Section 13 Haematology
13.1 Anaemia
Introduction
Anaemia is a condition in which the absolute number of red cells in the circulation is abnormally low. The diagnosis is usually made on the basis of the full blood count (FBC). This, together with the blood film, offers qualitative as well as quantitative data on the blood components, and a set of normal values is shown in Table 13.1.1.
| Haemoglobin (Hb) | |
| Males | 13.5–18 g/dL |
| Females | 11.5–16.5 g/dL |
| Red blood cell count | |
| Males | 4500–6500 × 109/L |
| Females | 3900–5600 × 109/L |
| Haematocrit | |
| Males | 42–54% |
| Females | 37–47% |
| MCH | 27–32 pg |
| MCHC | 32–36 g/dL |
| MCV | 76–98 fL |
| Reticulocytes | 0.2–2% |
| White blood cells | 4–11 × 109/L |
| Neutrophils | 1.8–8 × 109/L |
| Eosinophils | 0–0.6 × 109/L |
| Basophils | 0–0.2 × 109/L |
| Lymphocytes | 1–5 × 109/L |
| Monocytes | 0–0.8 × 109/L |
| Platelets | 150–400 × 109/L |
MCH, Hb divided by RBC; MCHC, Hb divided by HCT; MCV, HCT divided by RBC.
Most automated counting machines now give the red cell distribution width (RDW), a measure of degree of variation of cell size.
The overriding functional importance of the red cell resides in its ability to transport oxygen, bound to the haemoglobin molecule, from the lungs to the tissues. Functionally, anaemia may be regarded as an impairment in the supply of oxygen to the tissues and the adverse effects of anaemia, from whatever cause, are a consequence of the resultant tissue hypoxia. Anaemia is not a diagnosis: rather, it is a clinical or a laboratory finding that should prompt the search for an underlying cause (Table 13.1.2).
Table 13.1.2 Causes of anaemia
| Haemorrhage |
| Traumatic |
| Non-traumatic |
| Acute or chronic |
| Production defect |
| Megaloblastic anaemia |
| Vitamin B12 deficiency |
| Folate deficiency |
| Aplastic anaemia |
| Pure red cell aplasia |
| Myelodysplastic syndromes |
| Invasive marrow diseases |
| Chronic renal failure |
| Decreased RBC survival (haemolytic anaemia) |
| Congenital |
| Spherocytosis |
| Elliptocytosis |
| Glucose-6-phosphate-dehydrogenase deficiency |
| Pyruvate kinase deficiency |
| Haemoglobinopathies: sickle cell diseases |
| Acquired autoimmune haemolytic anaemia, warm |
| Acquired autoimmune haemolytic anaemia, cold |
| Microangiopathic haemolytic anaemias |
| RBC mechanical trauma |
| Infections |
| Paroxysmal nocturnal haemoglobinuria |
RBC, red blood cell.
ANAEMIA SECONDARY TO HAEMORRHAGE
Aetiology
By far the most common cause of severe anaemia encountered in the emergency department (ED) is haemorrhage. Therefore, the assessment of the anaemic patient is often chiefly concerned with the search for a site of blood loss. The most common causes of haemorrhage are outlined in Table 13.1.3. However, the emergency physician must remain alert to the possibility that the patient is not bleeding but manifesting a rarer pathological condition.
Table 13.1.3 Common causes of haemorrhage in the emergency department
| Trauma |
| Blunt trauma to mediastinum |
| Pulmonary contusions/haemopneumothorax |
| Intraperitoneal injury |
| Retroperitoneal injury |
| Pelvic disruption |
| Long bone injury |
| Open wounds: inadequate first aid |
| Non-trauma |
| Gastrointestinal haemorrhage |
| Oesophageal varices |
| Peptic ulcer |
| Gastritis/Mallory–Weiss |
| Colonic/rectal bleeding |
| Obstetric/gynaecological bleeding |
| Ruptured ectopic pregnancy |
| Menorrhagia |
| Threatened miscarriage |
| Antepartum haemorrhage |
| Postpartum haemorrhage |
| Other |
| Epistaxis |
| Postoperative |
| Secondary to bleeding diathesis |
Clinical features
In the context of trauma the history often gives clear pointers to both sites and extent of blood loss. Consideration of the mechanism of injury may allow anticipation of occult pelvic, intraperitoneal or retroperitoneal bleeding. Intracranial bleeding is never an explanation for hypovolaemic shock in an adult. In the context of non-trauma it is essential to obtain an obstetric and gynaecological history in women of childbearing age. The remainder of the formal history may supply information essential in determining the aetiology of anaemia. The past medical history may point to a known haematological abnormality or to a chronic disease process. A drug and allergy history is always relevant. Many drugs cause marrow suppression, haemolytic anaemia and bleeding. The family history points to hereditary disease; the social history may alert the clinician to an unusual occupational exposure in the patient’s past or, more likely, to recreational activities liable to exacerbate an ongoing disease process. The systems review is particularly relevant to the consultation with middle-aged or elderly male patients, who must be asked about symptoms of altered bowel habit and weight loss.
Clinical investigations
Red cell morphology, particularly the mean corpuscular volume (MCV), can help elucidate the cause of anaemia. The finding of a pancytopenia suggests a problem in haematopoiesis, rather than haemolysis or blood loss. In women of childbearing age, assay of blood or urine β-HCG is important.
Treatment
The principles of management of haemorrhage are as follows:
The indications for red cell transfusion are discussed in Chapter 13.5. The faster the onset of the anaemia, the greater the need for urgent replacement. Patients who are tolerating their anaemia may require no more than an appropriate diet with or without the addition of haematinics. Elderly patients with severe bleeding often need red cells urgently. Excessive administration of colloid and/or crystalloid precipitates left ventricular failure, and it can then be difficult to administer red cells.
ANAEMIA SECONDARY TO DECREASED RED CELL PRODUCTION
Megaloblastic anaemia
The finding of a raised MCV is common in the presence or absence of anaemia. Alcohol abuse is a frequent underlying cause, and other causes are listed in Table 13.1.4. MCVs greater than 115 fL are usually due to megaloblastic anaemia, which in turn is usually due to either vitamin B12 or folate deficiency. Vitamin B12 and folate are essential to DNA synthesis in all cells. Deficiencies manifest principally in red cell production because of the sheer number of red cells that are produced. B12 deficiency is usually the result of a malabsorption syndrome, whereas folate deficiency is of dietary origin. Tetrahydrofolate is a co-factor in DNA synthesis and, in turn, the formation of tetrahydrofolate from its methylated precursor is B12-dependent. Unabated cytoplasmic production of RNA in the context of impaired DNA synthesis appears to produce the enlarged nucleus and abundant cytoplasm of the megaloblast. These cells, when released to the periphery, have poor function and poor survival.
| Alcohol |
| Drugs |
| Hypothyroidism |
| Liver disease |
| Megaloblastic anaemias (B12 and folate deficiency) |
| Myelodysplasia |
| Pregnancy |
| Reticulocytosis |
The work-up for folate deficiency is similar to that for B12. Occasionally, patients require investigation for a malabsorption syndrome (tropical sprue, coeliac disease), which includes jejunal biopsy. Folate deficiency is common in pregnancy because of the large folate requirements of the growing fetus. It can be difficult to diagnose because of the maternal physiological expansion of plasma volume and also of red cell mass, but diagnosis and treatment with oral folate supplements are important because of the risk of associated neural tube defects.
Other causes of decreased red cell production
The myelodysplastic syndromes are a group of disorders primarily affecting the elderly. In these states there is no reduction in marrow cellularity but the mature red cells, granulocytes and platelets generated from an abnormal clone of stem cells are disordered and dysfunctional. There is peripheral pancytopenia. These disorders are classified according to observed cellular morphology (Table 13.1.5). These conditions were once termed ‘preleukaemia’, and one-third of patients progress to acute myeloid leukaemia.
Table 13.1.5 Classification of the myelodysplastic syndromes
| Refractory anaemia |
| Refractory anaemia with ringed sideroblasts |
| Refractory anaemia with excess of blasts |
| Chronic myelomonocytic leukaemia |
ANAEMIA SECONDARY TO DECREASED RED CELL SURVIVAL: THE HAEMOLYTIC ANAEMIAS
Patients whose main problem is haemolysis are encountered rarely in the ED. The most fulminant haemolytic emergency one could envisage is that following transfusion of ABO-incompatible blood (discussed in Ch. 13.5), a vanishingly rare event where proper procedures are followed. Haemolysis and haemolytic anaemia are occasionally encountered in decompensating patients with multisystem problems. Rarely, first presentations of unusual haematological conditions occur.
Some of the haemolytic anaemias are hereditary conditions in which the inherited disorder is an abnormality intrinsic to the red cell, its membrane, its metabolic pathways or the structure of the haemoglobin contained in the cells. Such red cells are liable to be dysfunctional, and to have increased fragility and a shortened lifespan. Lysis in the circulation may lead to clinical jaundice as bilirubin is formed from the breakdown of haemoglobin. Lysis in the reticuloendothelial system generally does not cause jaundice but may produce splenomegaly. The anaemia tends to be normochromic normocytic; sometimes a mildly raised MCV is due to an appropriate reticulocyte response from a normally functioning marrow. Serum bilirubin may be raised even in the absence of jaundice. Urinary urobilinogen and faecal stercobilinogen are detectable and serum haptoglobin is depleted. The antiglobulin (Coombs’) test is important in the elucidation of some haemolytic anaemias. In this test, red cells coated in vivo (direct test) or in vitro (indirect test) with IgG antibodies are washed to remove unbound antibodies, then incubated with an antihuman globulin reagent. The resultant agglutination is a positive test.
Sickle cell anaemia
Pain relief should commence early. A morphine infusion may be required for patients with severe ongoing pain. Other supportive measures are dictated by the presentation. Intravenous fluids are particularly important for patients with renal involvement. Aim to establish a urine output in excess of 100 mL/h in adults. Antibiotic cover may be required in the case of febrile patients with lung involvement. It may be impossible to differentiate between pulmonary vaso-occlusion and pneumonia. Many patients with sickle cell disease are effectively splenectomized owing to chronic splenic sequestration with infarction, and are prone to infection from encapsulated bacteria. The choice of antibiotic depends on the clinical presentation. Indications for exchange transfusion are shown in Table 13.1.6. The efficacy of exchange transfusion in painful crises remains unproven.
Table 13.1.6 Indications for exchange transfusion in sickle cell crisis
| Neurological presentations: TIAs, stroke, seizures |
| Lung involvement (PaO2 < 65 mmHg with FiO2 60%) |
| Sequestration syndromes |
| Priapism |
TIA, transient ischaemic attack
Haemoglobin S-C disease
Sickle trait or Hb S-C disease occurs in up to 10% in the black population. The clinical presentation resembles that of sickle cell disease but is usually less severe.
Microangiopathic haemolytic anaemia
In this important group of conditions intravascular haemolysis occurs in conjunction with a disorder of microcirculation. Important causes are shown in Table 13.1.7.
| Disseminated intravascular coagulation |
| Haemolytic uraemic syndrome |
| HELLP |
| Malignancy |
| Malignant hypertension |
| Snake envenoming |
| Thrombotic thrombocytopenic purpura |
| Vasculitis |
Haemolytic uraemic syndrome and thrombotic thrombocytopenic purpura
In adults, the presentation is usually one of a neurological disturbance (headache, confusion, obtundation, seizures or focal signs). The blood film reveals anaemia, thrombocytopenia, reticulocytosis and schistocytes. Coombs’ test is negative.
Other causes of haemolysis
Haemolysis may be due to mechanical trauma, as in ‘March haemoglobinuria’. Artificial heart valves can potentially traumatize red cells. Historically, ball-and-cage type valves have been most prone to cause haemolysis, whereas disc valves are more thrombogenic. Improvements in design have made cardiac haemolytic anaemia very rare. Haemolysis is sometimes seen in association with a number of infectious diseases, notably malaria. Other infections that have been implicated are listed in Table 13.1.8. Certain drugs and toxins are associated with haemolytic anaemia (Table 13.1.9). The haemolytic anaemia that is commonly seen in patients with severe burns is attributed to direct damage to the red cells by heat.
| Babesiosis |
| Bartonella |
| Clostridia |
| Cytomegalovirus |
| Coxsackie virus |
| Epstein-Barr virus |
| Haemophilus |
| Herpes simplex |
| HIV |
| Malaria, especially Plasmodium falciparum (Blackwater fever) |
| Measles |
| Mycoplasma |
| Varicella |
Table 13.1.9 Drugs and toxins associated with haemolysis
| Antimalarials |
| Arsine (arsenic hydride) |
| Bites: bees, wasps, spiders, snakes |
| Copper toxicity |
| Dapsone |
| Lead (plumbism) |
| Local anaesthetics: lidocaine, benzocaine |
| Nitrates, nitrites |
| Sulfonamides |
Bain BJ. Morphology in the diagnosis of red cell disorders. Hematology. 2005;10S(1):178-181.
Bayless PA. Selected red cell disorders. Emergency Medicine Clinics of North America. 1993;11(2):481-493.
Bojanowski C. Use of protocols for ED patients with sickle cell anaemia. Journal of Emergency Nursing. 1989;15:83-87.
Brookoff D, Polomano R. Treating sickle cell pain like cancer pain. Annals of Internal Medicine. 1992;116(5):364-368.
Carbrow MB, Wilkins JC. Haematologic emergencies. Management of transfusion reactions and crises in sickle cell disease. Postgraduate Medicine. 1993;93(5):183-190.
Erslev A. Erythropoietin. New England Journal of Medicine. 1991;316:101.
Evans TC, Jehle D. The red blood cell distribution width. Journal of Emergency Medicine. 1991;9(suppl 1):71-74.
Friedman EW, Webber AB, Osborn HH, et al. Oral analgesia for treatment of painful crisis in sickle cell anaemia. Annals of Emergency Medicine. 1986;15:787-791.
Gaillard HM, Hamilton GC. Hemoglobin/hematocrit and other erythrocyte parameters. Emergency Medicine Clinics of North America. 1986;4(1):15-40.
Gregory SA, McKenna R, Sassetti RJ, et al. Hematologic emergencies. Medical Clinics of North America. 1986;70(5):1129-1149.
Losek JD, Hellmich TR, Hoffman GM. Diagnostic value of anemia, red blood cell morphology, and reticulocyte count for sickle cell disease. Annals of Emergency Medicine. 1992;21(8):915-918.
Pollack CV. Emergencies in sickle cell disease. Emergency Medicine Clinics of North America. 1993;11(2):365-378.
Powers RD. Management protocol for sickle-cell disease patients with acute pain: impact of emergency department and narcotic use. American Journal of Emergency Medicine. 1986;4(3):267-268.
Thomas C, Thomas L. Anemia of chronic disease: pathophysiology and laboratory diagnosis. Laboratory Hematology. 2005;11(1):14-23.
13.2 Neutropenia
Introduction
Neutropenia is defined as a decrease in the number of circulating neutrophils. The neutrophil count varies with age, sex and racial grouping. The severity of neutropenia is usually graded as follows:1
Pathophysiology and aetiology
For a previously normal individual to become neutropenic there must be decreased production of neutrophils in the marrow, decreased survival of mature neutrophils or a redistribution of neutrophils from the circulating pool. The important causes are shown in Table 13.2.1.
| Decreased production |
| Aplastic anaemia |
| Leukaemias |
| Lymphomas |
| Metastatic cancer |
| Drug-induced agranulocytosis |
| Megaloblastic anaemias |
It is a defect in neutrophil production that is most likely to prove life threatening. Consumption of neutrophils in the periphery, as occurs early in infectious processes, is likely to be rapidly compensated for by a functioning marrow. Fortunately, most of the primary diseases of haematopoiesis are rare, and in practice many of the acquired neutropenias are drug induced. Processes interfering with haematopoiesis, often involving autoimmune mechanisms, may affect neutrophils both in the marrow and in the periphery. Some drugs cause neutropenia universally but many more reactions are idiosyncratic, be they dose-related or independent of dose. Some commonly implicated drugs are listed in Table 13.2.2. Cancer chemotherapy drugs are now recognized as the commonest cause of neutropenia.
Table 13.2.2 Drugs commonly associated with neutropenia
| Antibiotics: chloramphenicol, sulfonamides, isoniazid, rifampicin, β-lactams, carbenicillin |
| Antidysrhythmic agents: quinidine, procainamide |
| Antiepileptics: phenytoin, carbamazepine |
| Antihypertensives: thiazides, ethacrynic acid, captopril, methyldopa, hydralazine |
| Antithyroid agents |
| Chemotherapeutic agents: especially methotrexate, cytosine arabinoside, 5-azacytidine, azothioprine, doxorubicin, daunorubicin, hydroxyurea, alkylating agents |
| Connective tissue disorder agents: phenylbutazone, penicillamine, gold |
| H2-receptor antagonists |
| Phenothiazines, especially chlorpromazine |
| Miscellaneous: imipramine, allopurinol, clozapine, ticlopidine, tolbutamide |
Clinical features
Neutropenia is frequently anticipated based on the clinical presentation, such as fever developing in the context of cancer chemotherapy, by far the most common scenario in which severe neutropenia is seen in the ED. Alternatively, it may be identified in the course of investigation for a likely infective illness, or it might be an incidental finding during investigation for an unrelated condition.
Chronic neutropenia may be asymptomatic unless secondary or recurrent infections develop. Acute severe neutropenia may present with fever, sore throat, and mucosal ulceration or inflammation.2 Symptoms or signs of an associated disease process may also be present, such as pallor from anaemia, or bleeding from thrombocytopenia, as might occur in conditions causing pancytopenia.
Physical examination may reveal necrotizing mucosal lesions, pallor, petechial rashes, lymphadenopathy, bone tenderness, abnormal tonsillar or respiratory findings, spleno- or other organomegaly.2 Careful examination of the skin of the back, the lower limbs and the perineum for evidence of infection is important. The presence of indwelling venous access devices should be noted and insertion sites inspected for evidence of inflammation or infection.
Treatment
Empiric broad-spectrum antibiotic therapy should be started in the ED after drawing blood for culture in any patient with fever and confirmed significant neutropenia. This strategy has played a pivotal role in reducing mortality rates in febrile neutropenia.3 There is no clear consensus approach to which particular empiric antibiotic regime should be used. Practices vary widely amongst institutions and regions, and are influenced by local patterns of infection, prevalence and risk of inducing resistant organisms, and acquisition costs.4 In general antibiotics should provide good cover for both Gram-positive and Gram-negative organisms. With increased use of indwelling venous access devices for cancer chemotherapy, there has been an increase in the incidence of sepsis due to Gram-positive organisms such as coagulase negative staphylococci, S. aureus and MRSA.5 Although occurring infrequently, bacteraemia due to Pseudomonas aeruginosa is associated with a high morbidity and mortality and therefore should also be covered.6 A reasonable initial regime might include ticarcillin/clavulanate plus either a cephalosporin, such as ceftazidime, or an aminoglycoside, such as gentamicin. The addition of vancomycin might be considered if the patient is in shock, is known to be colonized with MRSA or has clinical evidence of a catheter-related infection in a unit with a high incidence of MRSA. Empiric antifungal therapy is not generally required unless there is persistent fever in high-risk patients beyond 96 h of antibacterial therapy.6
Disposition
The presence of significant neutropenia with fever generally mandates admission to hospital. Patients with severe acute neutropenia without an established aetiology will also generally require admission regardless of the presence or absence of fever. Both the haematological abnormality and the likely presence of infection require investigation. Sometimes the aetiology of the neutropenia will be evident; on other occasions marrow aspiration and biopsy will be required.
There is emerging evidence that a subset of febrile neutropenic patients can be identified who are at low risk of life-threatening complications and in whom duration of hospitalization and intensity of treatment may be safely reduced.7 Strategies that involve outpatient treatment of low-risk patients with oral antibiotics have also been evaluated.3 Such regimens are reliant upon accurate prediction of risk, as well as the availability of structured programmes and resources, and are not yet in widespread use.
Prognosis
The prognosis of the neutropenic patient is largely dependent upon the underlying aetiology of the condition. Febrile neutropenia has in the past been associated with a significant mortality rate which varies depending on the organism causing the infection. Improvements in therapy, such as rapid treatment with empiric broad-spectrum antibiotics, have significantly reduced mortality rates from this condition. Overall mortality rates for patients with febrile neutropenia have reduced from more than 20% to less than 4% in recent data sets.3,7
1 Palmblad J, Papadaki HA, Eliopoulos G. Acute and chronic neutropenias. What’s new? Journal of Internal Medicine. 2001;250:476-491.
2 Dale DC. Neutropenia and neutrophilia. In Lichtman MA, et al, editors: Williams haematology, 7th edn, New York: McGraw-Hill, 2006.
3 Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clinical Infectious Diseases. 2005;40:S240-S245.
4 Glasmacher A, von Lilienfeld-Toal M, Schulte S, et al. An evidence-based evaluation of important aspects of empirical antibiotic therapy in febrile neutropenic patients. Clinical Microbiology and Infection. 2005;11(suppl 5):17-23.
5 Picazo JJ. Management of the febrile neutropenic patient: a consensus conference. Clinical Infectious Diseases. 2004;39:S1-S6.
6 . Severe sepsis: empirical therapy (no obvious source of infection): febrile neutropenic patients. eTG, Therapeutic Guidelines Limited,, 2007.
7 Chisholm JC, Dommett R. The evolution towards ambulatory and day-case management of febrile neutropenia. British Journal of Haematology. 2006;135:3-16.
8 Waladkhani AR. Pegfilgrastim 2004: a recent advance in the prophylaxis of chemotherapy-induced neutropenia. European Journal of Cancer Care. 2004;13:371-379.
9 Bishton M, Chopra R. The role of granulocyte transfusions in neutropenic patients. British Journal of Haematology. 2004;127:501-508.
13.3 Thrombocytopenia
Introduction
Thrombocytopenia is defined as a reduction in the number of circulating platelets, the normal circulating platelet count being 150–400 × 109/L. It is the most common cause of abnormal bleeding.1 Like anaemia, thrombocytopenia itself is not a diagnosis, but rather a manifestation of another underlying disease process.
Aetiology
The clinically important causes of thrombocytopenia are outlined in Table 13.3.1. Diagnoses are classified by pathological process. It should be noted that more than one pathological process may be present.
| Pseudothrombocytopenia |
|---|
| Increased platelet destruction |
|---|
| Decreased platelet production |
|---|
Artifactual thrombocytopenia
Artifactual thrombocytopenia results from an underestimation of the platelet count as measured by an automated particle counter. The most common mechanism is platelet clumping. Clumping is most often due to the anticoagulant EDTA, but may also result from auto-antibodies such as cold agglutinins. The presence of giant platelets and platelet satellitism may also yield falsely low automated platelet counts.2
Immune-related thrombocytopenia
Idiopathic thrombocytopenic purpura
Idiopathic thrombocytopenic purpura (ITP) is defined as an isolated thrombocytopenia (low platelet count with an otherwise normal complete blood count and peripheral blood smear) in a patient with no clinically apparent associated conditions that can cause thrombocytopenia.3 It is a common cause of low platelet count and abnormal bleeding in both children and adults. ITP is thought to be caused by the development of auto-antibodies to platelet membrane antigens.
Treatment is aimed at modulating the immune response and reducing the rate of platelet destruction and is indicated in all patients who have counts less than 20 × 109/L, and those with counts less than 50 × 109/L accompanied by significant mucous membrane bleeding. First phase treatment includes parenteral glucocorticoids and intravenous IgG. Splenectomy is usually reserved for patients who do not respond to medical therapy and have ongoing bleeding symptoms.3 Platelet transfusions may cause temporary increases in platelet count and may be used in cases of life-threatening haemorrhage but are otherwise not usually indicated.4
Drug-related thrombocytopenia
A large number of drugs have been reported to cause immune-related thrombocytopenia. By far the most commonly implicated are quinine, quinidine and heparin. Heparin is associated with a syndrome of thrombosis due to diffuse platelet activation accompanied by a consumptive thrombocytopenia. Some platelet inhibitors, particularly ticlopidine and less commonly clopidogrel, are associated with severe thrombocytopenia and other signs and symptoms of thrombotic thrombocytopenic purpura. Recently developed monoclonal antibodies, such as infliximab (anti-tumour necrosis factor-α antibody), efalizumab (anti-CD11α antibody) and rituximab (anti-CD20 antibody) are also associated with an acute, severe, but usually self-limited thrombocytopenia.5
In most cases of drug-related thrombocytopenia, recovery occurs rapidly after withdrawal of the offending agent. The exception is patients with gold sensitivity, who may remain thrombocytopenic for months due to the slow clearance of this drug.6
Post-transfusion purpura
Post-transfusion purpura is clinically distinct from thrombocytopenia due to dilution of platelets following massive transfusion. It is an acute, severe thrombocytopenia occurring about 1 week after blood transfusion and is associated with a high titre of platelet-specific alloantibodies. It is most commonly reported in multiparous women following their first blood transfusion. The mechanism for alloantibody formation is unclear. Spontaneous recovery occurs within weeks, although fatalities from severe haemorrhage have been reported.6
Non-immune platelet destruction
Thrombotic thrombocytopenic purpura
TTP can occur sporadically as an idiopathic disorder or may be associated with pregnancy, epidemics of verotoxin-producing Escherichia coli and Shigella dysenteriae, malignancy, chemotherapy, marrow transplantation, and drug-dependent antibodies. Treatment with plasma exchange has dramatically influenced the outcome of TTP. Mortality has fallen from more than 90% prior to introduction of plasma exchange to less than 20% with this treatment.7
Thrombocytopenia in pregnancy
Gestational thrombocytopenia develops during an otherwise normal pregnancy and is clinically distinct from autoimmune thrombocytopenias such as ITP. It is thought to be due to decreased platelet survival consequent to activation of the coagulation system. Thrombocytopenia is usually mild and there is no corresponding thrombocytopenia in the infant. The platelet count returns to normal after delivery, although thrombocytopenia may recur in subsequent pregnancies.8
Autoimmune thrombocytopenias, on the other hand, are often associated with more severe reductions in the platelet count. Antiplatelet antibodies are capable of crossing the placenta and may result in significant thrombocytopenia in the fetus and newborn. This can lead to complications, such as intracranial haemorrhage, during the delivery. Treatment of the mother with autoimmune thrombocytopenia is similar in principle to the treatment of non-pregnant cases.8
In the context of pregnancy, thrombocytopenia may also be seen as part of the HELLP (haemolysis, elevated liver enzymes, low platelets) and pre-eclampsia syndromes. The two syndromes are thought to be related. Common to both is a process of microvascular endothelial damage and intravascular platelet activation. This leads to release of thromboxane A and serotonin, which provoke vasospasm, platelet aggregation and further endothelial damage.9 In both syndromes, the process is terminated by delivery.
Disseminated intravascular coagulation
Thrombocytopenia is one manifestation of the syndrome of disseminated intravascular coagulation (DIC). DIC is an acquired syndrome of diffuse intravascular coagulation up to the level of fibrin formation, accompanied by secondary fibrinolysis or inhibited fibrinolysis.10 It occurs in the course of severe systemic diseases or may be provoked by toxins such as snake venoms.
Massive blood transfusion and thrombocytopenia
Massive blood transfusion is defined as the transfusion of a volume equivalent to the patient’s normal blood volume within a 24-h period. Thrombocytopenia results from dilution of the patient’s remaining platelets and, where whole blood is used, decreased survival of platelets in stored blood. It is possibly the most important factor contributing to the haemostatic abnormality seen in massively transfused patients. Platelet transfusion should be reserved for cases where the platelet count falls below 50 × 109/L.4
Hypersplenism
Hypersplenism refers to the thrombocytopenia due to pooling in patients with splenic enlargement. It is the primary cause of thrombocytopenia in hepatic cirrhosis, portal venous hypertension, and congestive splenomegaly. In these cases, thrombocytopenia is rarely severe and not usually of clinical importance.2
Transient thrombocytopenia has been described in patients suffering severe hypothermia and is due to splenic sequestration. Platelet counts usually return to normal within days of rewarming.2
Clinical features
Spontaneous bleeding related to thrombocytopenia typically manifests as cutaneous petechiae and/or purpura, most commonly in dependent areas such as the legs and buttocks.1,3 Other spontaneous manifestations include multiple small retinal haemorrhages, epistaxis, gingival and gastrointestinal bleeding. Bleeding following trauma or surgery in thrombocytopenic patients is often immediate and may respond to local methods of haemostasis. In contradistinction to this the bleeding associated with coagulation disorders is most commonly in the form of large haematomata or haemarthroses that occur spontaneously or develop hours to days following trauma.1
In addition to the haemorrhagic manifestations of platelet insufficiency, patients with thrombocytopenia may present with the clinical features of the underlying causative disorder. Splenic enlargement may be present in cases where thrombocytopenia is due to hypersplenism but is not a feature of immune-related thrombocytopenia.
The level of platelets associated with clinically significant abnormal bleeding is not precisely defined. It varies depending on the platelets’ functional integrity, and with the presence or absence of other risk factors, such as coagulation disorder, trauma, and surgery. There is evidence that platelet counts above 5 × 109/L are sufficient to prevent bleeding when the platelets are functionally normal and there are no other risk factors. Severe haemorrhage is uncommon at platelet counts above 20 × 109/L and in the setting of surgery the risk of abnormal haemorrhage is reduced at counts above 50 × 109/L.4
Clinical investigation
The FBC and examination of the blood film are diagnostic of thrombocytopenia. The pattern of deficiency should be considered. Isolated thrombocytopenia refers to a low platelet count in the presence of an otherwise normal FBC and blood film. In these cases, FBC combined with a careful clinical history and examination is often sufficient to lead to a final diagnosis.3 Coexistent anaemia and/or leukopenia suggest bone marrow dysfunction as the primary aetiological process.
Treatment
Treatment for specific causes of thrombocytopenia has already been discussed. Bleeding in the face of low platelet count may be responsive to local methods of haemostasis if the remaining platelets are functionally normal and there is no other disorder of coagulation present. Individual case reports provide some support for the use of recombinant Factor VIIa as an enhancer of haemostasis in the treatment of bleeding in the context of severe thrombocytopenia, although evidence from randomized clinical trials is lacking.11 Platelet transfusion may be helpful in cases of severe haemorrhage and is sometimes used prophylactically to prevent bleeding in patients with very low platelet counts.
Platelet transfusion is primarily indicated in patients in whom thrombocytopenia is due to impaired platelet production and who are bleeding or have very low counts. The threshold for prophylactic transfusion in these patients is controversial. It is indicated when the platelet count is below 5 × 109/L, but is probably not indicated above this level unless other risk factors for bleeding are present.4
Platelet transfusion is rarely indicated in immune-related thrombocytopenias as the transfused platelets are rapidly destroyed. Transfusion of platelets may aggravate TTP.4 In DIC, platelet transfusion has not been proven to be effective but may be indicated in bleeding patients. There is little evidence to support the suggestion that blood component therapy aggravates DIC.12 In cases of massive blood transfusion, platelets are not routinely indicated unless there is ongoing bleeding and the platelet count is below 50 × 109/L.4
Raising the platelet count to 20–50 × 109/L is sufficient to prevent serious bleeding. In patients undergoing surgery or other invasive procedures counts up to 60–100 × 109/L may be required. A useful rule of thumb is that in a 70 kg adult, transfusion of one unit of platelets will increase the platelet count by 11 × 109/L.4
At present, platelet preparations for transfusion are stored in liquid at 22 °C. Problems include the continued risk of febrile non-haemolytic reactions, transmission of infectious agents and graft-versus-host disease. Alternatives to conventional liquid storage include frozen storage, cold liquid storage, photochemical treatment and lyophilized platelets. None of these methods is currently widely available. Several platelet substitutes have been developed but remain untested in the clinical setting. Some examples are red cells with surface-bound fibrinogen, fibrinogen-coated albumin microcapsules and liposome-based haemostatic agents.13
Disposition
Disposition will depend on the presence and extent of abnormal bleeding, the degree of thrombocytopenia and the underlying aetiology. In general, patients who present with abnormal bleeding and a low platelet count should be admitted for further evaluation and treatment. In the absence of bleeding, patients who have isolated thrombocytopenia with counts above 20 × 109/L may be investigated on an outpatient basis.3
1 Rodgers GM, Bithell TC. The diagnostic approach to the bleeding disorders. In: Lee GR, Wintrobe MM, Lee GR, et al, editors. Wintrobe’s clinical hematology. 10th edn. Maryland: Lippincott Williams & Wilkins; 1999:1557-1578.
2 George JN. Thrombocytopenia: pseudothrombocytopenia, hypersplenism, and thrombocytopenia associated with massive transfusion. In: Beutler E, Beutler E, Williams WJ, editors. Williams Hematology. 5th edn. New York: McGraw-Hill; 1995:1355-1360.
3 American Society of Hematology. ITP Practice Guideline Panel. Diagnosis and treatment of idiopathic thrombocytopenic purpura. American Family Physician. 1996;54(8):2437-2447. 24512452
4 Mollison PL, Engelfriet CP, Contreras M. Blood transfusions in clinical medicine, 10th edn. Oxford: Blackwell Science, 1997.
5 Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. New England Journal of Medicine. 2007;357(6):580-587.
6 George JN, El-Harake M, Aster RH. Thrombocytopenia due to enhanced platelet destruction by immunological mechanisms. In: Beutler E, et al, editors. Williams hematology. 5th edn. New York: McGraw-Hill; 1995:1315-1354.
7 George JN, El-Harake M. Thrombocytopenia due to enhanced platelet destruction by nonimmunological mechanisms. In: Beutler E, et al, editors. Williams hematology. 5th edn. New York: McGraw-Hill; 1995:1290-1314.
8 Schwartz KA. Gestational thrombocytopenia and immune thrombocytopenias in pregnancy. Hematology and Oncology Clinics of North America. 2000;14(5):1101-1116.
9 Padden MO. HELLP syndrome: recognition and perinatal management. American Family Physician. 1999;60(3):829-836.
10 Ten Cate H. Pathophysiology of disseminated intravascular coagulation in sepsis. Critical Care Medicine. 2000;28(9 suppl):S9-S11.
11 Goodnough LT, Lublin DM, Zhang L, et al. Transfusion medicine service policies for recombinant factor VIIa administration. Transfusion. 2004;44:1325-1331.
12 Levi M, de Jonge E, van der Poll T. Novel approaches to the management of disseminated intravascular coagulation. Critical Care Medicine. 2000;28(9 suppl):S20-S24.
13 Lee DH, Blajchman MA. Novel treatment modalities: new platelet preparations and substitutes. British Journal of Haematology. 2001;114:496-505.
13.4 Haemophilia
Introduction
Haemophilia is a group of congenital disorders of blood coagulation that arise as a result of a deficiency of clotting factor proteins, which are essential to the normal intrinsic coagulation pathway. The classic form, haemophilia A, is attributable to deficiency of Factor VIII while haemophilia B (also known as Christmas disease) is attributable to deficiency of Factor IX. Both these diseases have a classic X-linked pattern of inheritance and thus affect males while female carriers may also have mild deficiency of the appropriate coagulation factor.
Pathophysiology
Clinical features
The most common manifestations of haemophilia are:
Patients may also present with a complication of therapy. Most haemophiliac patients treated before 1985 have been exposed to pathogenic viruses, of which the most important are hepatitis C, hepatitis B and HIV. Of those who received plasma prior to the mid-1980s, 90% are hepatitis B positive, 85–100% are hepatitis C positive, and 60–90% are HIV positive.
Treatment
Products currently available for the treatment of haemophilia include:
Options for adequate analgesia include:
One unit of Factor VIII concentrate provides the amount of Factor VIII activity in 1 mL of normal plasma. Given that a 70-kg adult has a plasma volume of 3500 mL, we can expect that an infusion of 3500 units of Factor VIII will produce 100% Factor VIII activity in a haemophiliac with negligible activity prior to treatment. The half-life of Factor VIII is approximately 12 h. Accordingly, a further dose of 1750 units in 12 hours’ time will again restore 100% activity.
Treatment of bleeding
Minor bleed (e.g. spontaneous haemarthrosis or muscle bleed):
Moderate bleed (e.g. epistaxsis, traumatic haemarthrosis, excluding hip):
Major bleed (e.g. intracerebral, hip, neck, throat, psoas muscle):
Disposition
von Willebrand disease
Factor VIII has an intimate association with von Willebrand factor (vWF). This is an adhesive glycoprotein secreted by endothelium and megakaryocytes, which is required for the normal instigation of platelet plug formation and for stabilization and transport of Factor VIII within the circulation. Thus von Willebrand disease (vWD) is a result of dysfunction, reduction, or a complete lack of the vWF and is often associated with low Factor VIII activity. It is the most common inherited bleeding disorder, affecting 0.1–1% of the population, and affects males and females equally.
Three types of von Willebrand disease are recognized:
Useful contacts
Websites
Australian Haemophilia Centre Directors’ Organisation: www.ahcdo.org.au
Haemophilia Foundation Australia: www.haemophilia.org.au
Canadian Hemophilia Society: www.hemophilia.ca
Hemophilia Federation of America: www.hemophiliafed.org
Haemophilia Foundation Australia: www.haemophilia.org.au
Haemophilia Foundation of New Zealand: www.haemophilia.org.nz
Contact numbers for advice/referrals
NSW
QLD
SA
VIC
Bell BA, Birch K, Glazer S. Experience with recombinant factor VIIA in an infant with haemophilia with inhibitors to FVIII:C undergoing emergency central line placement. A case report. American Journal of Pediatrics, Hematology and Oncology. 1993;15(1):77-79.
Bush MT, Roy N. Hemophilia emergencies. Journal of Emergency Nursing. 1995;21(6):531-538.
De Behnke DJ, Angelos MG. Intracranial hemorrhage and hemophilia: case report and management guidelines. Journal of Emergency Medicine. 1990;8(4):423-427.
Pfaff JA, Geninatti M. Hemophilia. Emergency Medicine Clinics of North America. 1993;11(2):337-363.
Warrier I, Ewenstein BM, Koerper MA, et al. Factor IX inhibitors and anaphylaxis in hemophilia B. Journal of Pediatrics, Hematology and Oncology. 1997;19(1):23-27.
13.5 Blood and blood products
Introduction
Blood is a living tissue composed of blood cells suspended in plasma; it transports nutrients and oxygen, and facilitates temperature control. An average 70-kg male has a blood volume of about 5 L. The cellular elements comprise red blood cells, white blood cells and platelets, and make up about 45% of the volume of whole blood. Plasma, which is 92% water, makes up the remaining 55%.
Early attempts at blood transfusion were thwarted by adverse reactions. In 1900 Karl Landsteiner demonstrated the ABO blood group system and explained many of the observed severe incompatibility reactions (Table 13.5.1). He won the Nobel prize for medicine in 1930 and went on to discover the Rhesus factor in 1940. The next major advance in transfusion medicine occurred with the development of long-term anticoagulants such as sodium citrate, which allowed extended preservation of blood. Development of refrigeration procedures allowed storage of anticoagulated blood. The addition of a citrate-glucose solution extended the viability of collected blood to several days. The ability to preserve blood for longer than a few hours paved the way for the establishment of the first blood bank in a Leningrad hospital in 1932.
| ABO blood group | Antigens on red cells | Antibody in serum |
|---|---|---|
| O | None | Anti-A, Anti-B |
| A | A | Anti-B |
| B | B | Anti-A |
| AB | A, B | None |
In the Australian urban hospital setting, 50% of packed red cells are used for the treatment of anaemia, 22% pre- or perioperatively and 13% for abnormal, excessive or continued bleeding.1 Medical oncology uses 78% of all platelets.2 Forty-one per cent of all FFP is used to correct coagulopathy associated with surgery, 27% to correct coagulopathy in bleeding, 16% to reverse haemostatic disorders in patients having massive blood transfusion, 11.5% for reversal of warfarin effect and the remaining 4.5% for a number of miscellaneous conditions, including liver disease and disseminated intravascular coagulation (DIC).
Packed red blood cells
Packed red cells are the blood product most commonly prescribed in the ED, the usual indication being the replacement of acute blood loss.3 Transfusion of packed red cells is indicated where the patient’s oxygen-carrying capacity is so impaired that control of bleeding alone, if indeed it can be readily achieved, is regarded as insufficient to take the patient out of danger. Occasionally it may be necessary to transfuse a patient with a primary haematological condition, a failure of erythropoiesis or a haemolytic process. The indication for transfusion is the same as for haemorrhage: a severe reduction in oxygen-carrying capacity. Complex multisystem failure, such as DIC or septic shock, may result in simultaneous blood loss, circulatory collapse, haemolysis and a coagulopathy. Transfusion therapy may be life saving in this context (Table 13.5.2).
| Haemorrhage |
| Dilutional anaemia following severe burns |
| Iron-deficiency anaemia |
| Megaloblastic anaemia |
| Anaemia of chronic disorders |
| Chronic renal failure |
| Failure of erythropoiesis |
| Sickle cell disease |
| Septic shock |
| Disseminated intravascular coagulopathy |
Fluid administration is the cornerstone of management during initial trauma reception and resuscitation, as hypovolaemia is one of the leading causes of death in trauma. Fluid resuscitation must be rapid and appropriate, and should be reassessed and adjusted at regular intervals. This has led to a significant decrease in morbidity and mortality from major trauma.4
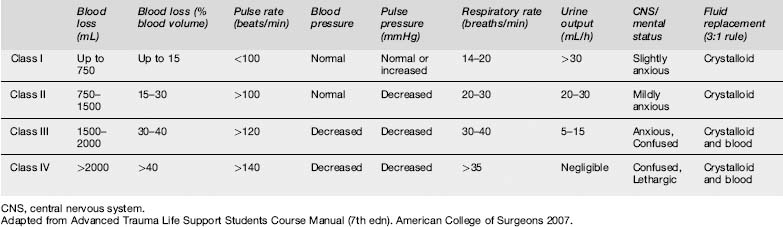
The American College of Surgeons estimates fluid and blood loss by using six physiological parameters to grade patients into classes I–IV (Table 13.5.3). This allows more accurate characterization of a patient’s immediate haemodynamic status than do laboratory parameters such as haemoglobin and haematocrit. It is recommended that patients in groups III and IV receive blood.
Transfusion is not indicated when alternative haematinic therapy is deemed safe and appropriate. A moderately anaemic patient who is asymptomatic and not bleeding, with some reserve oxygen-carrying capacity, does not require blood transfusion. A haemoglobin of 7 g/dL is sometimes taken as the failsafe point in the decision whether to transfuse, although of course the patient’s unique circumstances need to be taken into account: treat the patient, not the number. The National Health and Medical Research Council together with the Australasian Society of Blood Transfusion have published transfusion guidelines for red blood cells and other products (Table 13.5.4).
Table 13.5.4 Guidelines for transfusion of blood components
| Indications | Considerations |
|---|---|
| Red blood cells | |
| Hb | |
| <70 g/L | Lower thresholds may be acceptable in patients without symptoms and/or where specific therapy is available |
| 70–100 g/L | Likely to be appropriate during surgery associated with major blood loss or if there are signs or symptoms of impaired oxygen transport |
| >80 g/L | May be appropriate to control anaemia-related symptoms in a patient on a chronic transfusion regimen or during marrow suppressive therapy |
| >100 g/L | Not likely to be appropriate unless there are specific indications |
| Platelets | |
| Bone marrow failure | At a platelet count of <10 × 109/L in the absence of risk factors and <20 × 109 in the presence of risk factors (e.g. fever, antibiotics, evidence of systemic haemostatic failure) |
| Surgery/invasive procedure | To maintain platelet count at >50 × 109/L. For surgical procedures with high risk of bleeding (e.g. ocular or neurosurgery) it may be appropriate to maintain at 100 × 109/L |
| Platelet function disorders | May be appropriate in inherited or acquired disorders, depending on clinical features and setting. In this situation, platelet count is not a reliable indicator |
| Bleeding | May be appropriate in any patient in whom thrombocytopenia is considered a major contributory factor |
| Massive haemorrhage/transfusion | Use should be confined to patients with thrombocytopenia and/or functional abnormalities who have significant bleeding from this cause. May be appropriate when the platelet count is <50 × 109/L (<100 × 109/L in the presence of diffuse microvascular bleeding) |
| Fresh frozen plasma | |
| Single factor deficiencies | Use specific factors if available |
| Warfarin effect | In the presence of life-threatening bleeding. Use in addition to vitamin-K-dependent concentrates |
| Acute DIC | Indicated where there is bleeding and abnormal coagulation. Not indicated for chronic DIC |
| TTP | Accepted treatment |
| Coagulation inhibitor deficiencies | May be appropriate in patients undergoing high-risk procedures. Use specific factors if available |
| Following massive transfusion or cardiac bypass | May be appropriate in the presence of bleeding and abnormal coagulation |
| Liver disease | May be appropriate in the presence of bleeding and abnormal coagulation |
| Cryoprecipitate | |
| Fibrinogen deficiency | May be appropriate where there is clinical bleeding, an invasive procedure, trauma or DIC |
TTP, idiopathic thrombocytopenia purpura ;DIC, disseminated intravascular coagulation.
Adapted from the National Health and Medical Research Council and Australasian Society Clinical Practice Guidelines on appropriate use of blood components http://www.nhmrc.gov.au/publications/synopses/-files/cp82.pdf
Prior to transfusion, except in an extreme emergency, the patient’s informed consent should be sought, obtained and documented. Rarely, patients may be encountered who refuse transfusion, either on religious grounds or for fear of adverse reactions, particularly with respect to the transfer of viral agents. This can pose problems if the patient appears incompetent to give or withhold informed consent. The situation of an exsanguinating minor whose parents refuse permission to transfuse is particularly difficult. Court orders can be obtained to treat minors without parental consent, and in such an extreme situation many doctors would feel justified in applying to obtain one, even retrospectively.
Effect of storage on red blood cells
Although it makes intuitive sense that blood loss should be replaced by blood products, there is evidence that the immediate observed benefit is from volume replacement rather than improved oxygen carriage.5,6 Red blood cells may not be fully functional until 2–6 h after transfusion because storage affects the oxygen-carrying capacity of blood. This is probably due to decreased intracellular 2,3-diphosphoglycerate (2,3-DPG), loss of red cell viability, decreased red cell deformability, relative acidosis and potassium leakage.7
Storage reduces 2,3-DPG levels, leading to a leftward shift of the oxyhaemoglobin dissociation curve and increased affinity of oxygen binding. The transfused red cell does regenerate 2,3-DPG to normal levels but this can take 6–24 h post transfusion. With increasing age of stored red cells, levels of 2,3-DPG progressively fall such that by 5–6 weeks the level is 10% of normal. It is still uncertain whether this abnormality is physiologically important, even in critically ill patients.8
When red cells are transfused, some of the cells are removed from the circulation within a few hours, with the rest surviving normally; as the storage time increases to 42 days, more cells are removed immediately after transfusion. This loss of viability is highly dependent upon the anticoagulant-preservative solution used.9
Potassium gradually leaks out of stored red cells and this raises the plasma potassium by approximately 1 meq/L per day.10
Choice of red cell product
The choice of red cell product is determined by time and safety considerations. O-negative red cells, the universal donor group, are readily available in most major hospitals. Supplies of O-negative blood are limited and the product should be used with care. It is preferable that blood be collected prior to infusion of such cells so as to characterize the recipient’s blood group serology. Premenopausal female patients should be given group O Rhesus negative, Kell negative blood in an emergency situation in order to avoid sensitization and possibility of haemolytic disease of newborn in subsequent pregnancies. Male patients, however, can be transfused either Rhesus positive or negative blood. The incidence of adverse reaction using this type of blood is approximately 3%. O-negative may be given immediately on arrival where the patient has not responded to adequate crystalloid given during transport, or the patients are in class III or IV shock.11 By contrast, the provision of group-specific blood requires matching a blood sample to the major (ABO) and Rhesus D compatibility groups only. Group-specific blood can be available for transfusion within 35 min depending on the logistic support and staffing levels within the haematology laboratory. It has an incidence of adverse reaction similar to O-negative blood. As O-negative blood is usually in short supply it is preferable where possible to infuse group-specific blood. A more comprehensive cross match where there are no atypical antibodies identified in the initial screening can take 30 min or more and the incidence of adverse transfusion reaction is reduced to 0.01%. Due to the time required for sample collection and cross matching, it is generally not possible to provide fully cross-matched blood within the first 30 min of trauma reception.
Precautions when cross-matching and transfusing blood
Although most patients do not require transfusion in the ED, it is often appropriate to ‘group and hold’ or cross-match the patient while in the department. Many hospitals have written protocols detailing the anticipated requirements for a given surgical procedure. Documentation should be meticulous. It is preferable that the person drawing the blood for cross-matching should also fill in and sign the laboratory request form. Most severe incompatibility reactions to blood transfusion result not from exposure to unusual antigens but from an administrative error. Any systematic change in documentation protocols, for example the adoption of an electronic record, needs to be accompanied by an obsessive risk management strategy.
Adverse reactions to transfusion
The principal adverse reactions to blood transfusion are listed in Table 13.5.5. Serious adverse reactions are relatively rare (Table 13.5.6) although some are more likely to occur when blood is administered urgently.
| Immunological transfusion reactions | Transmission of infection |
|---|---|
HIV, human immunodeficiency virus; HTLV, human T-cell lymphotropic virus.
Table 13.5.6 Incidence of adverse transfusion reactions (per unit packed red cells transfused)
| Adverse transfusion reaction | Incidence | Mortality |
|---|---|---|
| Bacterial sepsis | 1 in 40 000–500 000 | 1 in 4–8 million |
| Acute haemolytic reaction | 1 in 12 000–38 000 | 1 in 600 000–1.5 million |
| Delayed haemolytic reaction | 1 in 1000–12 000 | 1 in 2.5 million |
| Anaphylaxis | 1 in 20 000–50 000 | |
| Transfusion-related acute lung injury | 1 in 5000–100 000 | 1 in 5 million |
| Fluid overload | 1 in 100–700 | |
| Transfusion-associated graft versus host disease | Rare | 90% fatality |
Adapted from Australian Red Cross website: http://www.transfusion.com.au/TRANSFUSIONPOCKETGUIDES/Pocket_RiskConsent.asp (accessed 25th February 2008).
Immunological transfusion reactions
Immunological transfusion reactions may be immediate or delayed in onset.
Immediate
Acute haemolytic reactions
Acute haemolytic transfusion reactions (ATHRs) result from the rapid destruction of donor red cells by preformed recipient antibodies and are a medical emergency. They are usually due to ABO incompatibility and most often the result of clerical or procedural error.12 Some acquired allo-antibodies, such as anti-Rh or anti-Jka, are occasionally implicated, but AHTRs more typically occur when a group O recipient is transfused with non-group O red cells. This may lead to DIC, shock and acute tubular necrosis precipitating acute renal failure. These reactions usually manifest with fever and rigors, lumbar pain, crushing chest pain, tachycardia, hypotension and haemoglobinaemia with subsequent haemoglobinuria. The symptoms usually develop within the first 30 min of transfusion.
Anaphylactoid transfusion reactions
Treatment of an anaphylactoid transfusion reaction consists of immediate cessation of transfusion and standard treatment of anaphylaxis including, oxygen fluids and adrenaline (see Ch. 28.7).
Transfusion-related acute lung injury
This complication is characterized by onset of acute respiratory distress, hypoxaemia, hypotension, tachycardia, fever and pulmonary oedema, initially without signs of left ventricular failure. The central venous pressure is normal, which helps distinguish the condition from transfusion-associated circulatory overload. Treatment is supportive. High-dose steroid therapy has been used but appears to be ineffective.13 The blood bank needs to be notified and the donor identified.
Transmission of infection
In terms of viral safety Australia has one of the safest blood supplies in the world (Table 13.5.7).
Table 13.5.7 Risks of transfusion transmitted infection (per unit tested blood transfused)
| Infection | Residual risk |
|---|---|
| CMV | 1 in 127 000 |
| Hepatitis B | Approximately 1 in 660 000 |
| Syphilis | Considerably less than 1 in a million |
| Hepatitis C | <1 in 10 million |
| HIV | <1 in 10 million |
| HTLV I and II | <1 in 10 million |
| Variant CJD | Possible and cannot be excluded |
CMV, cytomegalovirus; HIV, human immunodeficiency virus; HTLV, human T-cell lymphotropic virus; CJD, Creutzfeldt–Jakob disease.
Adapted from Australian Red Cross website: http://www.transfusion.com.au/TRANSFUSIONPOCKETGUIDES/Pocket_RiskConsent.asp (accessed 25th February 2008).
Hypothermia
Red blood cells are stored at 4 °C. Rapid infusion of large volumes of stored blood can contribute to hypothermia. Blood warmers should be used during massive blood transfusion. In addition, other intravenous fluids should be warmed and other measures instituted to maintain patient body temperature (see Ch. 28.2).
Dilutional coagulopathies
Clinically significant depletion of coagulation proteins and platelets is a complication of massive transfusion, secondary to dilution and the consumptive coagulopathy of trauma. Stored red cells are deficient in platelets and clotting factors, and transfusion of large amounts can complicate bleeding when not accompanied by assessment and correction of coagulation disturbances. Coagulation parameters including the prothrobin time (PT), activated partial thromboplastin time (APTT), platelet count and fibrinogen level should be monitored and corrected if deficiencies occur in the presence of abnormal bleeding.
Management of transfusion reactions
Platelets
Platelets are transfused to prevent or treat haemorrhage in patients with thrombocytopenia or defects in platelet function. Specific indications for platelet transfusion are shown in Table 13.5.4. Use of platelets is not generally considered appropriate in the treatment of immune-mediated platelet destruction, thrombotic thrombocytopenic purpura, haemolytic uraemic syndrome or drug-induced or cardiac bypass thrombocytopenia without haemorrhage.3
Fresh frozen plasma
FFP is prepared from anti-coagulated blood by separating the plasma from the blood cells through centrifugation of whole blood or apheresis. It is stored frozen until used. It contains all coagulation factors including small amounts of Factor V and approximately 200 units of Factor VIII. Fresh frozen plasma can be stored at below −25 °C for up to 12 months. Indications for use of FFP are shown in Table 13.5.2.
The appropriate dose depends on the clinical indication, patient size and results of laboratory tests. A general guide is 10–15 mL/kg per dose, but in some situations dosages greater than this may be required (e.g. dilutional coagulopathy in the context of massive transfusion). On average 1 mL of FFP/kg patient weight will raise most coagulation factors by 1%, therefore a dose of 10–15 mL/kg would be expected to increase levels by 10–15%.3 Compatibility testing is not required; however, ABO compatible plasma should be used wherever possible. Group AB plasma can be used for all patients in an emergency.
Cryoprecipitate
Cryoprecipitate is prepared by thawing FFP to between 1 and 6 °C and recovering the precipitable protein fraction. It contains most of the Factor VIII, fibrinogen, Factor XIII, von Willebrand factor and fibronectin from the FFP. It may be stored for up to 12 months at 25 °C or below. Once thawed it must be used immediately or stored at 2–6 °C for up to 24 h. Cryoprecipitate is indicated in fibrinogen deficiency with clinical bleeding or prior to an invasive procedure, and in DIC.3 Cryoprecipitate is transfused to keep fibrinogen levels above 1.0 g/L in the acutely bleeding patient. Compatibility tests before transfusion are not necessary. It is preferable to use an ABO group compatible with the recipient’s red cells; however, ABO incompatible can be used with caution. Up to 4 units/10 kg body weight may be required to raise the fibrinogen concentration by approximately 0.5 g/L in the absence of continued haemorrhage.
The use of cryoprecipitate is not generally considered appropriate in the treatment of haemophilia, von Willebrand’s disease or deficiencies of Factor XIII or fibronectin, unless alternative therapies are unavailable.3
Massive transfusion
In the 1970s, massive transfusion was defined as more than 10 units of blood over a 24-h period. This is equivalent to approximately one patient blood volume in a person of average weight person.14 Recent reviews in the literature have expanded this definition, with some reports using up to 50 units of blood in 24 or 48 h.15
1 Rubin GL, Schofield WN, Dean MG, et al. Appropriateness of red blood cell transfusions in major urban hospitals and effectiveness of an intervention. Medical Journal of Australia. 2001;175:354-358.
2 Metz J, McGrath KM, Copperchini ML, et al. Appropriateness of transfusions of red cells, platelets and fresh frozen plasma. An audit in a tertiary care teaching hospital. Medical Journal of Australia. 1995;162:572-577.
3 Practice Guidelines on the Use of Blood Components. National Health and Medical Research Council and Australasian Society of Blood Transfusion Clinical, 2001 http://www.nhmrc.gov.au/publications/synopses/_files/cp78.pdf (accessed 4 August 2008).
4 Champion H, Bellamy RF, Roberts CP, et al. A profile of combat injury. Journal of Trauma. 54(suppl), 2003. s31-s19
5 Dabrowski GP, Steinberg SM, Ferrara JJ, et al. A critical assessment of endpoints of shock resuscitation. Surgical Clinics of North America. 2000;80:825-844.
6 Revell M, Greaves I, Porter K. Endpoints for fluid resuscitation in hemorrhagic shock. Journal of Trauma. 2003;54(suppl):s63-s67.
7 McKinley BA, Valdivia A, Moore FA. Goal orientated shock resuscitation for major torso trauma. Current Opinions in Critical Care. 2003;9:292-299.
8 Walsh TS, McArdle F, McLellan SA, et al. Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anaemic critically ill patients? Critical Care Medicine. 2004;32:364-371.
9 Council of Europe. Guide to the preparation, use and quality assurance of blood components, 11th edition, Strasbourg: Council of Europe Publishing; 2005:1-266. Recommendation R (95) 15
10 Simon GE, Bove JR. The potassium load from blood transfusion. Postgraduate Medicine. 1971;49:61-64.
11 Advanced Trauma Life Support Students Course Manual, 7th edn. American College of Surgeons, Chicago, 2007.
12 Serious Hazards of Transfusion Annual Report 2004; http://www.shotuk.org/SHOTREPORT2004.pdf (accessed 4 August 2008).
13 Popovsky MA, Chaplin HCJr, et al. Transfusion-related acute lung injury: a neglected, serious complication of hemotherapy. Transfusion. 1992;32:589-592.
14 Wilson RF, Mammen E, Walt AJ. Eight years of experience with massive blood transfusions. Journal of Trauma. 1971;11:275-285.
15 American Society of Anesthesiologists. Practice Guidelines for Blood Component Therapy. Baltimore: Williams & Wilkins, 2003.
16 Ho J, Sibbald WJ, Chin-Yee IH. Effects of storage on efficacy of red cell transfusion: when is it not safe? Critical Care Medicine. 2003;31:S687-S697.
17 Reiss RF. Hemostatic defects in massive transfusion: rapid diagnosis and management. American Journal of Critical Care. 2000;9:158-165.
18 Boffard KD, Riou B, Warren B, et al. NovoSeven Trauma Study Group. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. Journal of Trauma-Injury Infection & Critical Care. 2005;59(1):8-15.
19 Dutton RP, McCunn M, Hyder M, et al. Factor VIIa for correction of traumatic coagulopathy. Journal of Trauma-Injury Infection & Critical Care. 2004;57(4):709-718.
20 Levi M, Peters M, Buller HR. Efficacy and safety of recombinant factor VIIa for treatment of severe bleeding: a systematic review. Critical Care Medicine. 2005;33:883-890.