History
Current Medications
Current Symptoms
Physical Examination
Laboratory Data
Electrocardiogram
Findings
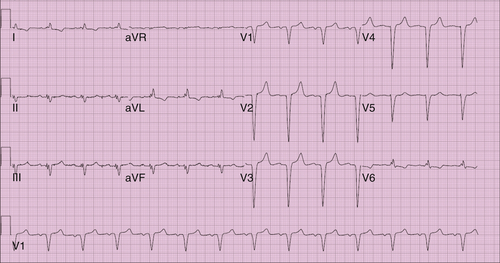
FIGURE 16-1 Electrocardiogram 3 days before surgery.
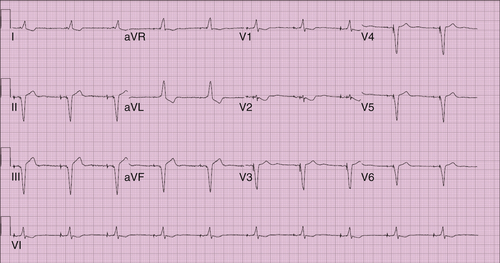
FIGURE 16-2 Electrocardiogram 20 days after surgery.
Comments
Findings
Comments
Chest Radiographs Findings
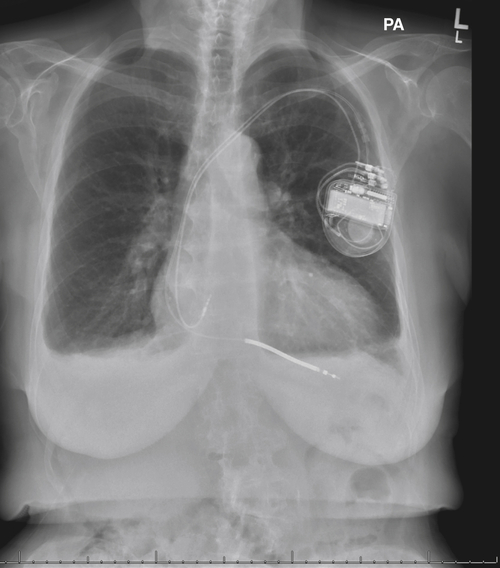
FIGURE 16-3 Anteroposterior chest radiograph obtained 3 days before surgery.
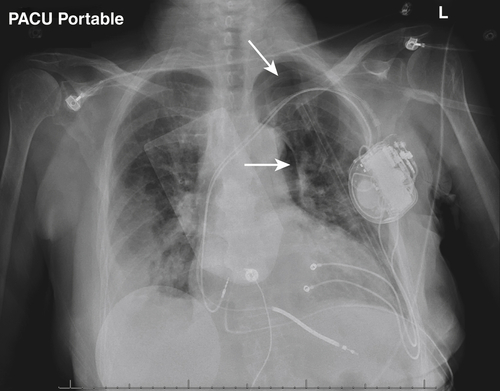
FIGURE 16-4 Postoperative anteroposterior chest radiograph. Arrows denote a very small postoperative pneumothorax.
Echocardiogram
5 Months Before Surgery
Findings
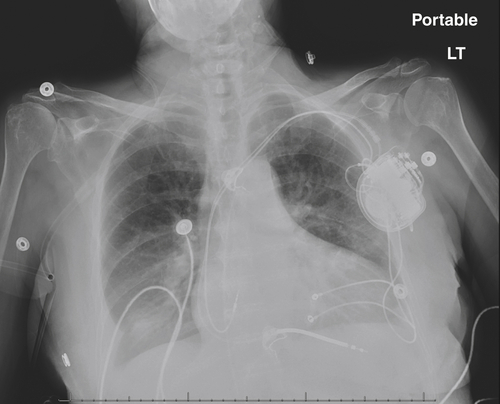
FIGURE 16-5 Anteroposterior chest radiograph obtained 3 days postoperatively. The small pneumothorax is no longer seen.
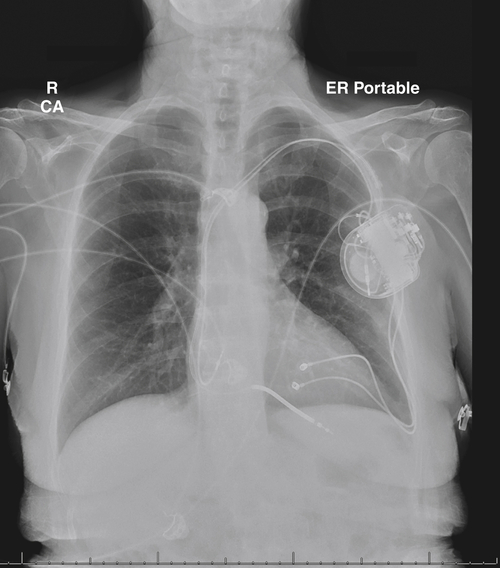
FIGURE 16-6 Anteroposterior chest radiograph obtained 20 days postoperatively.
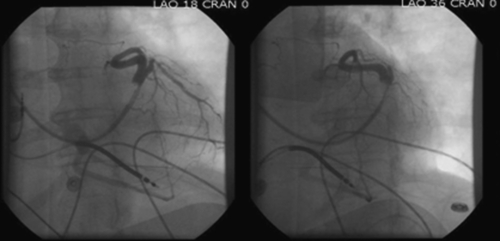
FIGURE 16-7 Venogram 2 months before surgery.
Postoperative Day 3
Findings
Venogram
Findings
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
Question
Discussion
Final Diagnosis
Plan of Action
Intervention
Outcome
Selected References
1. Abraham W.T. Cardiac resynchronization therapy for heart failure: biventricular pacing and beyond. Curr Opin Cardiol. 2002;17:346–352.
2. Alonso C., Leclercq C., d’Allonnes F.R. et al. Six year experience of transvenous left ventricular lead implantation for permanent biventricular pacing in patients with advanced heart failure: technical aspects. Heart. 2001;86:405–410.
3. Ansalone G., Giannantoni P., Ricci R. et al. Doppler myocardial imaging to evaluate the effectiveness of pacing sites in patients receiving biventricular pacing. J Am Coll Cardiol. 2002;39:489–499.
4. Fernandez A.L., Garcia-Bengochea J.B., Ledo R. et al. Minimally invasive surgical implantation of left ventricular epicardial leads for ventricular resynchronization using video-assisted thoracoscopy. Rev Esp Cardiol. 2004;57:313–319.
5. Gabor S., Prenner G., Wasler A. et al. A simplified technique for implantation of left ventricular epicardial leads for biventricular re-synchronization using video-assisted thoracoscopy (VATS). Eur J Cardiothorac Surg. 2005;28:797–800.
6. Mair H., Jansens J.L., Lattouf O.M. et al. Epicardial lead implantation techniques for biventricular pacing via left lateral mini-thoracotomy, video-assisted thoracoscopy, and robotic approach. Heart Surg Forum. 2003;6:412–417.
7. Mair H., Sachweh J., Meuris B. et al. Surgical epicardial left ventricular lead versus coronary sinus lead placement in biventricular pacing. Eur J Cardiothorac Surg. 2005;27:235–242.
8. Puglisi A., Lunati M., Marullo A.G. et al. Limited thoracotomy as a second choice alternative to transvenous implant for cardiac resynchronisation therapy delivery. Eur Heart J. 2004;25:1063–1069.
9. Shah R.V., Lewis E.F., Givertz M.M. Epicardial left ventricular lead placement for cardiac resynchronization therapy following failed coronary sinus approach. Congest Heart Fail. 2006;12:312–316.
10. Steinberg J.S., Derose J.J. The rationale for nontransvenous leads and cardiac resynchronization devices. Pacing Clin Electrophysiol. 2003;26:2211–2212.