additional methods of haemodynamic monitoring may be considered in these patients, with early involvement of the intensive care department.
Other non-invasive monitoring methods for cardiac output
Ultrasonic cardiac output monitor (USCOM)
This device was developed in Australia and introduced for clinical use in 2001. It provides non-invasive transcutaneous measurement of CO based on continuous-wave Doppler ultrasound.43 An ultrasound transducer is used to obtain a Doppler flow profile (velocity–time graph) from either the aortic (suprasternal notch) or the pulmonary (left of sternum, below the second intercostal space) window. The transducer is manipulated to obtain the best flow profile and audible feedback. CO is calculated from the product of the velocity–time integral (vti) and the cross-sectional area of the target valve.44
The device appears to perform well in terms of the time taken to become a competent operator, and the reproducibility of its readings.45,46 It appears to be a rapid and safe measure of CO and may assist in the prompt starting of EGDT by emergency physicians, even during a medical retrieval out of hospital.47 The correlation of USCOM with standard measures of CO, such as by thermodilution using a pulmonary artery catheter, has been reported as good.43Some concerns were raised that reliability is affected by patient pathology and the severity of their illness.46
Oesophageal Doppler
Measurement of CO using various Doppler-based techniques has been extensively studied.48 The main difficulties are an inability to obtain acceptable flow signals with the transthoracic approach, and problems in the measurement of the cross-sectional area using flow.49 The transoesophageal approach has been found to be more reliable than the transthoracic.50
The oesophageal Doppler device requires minimal training, and volume challenge protocols may be developed so that nursing staff can use them at the bedside.14 However, this technique is not well tolerated in awake patients43 and thus has limited application in the ED.
Echocardiography
Echocardiography may be used to determine left ventricular size, thickness and performance.52 Recently, it has also been reported to accurately identify patients who require fluids.53 The use of echocardiography has increased as the technology has improved, and as the utility of non-invasive techniques has become more accepted. There is a move towards training for the majority of intensive care specialists in this technique, and there is no reason why ED physicians should not also learn. Effective treatment decisions can be based on what is seen on the screen, and in subsequent assessment of left ventricular function.51
The major criticisms regarding the use of echocardiography for haemodynamic monitoring is that it cannot be done continuously.51 Other problems include the lack of skilled operators and the need to reassess variables after changes to patient management regimens. Thus, although promising, the usefulness of echocardiography for haemodynamic monitoring in the ED is uncertain at present.
Invasive devices
Central venous pressure monitoring
Central venous access was first performed in Germany in the late 1920s,54 but it was not until the 1950s, with the work of Brannon55 and Zimmerman,56 that the utility of the process was really appreciated. This ultimately led to the development of cardiac angiography, central blood oxygenation determination and pressure recordings.57 The technique, management and clinical relevance of continuous central venous pressure (CVP) monitoring were first described in 1962.58 This first step in bedside invasive cardiac monitoring allowed direct determination of right heart function and assessment of intravascular volume status.
However, correlation with left heart function was found to be unpredictable and unreliable in the critically ill.57 Hence the physiological meaning of the values obtained and their role in patient management are not clear. Problems may result from errors in measurement and failure to correctly understand the physiology involved.59
Central venous access is obtained in the ED by inserting a catheter into a peripheral or central vein. Central venous access is defined by the position of the catheter tip: to be central the tip should be positioned at the junction of the proximal superior vena cava and right atrium.60
There is no ideal insertion site. Selection depends on the experience of the operator, and patient factors such as body habitus, injuries sustained and coagulation profile.57 The main routes used are the internal jugular, subclavian and femoral veins.14
Indications for central venous access
Complications related to insertion can be divided into early and late. The most relevant early complications in the ED setting include pneumothorax, haemothorax, dysrhythmias and injury to surrounding structures, including arterial puncture, and nerve and tracheal injury. Late complications include catheter-related sepsis, superior vena cava erosion with cardiac tamponade, and thrombosis.14
The CVP is often used as a marker of preload and is considered an estimate of right atrial pressure (RAP).2 The normal CVP in the spontaneously breathing supine patient is 0–5 mmHg, whereas 10 mmHg is considered an upper limit of normal in those being mechanically ventilated.14 The CVP also correlates with left ventricular end-diastolic pressure (LVEDP) in a patient with normal heart and lungs. However, in disease states this relationship is frequently abnormal.57 Thus the CVP is only a rough guide to right ventricular preload, with emphasis on dynamic changes rather than absolute values.2,14
The Rivers’ study
The Rivers’ study6 demonstrated that in cases of septic shock, early aggressive resuscitation guided by CVP, MAP and continuous central venous oxygen saturation (ScvO2) monitoring reduced 28-day mortality rates from 46.5% to 30.5%. ScvO2 is measured on blood taken via the central venous catheter and reflects the balance between oxygen delivery and oxygen consumption.19 Normally oxygen extraction is about 25–30% and a ScvO2 >65% reflects an optimal balance.63,64 ScvO2 correlates well with mixed venous saturations (SvO2) obtained via a pulmonary artery catheter.65,66 Current guidelines recommend instituting goal-directed therapy in septic shock, especially when the ScvO2 is below 70%.17 The ScvO2 has also been shown to be significant in postoperative surgical patients in the ICU, with levels <70% being independently associated with a higher rate of complications61 and increased length of hospital stay.62
Continuous measurement of ScvO2 is feasible in the ED setting67 where central venous catheterization is commonly performed, and where the alternative of pulmonary artery insertion is not practical.19
Pulse contour techniques for cardiac output
The use of pulse contour techniques to obtain a continuous CO by analysis of the arterial waveform dates back over 100 years.2 Erlanger and Hooker68 first proposed a correlation between stroke volume and changes in arterial pressure, and suggested there was a correlation between CO and the arterial pulse contour. Advances in computer technology have since led to the development of complex algorithms relating the arterial pulse contour and CO.
PiCCO system of arterial waveform monitoring
The PiCCO system uses pulse contour analysis to provide a continuous display of CO according to a modified version of Wesseling’s algorithm.69,70 The patient requires a central line sited in either the internal jugular, the subclavian or the femoral veins, and an arterial catheter with a thermistor placed in one of the larger arteries, such as the femoral or axillary artery.71 The femoral site is preferable as it requires only one sterile field for both lines, but does require a 50 cm long venous line.
The PiCCO system combines the pulse contour method for continuous CO measurement and a transpulmonary thermodilution technique to offer complete haemodynamic monitoring.72 Transpulmonary thermodilution works on the principle that a known volume of thermal indicator (cold 0.9% NaCl) is injected into a central vein. The injectate rapidly disperses both volumetrically and thermally within the pulmonary and cardiac volumes. This volume of distribution is termed the intrathoracic volume. When the temperature signal reaches the arterial thermistor, a temperature difference is detected and a dissipation curve is generated. The Stewart Hamilton equation is applied to this curve and CO is calculated.
This transpulmonary thermodilution also gives measures of preload in terms of global end-diastolic blood volume (GEDV) as well as intrathoracic blood volume (ITBV).73 The extravascular lung water (EVLW) is also calculated and has been shown to be a sensitive indicator of pulmonary oedema.74 The technique of transpulmonary thermodilution has been compared to pulmonary artery thermodilution and confirmed to be as accurate.75 Following calibration by thermodilution the PiCCO continually quantifies various parameters.71
PiCCO parameters quantified
The last three parameters are relatively new, and the manufacturer has devised decision trees to guide their use in the clinical setting. The ITBV has been found to be a potentially more reliable and superior indicator of cardiac preload than pulmonary artery wedge pressure (PAWP)76 and has also been shown to be helpful in guiding fluid therapy.77
EVLW correlates with extravascular thermal volume in the lungs71 and with mortality. One study found that patients with an EVLW of up to 8–10 mL/kg had a mortality of 25%, which increased significantly to 75% if the EVLW was >10 mL/kg.78 The EVLW may also be used to guide fluid management, especially in those already known to have pulmonary oedema.79,80
The Cardiac Function Index (CFI) aids in evaluation of the contractile state of the heart and hence overall cardiac performance. It is a preload-independent variable and reflects the inotropic state of the heart. The CFI has the potential to become a routine parameter of cardiac performance.71
The main advantage of the PiCCO system is that it is less invasive than a pulmonary artery catheter, requiring only a central line and an arterial line, which most critically ill patients already have. This in turn leads to fewer complications.75 The data collected are also extensive and allow manipulation of haemodynamics using reliable parameters.
There are contraindications to using the PiCCO, for example when access to the femoral artery is restricted, such as in burns. The PiCCO may also give inaccurate thermodilution measurements in the presence of intracardiac shunts, an aortic aneurysm, aortic stenosis, pneumonectomy, and during extracorporeal circulation.71
The use of the PiCCO system in the ED is plausible. The technique is relatively non-invasive and uses access lines that are already used in the management of the critically ill. The device can both aid diagnosis and provide a monitoring tool for clinical decision making regarding fluid replacement.81
Pulmonary artery catheter
The pulmonary artery catheter (PAC) or Swan–Ganz catheter has long been considered the ‘gold standard’ method of monitoring the unstable circulation.2 Since its introduction in the 1970s, it was assumed that the extra information provided improved patient outcomes. However, various observational studies have now shown that its use does not improve outcome and may even be associated with a worse outcome.13 Hence, the use of the PAC without targeting specific endpoints confers no benefit to the patient. Conversely, the insertion of the PAC does not necessarily confer any disadvantage to the patient, except for the time, expertise and skill required to use it competently.7–9
Disadvantages of pulmonary artery catheters
The insertion of a PAC is time-consuming and requires skill and experience. The technique also has complications and the data generated are difficult to interpret.13 Current guidelines recommend that the PAC is not used routinely in the management of shock,17 and therefore its use in the ED should not be considered.
Conclusion
The real challenge in emergency medicine is to select those haemodynamic monitoring methods and technologies that are best suited to the clinical environment, and which are able to positively influence both the diagnosis and the subsequent management to improve patient outcome. Currently, the best approach is to begin with sound clinical assessment, and then to increase the invasiveness of monitoring in tandem with the patient’s suspected diagnosis and response.
Future developments
1 Gattinoni L, Valenza F, Carlesso E. Adequate haemodynamics: a question of time? In: Pinsky MR, Payen D, editors. Functional haemodynamic monitoring. Heidelberg: Springer Verlag; 2005:69-86.
2 Wilson J, Cecconi M, Rhodes A. The use of haemodynamic monitoring to improve patient outcome. In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. Berlin: Springer; 2007:471-478.
3 Young D, Griffiths J. Clinical trials of monitoring in anaesthesia, critical care and acute ward care: a review. British Journal of Anaesthesia. 2006;97:39-45.
4 Darovic GO, Stratton KL. Introduction to the care of critically ill and injured patients. In: Darovic GO, editor. Haemodynamic monitoring, invasive and noninvasive: clinical application. St. Louis: WB Saunders; 2002:3-8.
5 Bellomo R, Pinsky MR. Invasive haemodynamic monitoring. In: Tinker J, Browne D, Sibbald EJ, editors. Critical care: standards, audit and ethics. London: Edward Arnold; 1996:82-105.
6 Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England Journal of Medicine. 2001;345:1368-1377.
7 Rhodes A, Cusack RJ, Newman PJ, et al. A randomized, controlled trial of the pulmonary artery catheter in critically ill patients. Intensive Care Medicine. 2002;28:256-264.
8 Richard G, Warszawski J, Anguel N, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized control trial. Journal of the American Medical Association. 2003;290:2713-2720.
9 Harvey S, Harrison DA, Singer M, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomized controlled trial. Lancet. 2005;366:472-477.
10 Boyd O, Grounds RM, Bennett ED. A randomized clinical trial of the effect of deliberate perioperative increase of oxygen delivery on mortality of high-risk surgical patients. Journal of the American Medical Association. 1993;270:2699-2707.
11 Pearse R, Dawson D, Fawcett J, et al. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomized, controlled trial. Critical Care. 2005;9:R687-693.
12 Dos Santos CC, Slutsky AS. Protective ventilation of patients with acute respiratory distress syndrome. Critical Care. 2004;8:145-147.
13 Connors AF, Speroff T, Dawson NV, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. Journal of the American Medical Association. 1996;276:889-897.
14 Morgan TJ. Haemodynamic monitoring. Bersten AD, Soni N, editors. Oh’s intensive care manual, 10. Butterworth–Heinemann, Oxford, 2003, 79-94. 5th edn
15 Rushmer R. The cardiac output. In: Cardiovascular dynamics. Philadelphia: WB Saunders; 1961.
16 Bowdle TA, Freund PR, Rooke GA. Cardiac output. Spacelab’s Medical, 1993.
17 Antonelli M, Levy M, Andrews PJD, et al. Haemodynamic monitoring in shock and implications for management. International Consensus Conference, Paris, France, April 2006. Intensive Care Medicine. 2007, 575-590. 33
18 Hayes MA, Timmins AC, Yau EH, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. New England Journal of Medicine. 1994;330:1717-1722.
19 Nguyen HB, River EP. The clinical practice of early goal-directed therapy in severe sepsis and septic shock. Advances in Sepsis. 2005;4:126-131.
20 Dellinger RP, Carlet JM, Masur H, et al. Surviving Sepsis Campaign guidelines for the management of severe sepsis and septic shock. Critical Care Medicine. 2004;32:858-872.
21 McCaig LF, Burt CW. National hospital ambulatory medical care survey: 2002 Emergency Department Summary. Advance Data. 2004;340:1-34.
22 Nelson M, Waldrop RD, Jones J, et al. Critical care provided in an urban emergency department. American Journal of Emergency Medicine. 1998;16:56-59.
23 Linton RA, Linton NW, Kelly F. Is the clinical assessment of the circulation reliable in postoperative cardiac surgical patients? Journal of Cardiothoracic and Vascular Anaesthesia. 2002;16:394-400.
24 Stephan F, Flahault A., Dieudonne N, et al. Clinical evaluation of circulating blood volume in critically ill patients. British Journal of Anaesthesia. 2001;86:754-762.
25 Rackow EC, Connors AF. Controversies in pulmonary medicine. Invasive measurements are required for assessing haemodynamic status in critically ill patients. American Review of Respiratory Disease. 1988;138:1070-1072.
26 Eisenberg PR, Jaffe AS, Schuster DP. Clinical evaluation compared to pulmonary artery catheterization in haemodynamic assessment of critically ill patients. Critical Care Medicine. 1984;12:549-553.
27 Palazzo M, Editorial I. Circulating volume and clinical assessment of the circulation. British Journal of Anaesthesia. 2001;86:743-746.
28 Joly HR, Weil MH. Temperature of the great toe as an indication of the severity of shock. Circulation. 1969;39:131-138.
29 Palazzo M, Soni N. Critical care studies: redefining the rules. Lancet. 1998;352:1306-1307.
30 Task Force of the American College of Critical care Medicine SoCCM. Practice parameters for haemodynamic support of sepsis in adult patients with sepsis. Critical Care Medicine. 1999;27:639-660.
31 Nara AR, Burns MP, Downs WG. Blood pressure. Spacelab’s Medical, 1989.
32 Darovic GO. Arterial pressure monitoring. In: Darovic GO, editor. Haemodynamic monitoring, invasive and noninvasive: clinical application. WB Saunders; 2002:133-160.
33 Cohen JN. Blood pressure measurement in shock. Mechanism of inaccuracy in auscultatory and palpatory methods. Journal of American Medical Association. 1997;199:118-122.
34 Gardner R, Hollingsworth K. Optimizing the electrocardiogram and blood pressure monitoring. Critical Care Medicine. 1986;14:651-658.
35 Scheer BV, Perel A, Pfeiffer UJ. Clinical review: Complications and risk factors of peripheral arterial catheters used for haemodynamic monitoring in anaesthesia and intensive care medicine. Critical Care Medicine. 2002;6:198-204.
36 Bedford RF. Long-term radial artery cannulation: effects on subsequent vessel function. Critical Care Medicine. 1978;6:64-67.
37 Weiss BM, Gattiker RI. Complications during and following radial artery cannulation: a prospective study. Intensive Care Medicine. 1986;12:424-428.
38 Sfeir R, Khoury S, Khoury Gh, et al. Ischaemia of the hand after radial artery monitoring. Cardiovascular Surgery. 2003;4:456-458.
39 Davis FM, Stewart JM. Radial artery cannulation: a prospective study in patients undergoing cardiothoracic surgery. British Journal of Anaesthesia. 1980;52:41-47.
40 Soderstrom CA, Wasserman DH, Dunham CM, et al. Superiority of the femoral artery for monitoring. A prospective study. American Journal of Surgery. 1982;144:309-312.
41 Lindsay SL, Kerridge R, Collett B-J. Abscess following cannulation of the radial artery. Anaesthesia. 1987;42:654-657.
42 Tuck M. Arterial catheter failure. Anaesthesia and Intensive Care. 1996;24:119-120.
43 Tan HL, Pinder M, Parsons R, et al. Clinical evaluation of USCOM ultrasonic cardiac output monitor in cardiac surgical patients in intensive care unit. British Journal of Anaesthesia. 2005;94:287-291.
44 USCOM Ltd. Ultrasonic cardiac output monitors. Sydney, NSW: USCOM Ltd, 2005. 2000
45 Dey I, Sprivulis P. Emergency physicians can reliably assess emergency department patient cardiac output using the USCOM continuous wave Doppler cardiac output monitor. Emergency Medicine Australasia. 2005;17:193-199.
46 Steel L, Carroll R, Murgo M, et al. Non invasive ultrasonic cardiac output (USCOM): assessment of ease of use and inter-operator reproducibility. Poster presentation, 31st Australian and New Zealand Annual scientific Meeting on Intensive Care, Hobart, Tasmania, 2006.
47 Knobloch K, Hubrich V, Rohmann P, et al. Feasibility of preclinical cardiac output and systemic vascular resistance in HEMS in thoracic pain – the ultrasonic cardiac output monitor. Air Medical Journal. 2006;25:270-275.
48 Brown JM. Use of echocardiography for haemodynamic monitoring. Critical Care Medicine. 2002;30:1361-1364.
49 Morrow WR, Murphy DJ, Fisher DJ, et al. Continuous wave Doppler cardiac output: use in paediatric patients receiving inotropic support. Pediatric Cardiology. 1988;9:131-136.
50 Vandenbogaerde JF, Scheldewaert RG, Rijckaert DL, et al. Comparison between ultrasonic and thermodilution cardiac output measurements in intensive care patients. Critical Care Medicine. 1986;14:294-297.
51 Viellard-Baron A. The meaning of hemodynamic monitoring in patients with shock: role of echocardiography. In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. Berlin: Springer; 2007:493-500.
52 Darovic GO. Monitoring patients with pulmonary disease. In: Darovic GO, editor. Haemodynamic monitoring, invasive and noninvasive: clinical application. St. Louis: WB Saunders; 2002:421-469.
53 Combes A, Arnoult F, Trouillet JL. Tissue Doppler imaging estimation of pulmonary artery occlusion pressure in ICU patients. Intensive Care Medicine. 2004;30:75-81.
54 Forssmann W. The catheterization of the right side of the heart. Klinische Wochenschrift. 1929;8:2085.
55 Brannon ES, Weens HS, Warren JV. Atrial septal defect. Study of hemodynamics by the technique of right heart catheterization. American Journal of Medical Science. 1945;210:480-492.
56 Zimmerman HA, Scott RW, Becker NO. Catheterization of the left side of the heart in man. Circulation. 1950;1:357-362.
57 Kumar A, Darovic GO. Establishment of central venous access. In: Darovic GO, editor. Haemodynamic monitoring, invasive and noninvasive: clinical application. WB Saunders; 2002:161-175.
58 Wilson JN, Grow JB, Demong CV, et al. Central venous pressure in optimal blood volume maintenance. Archives of Surgery. 1962;85:55-61.
59 Dellinger RP. Central venous pressure: A useful but not simple measurement. Critical Care Medicine. 2006;34:2224-2227.
60 Whiteman ED. Complications associated with the use of central venous access devices. Current Problems in Surgery. 1996;33:331-340.
61 Pearse R, Dawson D, Fawcett J, et al. Changes in central venous saturation after major surgery, and association with outcome. Critical Care. 2005;9:R694-R699.
62 Boyle M, Steel E, Murgo M, et al. Incidence of low ScvO2 after standard post-operative intensive care management. Poster presentation, 27th International Symposium on Intensive Care and Emergency Medicine, Brussels, Belgium, 2007. Critical Care. 2007, S123. 11
63 Orlando R. Continuous monitoring of mixed venous oxygen saturation in septic shock. Journal of Clinical Monitoring. 1987;3:213-214.
64 Krafft P, Stelzer H, Hiemayr M, et al. Mixed venous oxygen saturation in critically ill septic patients. The role of defined events. Chest. 1993;103:900-906.
65 Ladakis C, Myrianthefs P, Karabinis A, et al. Central venous and mixed venous saturation in critically ill patients. Respiration. 2001;68:279-285.
66 Reinhart K, Kuhn HJ, Hartog C, et al. Continuous central venous and pulmonary venous saturation monitoring in critically ill. Intensive Care Medicine. 2004;30:1572-1578.
67 Rivers EP, Anders DS, Powell D. Central venous oxygen saturation monitoring in the critically ill patient. Current Opinion in Critical Care. 2001;7:204-211.
68 Erlanger J, Hooker DR. An experimental study of blood pressure and of pulse pressure in man. Johns Hopkins Hospital Records. 1904;12:145-378.
69 Wesseling KH, Jansen JRC, Settels JJ, et al. Computation of aortic flow from pressure in humans using a nonlinear, three-element model. Journal of Applied Physiology. 1993;74:2566-2573.
70 Rodig G, Prasser C, Keyl C, et al. Continuous cardiac output measurement: pulse contour analysis vs. thermodilution technique in cardiac surgical patients. British Journal of Anaesthesia. 1999;82:525-530.
71 Cottis R, Magee N, Higgins DJ. Haemodynamic monitoring with pulse-induced contour cardiac output (PiCCO) in critical care. Intensive Care Critical Care Nursing. 2003;19:301-307.
72 Belda FJ, Aguilar G, Perel A. Transpulmonary thermodilution for advanced cardiorespiratory monitoring. In: Vincent JL, editor. Yearbook of intensive care and emergency medicine. Berlin: Springer; 2007:501-510.
73 Salukhe TV, Wyncoll DLA. Volumetric haemodynamic monitoring and continuous pulse contour analysis – an untapped resource for coronary and high dependency care units? British Journal of Cardiology. 2002;9:20-25.
74 Schnidt SS, Westhoff TH, Hofmann C, et al. Effect of the venous catheter site on transpulmonary thermodilution measurement variables. Critical Care Medicine. 2007;35:783-786.
75 Sakka SG, Meier Hellmann A, Reinhart K. Assessment of intrathoracic blood volume and extravascular lung water by single transpulmonary thermodilution. Intensive Care Medicine. 2000;26:180-187.
76 Bindels AJGH, Van der Hoeven JG, Graafland AD, et al. Relationship between volume and pressure measurements and stroke volume in critically ill patients. Critical Care. 2000;4:193-199.
77 Buhre W, Weyland A, Buhre K, et al. Effects of the sitting position on the distribution of blood volume in patients undergoing neurosurgical procedures. British Journal of Anaesthesia. 2000;84:354-357.
78 Strum JA. Development and significance of lung water measurement in clinical and experimental practice. In: Lewis FR, Pfeiffer UJ, editors. Practical applications of fibreoptics in critical care monitoring. Berlin: Springer-Verlag; 1990:129-139.
79 Mitchell JP, Schuller D, Calandrino FS, Schuster D. Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. American Review of Respiratory Disease. 1992;145:990-998.
80 Bindels AJGH, Van der Hoevan JG, Meinders AE. Pulmonary artery wedge pressure and extravascular lung water in patients with acute cardiogenic pulmonary oedema requiring pulmonary oedema. American Journal of Cardiology. 1999;84:1158-1163.
81 Hofer CK, Ganter MT, Matter-Ensner S, et al. Volumetric assessment of left heart preload by thermodilution: comparing the PiCCO-VoLEF system with transoesophageal echocardiography. Anaesthesia. 2006;61:316-321.
82 Soller BR, Cingo N, Puyana JC, et al. Simultaneous measurement of hepatic tissue pH, venous oxygen saturation and haemoglobin by near infrared spectroscopy. Shock. 2001;15:106-111.
83 Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Critical Care Medicine. 1999;27:1369-1377.
2.4 Shock overview