Ocular Emergencies
Edited by Peter Cameron
16.1 Ocular emergencies
David V Kaufman, James K Galbraith and Mark J Walland
Introduction
Acute ocular presentations are common. A seemingly trivial trauma may mask a more serious underlying injury. Similarly, a relatively transient episode of visual loss with no abnormality found on examination may indicate potentially life-threatening cerebrovascular disease. Therefore, all eye presentations in an emergency department (ED) should be carefully evaluated with the necessary equipment.
Basic sight testing equipment should include a Snellen 6-metre chart and a black occlusive paddle with multiple pinhole perforations. A slit-lamp biomicroscope is needed for examination of the anterior segment and the removal of foreign bodies. A portable slit lamp for examining reclining patients can be a valuable asset in the emergency department. An intraocular pressure-measuring device, such as a Tono-pen or iCare tonometer which is portable, accurate and easily used, is desirable.
Emergency eye trolley setup
Examining equipment
Treating
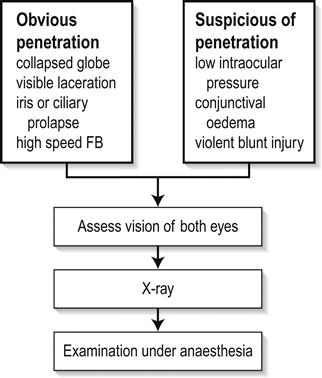
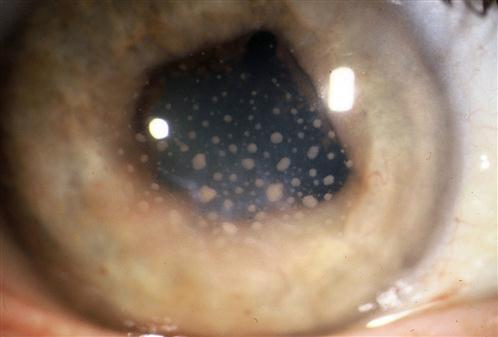
The incidence of injuries varies with the environment and protective measures taken. The major injuries result from blunt trauma or penetrating injuries to the globe, with or without the retention of a foreign body. Mechanical interference with eye movement may result from orbital injury, either haematoma or interference with muscle function. Similarly, neurotrauma may disturb the visual pathways or ocular motor nerves. It is necessary to elicit a history of the patient’s prior visual status, including the wearing of glasses or contact lenses and ocular medication. After an eye toilet to remove any debris from the eyelids, vision is tested by a distance Snellen chart, if necessary using a pinhole device as a rough focussing aid. Vision less than 6/60 Snellen may be graded by the patient’s ability to count fingers (CF) at a measured distance, discern hand movements (HM) or to project the direction of a light (PL) from various angles. The eye not being tested must be completely shielded by an opaque occluder. It is essential to assess early whether the patient has sustained a relatively minor superficial injury or a severe injury, which may be either blunt or penetrating. Reassurance and extreme gentleness in examining the eye will allow a more definite assessment to be made in the ED. Fresh local anaesthetic drops–preferably single use Minims – may be instilled to ease discomfort. With penetrating trauma, any external pressure on the eye may result in ocular structures being squeezed out of the wound, drastically worsening the prognosis. Desmarres retractors (Fig. 16.1.1) can be useful to open the lids yet avoid globe pressure. To open lids that are adherent due to blood or discharge, gently bathe with sterile saline. Wipe the eyelid skin dry and apply gentle distractive pressure to skin below the brow and below the lower lid, i.e. over bony orbital rim to open the lids. All patients in whom a penetrating injury is suspected require X-ray or computed tomography (CT) scanning to exclude a radiopaque intraocular foreign body (IOFB). If there is any possibility of metallic IOFB, magnetic resonance imaging (MRI) scans are contraindicated. When an adequate examination cannot be made, or where occult perforation is suspected, examination under anaesthesia is mandatory. The corneal epithelium is easily dislodged by a glancing blow from fingers, twigs, stones or a paper edge. The trauma produces an acute sensation of a foreign body, with light sensitivity and excessive tearing. After fluorescein staining, the size of the epithelial defect is recorded. Antibiotic ointment (chloramphenicol) is instilled and an eye pad applied if local anaesthetic was used. The condition heals spontaneously within 24–48 h. Large abrasions produce reflex ciliary spasm, which may require short-acting mydriatics, such as homatropine 2%, in addition to oral analgesia to relieve the pain. Under no circumstances can a patient ever be discharged with local anaesthetic drops for their own use. Small ferrous particles rapidly oxidize when adherent to the corneal epithelium, producing a surrounding rust ring within hours. The rusted particle requires removal under adequate topical anaesthesia using a slit-lamp microscope. An adherent rust ring may be loosened by the application of antibiotic ointment and padding for 24 h, after which it is easily shelled out with the edge of a fine hypodermic needle. Mechanical dental burrs can be difficult to sterilize and may cause large areas of epithelial removal and delay return to work. Wooden splinters are particularly dangerous as they may easily penetrate the eye and cause violent suppuration. In all suspected foreign body injuries, the upper and lower lids should be everted and examined with suitable lighting and magnification. The conjunctival fornices may be swept gently with a moist cotton bud under topical anaesthesia. Technique for upper eyelid eversion: the patient must look down at all times; grasp the upper lid lashes and draw the lid down, then with a cotton bud in the other hand, depress the lid 11 mm above the central lid margin (i.e. above the tarsal plate) and counter-rotate the grasped lashes and lid around this cotton-bud fulcrum. The lashes may be held against the superior orbital margin with a finger and the cotton bud removed. When the examination is complete, release the lid and allow the patient finally to look up and the lid will revert to the normal position. Unless large, it is rare that these require suturing. It is, however, vital to ensure that this superficial laceration does not hide a deeper penetration of the globe. After instillation of local anaesthetic, the conjunctiva may be gently moved aside with a cotton bud to examine the bed of the laceration. A careful history is important in assessing penetrating injury, including prior visual status and the use of contact lenses or spectacles. Occupational trauma may be due to high-speed penetrating metal fragments. Agricultural trauma often involves heavily contaminated implements. Australian seatbelt legislation has markedly reduced the incidence of penetrating eye injuries in road trauma, but eye problems still occur from violent head and facial trauma, in addition to the neurological complications of head injury [1,2]. Examination of the eye involves the instillation of sterile topical anaesthetic drops, followed by a gentle eye toilet removing debris, clot and glass from the face and lids. The lids should be opened without pressure (Fig. 16.1.2). The penetration may be evidenced by an obvious laceration or presence of prolapsed tissue with collapse of the globe. Conjunctival oedema (chemosis) and low intraocular pressure (IOP) may indicate an occult perforation or bursting injury. When a penetrating injury is either suspected or established, the patient must be transferred without delay to a centre where appropriate surgical facilities are available. During transport, the eye should be covered with a sterile pad and a plastic cone. Vomiting should be prevented with antiemetics and the fasted patient given intravenous fluids as necessary. Extruded tissue or projectiles should not be removed: intraocular contents will surely follow. Removal can only be undertaken in the controlled environment of an operating theatre. Prognosis depends on the extent of globe disruption. Concussion of the globe may cause tearing of the iris root, resulting in blood in the anterior chamber (hyphaema). A hyphaema greater than one-third of the anterior chamber usually indicates some damage to the drainage angle and may also be associated with concussive lens damage. Uninterrupted absorption of the hyphaema is essential and is aided by sedation and an admission to hospital. Traditional treatment is to pad the affected eye and to nurse the patient semirecumbent to encourage sedimentation of the blood in the anterior chamber to clear as much of the angle as possible. A hyphaema may cause considerable pain due to raised IOP. To lower the pressure, oral or intravenous acetazolamide 500 mg initially is required. Atropine drops to ‘splint’ the ocular interior are logical but theoretically risk a re-bleed with the initial dilating effect. Pain is relieved by paracetamol or narcotics, with antiemetics if necessary. Aspirin in any form should be avoided as it increases the risk of secondary haemorrhage. The patient should remain in bed until the blood has completely cleared from the anterior chamber. Bleeding recurs in up to 10% of patients, usually due to early mobilization in those with extensive iris damage. Total hyphaema has a poor prognosis because of secondary glaucoma, field loss and corneal opacification. When angle damage occurs, long-term follow up after hyphaema is required to determine whether the IOP is raised. The fundus requires careful examination after the hyphaema has cleared completely, to exclude a traumatic retinal tear, which may be heralded by sudden onset of flashes and floaters. Smaller hyphaemas may be managed on an outpatient basis, perhaps with rest at home and the use of atropine drops and can be considered only if daily IOP monitoring is not required. The first principle of management at the location where the injury was sustained is copious irrigation of the eyes for at least 10 min with running water. Chemical trauma requires priority assessment on arrival at an emergency centre and immediate irrigation if this has not been done or was inadequate. Alcohol and solvent burns occur from splashes while painting and cleaning. Although the epithelium is frequently burnt, it regenerates rapidly. The condition is very painful initially, but heals with topical antibiotic and patching for 48 h. Alkali and acid burns are potentially more serious because of the ability of the burning agent to alter the pH in the anterior chamber of the eye and inflict chemical damage on the iris and lens. Caustic soda, lime and plaster, commonly used in industry, may inflict painful, deep and destructive ocular burns. Splashes of acids, such as sulphuric and hydrochloric, if concentrated, will cause equally destructive injury. Assessment of the ocular burn should be done using topical anaesthetic drops and fluorescein staining to determine the area of surface injury. The eyelids should be everted and the fornices carefully examined and swept gently with a cotton bud to ensure there is no particulate caustic agent remaining. Chemical burns where the epithelium is intact or minimally disturbed can usually wait 24 h before review by an ophthalmologist. Burns involving more than one-third of the epithelium and the corneal edge, with any clouding of the cornea, are potentially more serious as subsequent melting of the cornea by collagenase action may ensue. These burns should all be further irrigated in the ED with a buffered sterile solution, such as lactated Ringer’s (Hartmann’s). The irrigation should continue until the tears are neutral to litmus testing. More serious caustic injuries have shown a significant improvement in outcome with the introduction of 10% citrate and ascorbate drops, commencing 2-hourly for 48 h and reducing over the week, in combination with 1 g oral ascorbic acid daily. This regimen has an inhibitory effect on corneal melting. Topical antibiotic (e.g. chloramphenicol) is used; topical steroid is used under ophthalmic supervision. Exposure of the eyes to prolonged or severe ultraviolet radiation results in widespread punctate epithelial loss from the corneal surface. This is most frequently seen in welders who have not used sufficient eye protection while working or workers who have been inadvertently adjacent to welding. Patients complain of moderate to severe ocular discomfort with excessive watering and foreign body sensation. This usually occurs some hours after exposure to the inciting ocular radiation. Ocular examination shows widespread punctate fluorescein staining, usually with no ulceration and no evidence of foreign body present. It is usual to elicit a history of exposure to welding. Other instances in which excessive ultraviolet radiation may be encountered are in alpine snowfields and tanning beds. Treatment is supportive with oral analgesia and explanation of the cause and likely time course. The symptoms generally settle within 24–48 h as the epithelium recovers. While local anaesthetics give short-term relief, it is not appropriate to use these as a treatment. Acute primary angle-closure (APAC) is characterized by an acute impairment of the outflow of aqueous from the anterior chamber in an anatomically predisposed (crowded) eye. This results in a rapid and severe elevation in IOP. Usual IOP lies between 10 and 21 mmHg but, in cases of APAC, can rise to>60 mmHg. This is manifested as severe pain, blurring of vision and redness. The pain may be severe enough to cause nausea and vomiting and may be poorly localized to the eye. Visual disturbance can be preceded by halos around lights and, in established cases, is due to corneal oedema. Relative hypoxia of the pupillary sphincter due to elevated pressure results in a pupil unresponsive to light stimulation. The pupil is classically fixed and mid-dilated. The associated inflammation induces congestion of conjunctival and episcleral vessels. The term ‘acute angle-closure glaucoma’ is no longer regarded as appropriate, as there may be no optic nerve head cupping or visual field loss – the features that define glaucoma – at the acute presentation. Treatment of APAC is aimed at lowering the IOP and allowing the flow of aqueous from the posterior to the anterior chamber. Acetazolamide 500 mg IV and/or topical apraclonidine or brimonidine may be effective in acutely lowering the pressure and thereby reducing pain. If ineffective, subsequent constriction of the pupil with 2% pilocarpine, a parasympathomimetic, may alleviate the forward bowing of the iris, relieve the pupil block and re-establish aqueous flow and angle drainage. One drop is initially instilled every 5 min for 15 min and then half-hourly. If the pressure is very high, however, the ischaemia induced will render the pupillary sphincter unresponsive to the pilocarpine. In these cases, it may be necessary to move to early laser treatment. A peripheral iridotomy (PI) is performed using the yttrium:aluminium:garnet (YAG) laser to allow aqueous permanently to bypass the pupil and remove the risk of further episodes of APAC. This may be done acutely or electively. The anatomical predisposition to APAC is usually bilateral and a PI is also performed in the other eye as an elective procedure. Until this is done, miotics are instilled (G. pilocarpine 2% qid) in the unaffected eye to avoid the risk of APAC. Early YAG laser PI in the affected eye may be hampered by corneal oedema. Argon laser peripheral iridoplasty may be used in the acute phase for resistant attacks or where corneal oedema precludes YAG laser PI [3,4]. Acute iritis (AI) is an inflammatory response in the ciliary body and the iris. As part of this response there is an increase in vascular dilatation and permeability, with release of inflammatory mediators and cells that can damage intraocular structures. Acute iritis is usually an idiopathic condition with no systemic cause or association. Less commonly, associated conditions may include HLA-B27-related disease, sarcoidosis, inflammatory bowel disease, including ulcerative colitis and Crohn’s disease, connective tissue disorders, such as ankylosing spondylitis and ocular infection, including herpetic disease or toxoplasmosis. A complete history will often give clues to these associations. Acute iritis is generally unilateral, although bilateral involvement is seen. It is characterized by pain, redness and visual disturbance. The pain is constant and exacerbated by light owing to movement of the inflamed iris. Dilatation of the conjunctival and episcleral vessels is apparent, particularly in the vessels adjacent to the corneal limbus, often referred to as limbal flush. Visual acuity can be reduced by varying degrees depending on the severity of inflammation. The pupil is constricted due to irritation. Examination of the anterior segment with the slit lamp will reveal evidence of increased vascular permeability, seen as fibrin clumps, flare and inflammatory cells in the aqueous released from the vessels. In some cases, small collections of neutrophils can be seen aggregating on the posterior surface of the cornea as keratic precipitates (KP) (Fig. 16.1.3). In cases of severe inflammation, cells can accumulate in the inferior anterior chamber and a sediment level can be seen as a hypopyon. The IOP may be raised. Treatment of AI is directed towards resolution of the inflammatory response and limiting the ocular effects of this response. The mainstay of treatment is intensive, topical steroid eye drops (prednisolone acetate 1%, up to hourly in severe cases). In severe cases, orbital steroid injections or oral steroids may be necessary. Mydriatic eye drops (G. homatropine 2% qid) are used to break any lens–iris adhesions and to limit the extent of permanent adhesions. In ‘splinting’ the iris, these drops also provide pain relief by limiting pupil movement. As the degree of inflammation decreases on slit-lamp examination, the topical treatment is decreased in frequency. The long-term use of topical steroid drops is not without risk and can be associated with the development of glaucoma, cataract and concurrent ocular surface infection, such as herpes simplex keratitis. The surface of the eye is protected by several mechanisms from penetration by infectious agents, both bacterial and viral. The flow of tears over the surface washes debris away and contains antibodies and lysozymes. The smooth surface of the corneal epithelium hinders the adherence of infectious agents and the rapid repair of any defect in the epithelium limits the likelihood of penetration by such agents. If these defences are impaired in any way, there is the possibility of penetration into the corneal stroma and active infection may occur. Bacterial keratitis is characterized by a focus of infection with an associated inflammatory response. Patients complain of pain, redness, watering and a decrease in visual acuity. Fluorescein staining shows an area of ulceration over the infection, which appears as an opacity or area of whiteness within the cornea. Marked conjunctival and episcleral injection results in a unilateral red eye. Evidence of intraocular inflammation is usually present, with cells and flare being seen in the anterior chamber on slit-lamp examination. In severe cases, a collection of inflammatory cells can be seen in the inferior part of the anterior chamber as sediment, called a hypopyon. The most important aspect of management is to identify the infectious agent and to commence appropriate antibiotic treatment. A specimen is taken via a scraping for microbiological assessment, including Gram staining and culture. Under topical anaesthetic, using a preservative-free single-use dispenser of benoxinate or tetracaine, a sterile 23 G needle is used to gather a small specimen. This is transferred directly to glass slides and also plated on to HB and chocolate agar plates for culture. Fungal cultures may be indicated. Antibiotic therapy is not delayed until the results are available, but is commenced on a broad-spectrum basis, such as the intensive use of a fluoroquinolone eye drop (e.g. G. ciprofloxacin) on an hourly basis. Daily monitoring with slit-lamp examination is mandatory and severe infections require hospital admission. This regimen can be modified when culture and sensitivity results are available. Herpes simplex keratitis usually presents initially as an infection of the epithelial cell layer, although with recurrent episodes, stromal involvement may be seen. It is most often a unilateral infection. As with other herpetic infections, it is not possible to eradicate the virus, but limitation of inflammatory-mediated damage is important. Patients complain of foreign body sensation, redness, watering and a variable decrease in visual acuity. On examination, the areas of infected epithelium can be seen as a branching irregularity or dendrite on the surface of the eye (Fig. 16.1.4). Multiple dendrites may be scattered over the surface, particularly in immunocompromised patients. These are best seen when the cornea is stained with fluorescein and viewed by the slit lamp Treatment is directed to clearing the virus from the cornea to promote epithelial healing and limit stromal involvement and damaging corneal inflammation. A single pass with a sterile cotton bud rolled across the ulcer will deplete the viral load; an antiviral ointment (acyclovir) is then instilled five times daily until there is resolution of the epithelial lesions and then ceased as long-term usage may be toxic to the unaffected corneal epithelium. Steroid eye drops are contraindicated except under the strict supervision of an ophthalmologist. This highly contagious conjunctival infection is the commonest cause of viral conjunctivitis. Initial presentation is usually with a short history of discomfort or aching, watery discharge, redness, crusting of the eyelid margins and ocular foreign body sensation. While ultimately often bilateral, it may be unilateral on initial presentation. There is often a history of either recent upper respiratory tract infection or contact with someone with a current episode of adenoviral infection. Examination shows a follicular pattern of conjunctivitis, particularly in the subtarsal conjunctiva. In severe cases, subconjunctival haemorrhage and pseudomembrane formation can occur. The bulbar conjunctiva is often inflamed and a watery discharge is noted. There is often crusting of the lid margin and eyelashes. Uncommonly, the cornea can be involved with focal subepithelial infiltrates noted with minimal overlying epithelial disturbance. This is an immune response and can affect the visual acuity if in the visual axis. There is no evidence of intraocular inflammation and preauricular lymphadenopathy is often noted. The appearances may be asymmetrical. It is an acute, self-limiting disease (2–3 weeks) for which no specific treatment is required. There is no evidence that topical antibiotics or antiviral agents affect the course of the infection. Lid hygiene and cold compresses may be helpful for symptomatic relief. Topical steroid eyedrops are only given under strict ophthalmic supervision for corneal infiltrates if vision is affected. It is also important to stress the contagious nature of this condition and to encourage hand washing and the use of one’s own towel, pillow and face washer. Acute visual failure is any acute loss of visual acuity, visual field or colour vision. Most of the sinister causes of acute visual failure are painless (Table 16.1.1) and the absence of apparent distress may result in the patient being triaged in error to a non-acute review. Effective emergency management depends upon rapid recognition of those conditions for which acute therapy is available (Table 16.1.2). Some conditions have no effective therapy or are more appropriately managed on an outpatient basis. Table 16.1.1 Symptoms significant for cause in acute visual failure Table 16.1.2 Acute visual failure for which acute therapy is available Particular attention should be paid to the rapidity of onset, degree and location in space of visual loss, previous episodes and associated symptoms. One should distinguish history between acute onset and acute discovery of visual loss, as a patient may discover decreased vision from, for example, cataract, by inadvertently covering one eye for the first time. Testing of the visual acuity and visual field will clarify uni- or binocular involvement. Examination of the pupils is mandatory before pharmacological dilatation. Test for a relative afferent pupillary defect (RAPD), one of the few objective signs. When required, pupils will dilate in 10–15 min with tropicamide 1.0% drops, which last 1–2 h. Pupils should not be dilated if the patient requires monitoring for a head injury. Bilateral visual field loss usually implicates a retrochiasmal and, therefore, non-ocular cause. This visual field defect will, however, respect a vertical midline. In contrast, the retinal nerve fibre layer and retinal vascular elements within the eye are distributed around a horizontal midline and may thus involve a superior or inferior (i.e. horizontal) hemifield. Localized ocular pathology does not cause a visual field defect respecting a vertical midline. Bilateral acute, complete, visual failure is uncommon. Bilateral occipital infarction may present with bilateral blindness, but pupil responses would be expected to be intact. Rapidly progressive bilateral sequential visual loss from temporal arteritis is occasionally encountered. Other prechiasmal causes of bilateral, simultaneous, ocular involvement include toxic causes, such as poisoning with either quinine or methanol, where the patient presents with bilateral blindness and fixed, widely dilated pupils. Visual recovery in these cases is variable and the efficacy of a range of therapeutic interventions is controversial [5–7]. The history is typically of sudden, painless loss of vision in the affected eye over seconds. This may have been preceded by episodes of transient loss of vision (amaurosis fugax) in the previous days or weeks. Mean age of presentation is in the 70 s. Men are more frequently affected and the patient may be a ‘vasculopath’. Carotid disease is frequently implicated, with an embolus often being the cause of the obstruction, but its absence does not preclude the diagnosis, as the obstruction may lie behind the lamina cribrosa. Erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) should be tested when an embolus is not seen: temporal arteritis causes 5% of cases of CRAO. The visual acuity is drastically reduced, often to the level of light perception, with an RAPD present on the affected side. Fundus examination shows creamy-white retinal oedema (cloudy swelling) with a central red fovea – the ‘cherry-red spot’ – caused by the absence of oedema in the thinner retina at the fovea. The arterioles may be attenuated, with segmentation (‘cattle-trucking’) of the blood column. An embolus may be seen at any point along the retinal arterioles, from the disc to the periphery. Acute treatment proceeds on the assumption that the cause is embolic. The principles of therapy are, therefore, to vasodilate the retinal arterial circulation in order to promote dislodgement of the embolus from a proximal position and encourage its movement downstream to a less strategic site. All the measures currently used are directed to lowering the IOP, thereby relieving the compressive effect on the intraocular vasculature. Intravenous or oral acetazolamide 500 mg will lower IOP within 15–30 min; pulsed ocular compression (‘ocular massage’) involves cyclical sustained compression of the globe for 10–15 s before sudden release of this compression, continuing for 5–10 min. The release of pressure may result in a momentary marked increase in the perfusion pressure gradient and dislodge an embolus. The use of carbogen gas (95% oxygen/5% carbon dioxide) is now largely historical, but carbon dioxide rebreathing may be tried for its central vasodilatory effect. Definitive reduction of IOP is achieved with anterior chamber paracentesis by the removal of aqueous from the eye, but this is a procedure that cannot realistically be undertaken by the inexperienced and consultation by an ophthalmologist is needed. Timely intra-arterial fibrinolytic therapy is often beyond the logistical capabilities of many institutions and trials have so far shown a discouraging level of co-morbidity from the treatment [8–11]. The place of hyperbaric therapy is uncertain at this time [12]. Visual outcomes are generally poor in CRAO, but occasional successes justify aggressive intervention if the patient presents within 12 h. Non-acute management must include investigations to define the embolic source. Central or branch retinal vein occlusion may present as a painless blurring of vision that is not sudden. Patients are usually in the older age group, often with systemic hypertension, diabetes mellitus and glaucoma. Visual acuity varies with severity, as does the presence of an RAPD. The characteristic fundus appearance is of extensive intraretinal haemorrhage with a variable number of cotton wool spots (‘margherita pizza’). There may be disc oedema, with venous tortuosity and a generally congested appearance. If an insufficiently wide fundus view is obtained and this diagnosis missed, the patient may be subjected to unnecessary investigations to determine the cause of presumed ‘papilloedema’. There is no emergency management specific to the vein occlusion that will positively influence the visual outcome – systemic hypertension and raised IOP rarely require acute control – and the patient should therefore be referred to the next ophthalmic outpatient clinic. Arteritic anterior ischaemic optic neuropathy (AION) is the feared visual loss of giant cell (temporal) arteritis (GCA). The patient is commonly mid-70 s or older and more often female. Presentation is with profound vision loss in one eye. This may have been preceded by brief premonitory visual obscurations or double vision, to which the patient may not have ascribed significance. Systematic questioning may reveal specific features, such as jaw claudication, headache, scalp tenderness, anorexia, malaise, weight loss or night sweats, and there may be a history of polymyalgia rheumatica in up to 50% of cases. Giant cell arteritis is a systemic illness with the potential for devastating visual loss, as well as long-term life-threatening non-ophthalmic complications. Vision may be reduced at presentation to the level of perception of light only. An RAPD will be present. Total field loss in the affected eye is usual. The optic disc is almost invariably oedematous, but the fundus may be otherwise normal. Evidence of decreased acuity, colour vision deficits and disc oedema should also be sought in the other eye. Palpation of the temporal arteries will often be abnormal, with the pulses perhaps absent or the arteries thickened and tender. Clinical suspicion requires blood to be drawn for ESR and CRP. Treatment should then be started on an urgent basis and must not be delayed or deferred until after temporal artery biopsy. The biopsy will remain positive for at least several days despite steroids. Elevation of both ESR and CRP is highly specific for a diagnosis of GCA, but does not avoid the need for biopsy [13]. Urgent referral to an ophthalmologist is required. Prednisolone 1 mg/kg daily is an accepted dose, although recent experience has suggested that ‘pulse’ methylprednisolone 500 mg IV daily or twice daily over 1–2 h is safe and more efficacious in suppressing the inflammation and this has become standard therapy in a number of centres. This is generally used for 3 days and oral prednisolone is then substituted [14–16]. Treatment will be prolonged (at least 6 months) and should be undertaken in cooperation with a physician. Attention must be directed to the avoidance of steroid complications in this aged patient group. Non-arteritic AION is classically seen in males in the late 50s and 60s who have a history of cardiac or vascular disease, hypertension, diabetes or smoking. The visual presentation may be similar to that seen with GCA – although the visual acuity and field loss may not be as profound (may be a hemifield loss) and the specific systemic symptoms are absent – so that management must be as for arteritic AION until GCA is excluded. Retinal detachment is usually a result of retinal hole formation, with seepage of fluid into the subretinal space and lifting of the retina. This may occur as a result of trauma, but is more often seen in an older age group as a result of vitreous traction in spontaneous posterior vitreous detachment (PVD) and is predisposed to by high myopia (short-sightedness). Exudative retinal detachment is less common and is associated with underlying pathology. Shrinkage and detachment of the vitreous is common in the older population and produces a new onset of floaters – wispy spots, threads or ‘spider webs’ in the vision. As part of this process, vitreous traction on the retina may produce flashes of light (photopsia) seen particularly in the temporal periphery of vision in that eye. These flashes can usually be distinguished from the visual aura of migraine. While PVD is usually not serious in its own right, early elective ophthalmic review is required as it is not possible to exclude related changes predisposing to retinal detachment without dilated examination of the retinal periphery. With a history of flashes and floaters, the presence of pigmented cells (‘tobacco dust’) in the anterior vitreous should alert one to the possibility of a retinal hole and subsequent retinal detachment. A detached retina shows a visual field defect, which will be described as a shadow or curtain, corresponding to the area of detachment, i.e. inferior field defect equals superior retinal detachment. Vision loss is painless. There may be an RAPD, depending on the amount of retina involved. Treatment will usually require surgery. If the visual acuity is normal, the macula is likely to be still attached and referral to an ophthalmologist specializing in vitreo-retinal surgery is urgent. The common causes are proliferative diabetic retinopathy, chronic branch retinal vein occlusion, posterior vitreous detachment or trauma. Patients with an acute vitreous haemorrhage may have symptoms varying from a few floaters causing blurred vision to a total loss of vision to a level of light perception, depending on the density of the haemorrhage. Any loss of vision is painless. The red reflex may be poor and the view of the retina may be similarly impaired. Media opacities do not affect pupil light reflexes, so there should be no RAPD, unless the underlying retina is damaged or detached. The patient should be referred for early ophthalmic assessment – urgent if an RAPD is present – which may include B scan ultrasonography to exclude retinal detachment if the retinal view is inadequate. Anticoagulants should be avoided where possible. In 10–20% of cases of age-related macular degeneration (AMD), an exudative-type disease is seen and, in its most sinister form, this will involve subretinal neovascularization (SRNV). These patients may present with painless distortion of vision, particularly ‘metamorphopsia’ – a complaint that objects that they know to be straight appear curved. Visual acuity is reduced, depending on the stage of the disease; an RAPD is rarely seen owing to the relatively small area of retina involved, which manifests as a central scotoma on field testing. Macular drusen (yellow spots), retinal thickening and haemorrhage may be seen, with at least drusen usually also seen in the fellow eye. Acute symptoms must not be dismissed. With appropriate treatment, central vision may be preserved in a proportion of these patients. Rapid ophthalmic review is therefore appropriate. Recent advances in treatment with antivascular endothelial growth factor (VEGF) agents, such as ranibizumab (Lucentis) or bevacizumab (Avastin), have revolutionized the prognosis and sight can often now be preserved [17,18]. Optic neuritis classically presents in young females and may be the first presentation of a demyelinating illness. Visual symptoms are not usually sudden and presentation is thus seldom acute. The vision declines gradually over days, perhaps to the level of 6/36–6/60, with loss of colour vision being prominent. The common visual field defect is a central scotoma, but many variations are possible and an RAPD should always be present. If disc oedema is not seen, the diagnosis may be retrobulbar neuritis. There may be pain on medial or superior eye movement. Good spontaneous recovery has made the value of treating optic neuritis controversial: the results of the Optic Neuritis Treatment Trial [19] would suggest that there is no place for oral prednisolone alone in management. The benefit of ‘pulse’ intravenous methylprednisolone seems restricted to shortening the acute episode, without influencing the possibility of progression to multiple sclerosis or the final visual outcome [20]. However, there is usually no role for acute intervention and referral within a day or two to a neurologist is satisfactory.Ocular trauma
History
Examination
Visual acuity

Investigation
Management of specific injuries
Superficial injury
Corneal abrasion
Corneal foreign body
Conjunctival laceration
Penetrating injury
Blunt injury
Chemical burns
Flash burns
Acute inflammatory conditions
Acute primary angle-closure (glaucoma)
Acute iritis
Acute infectious keratitis
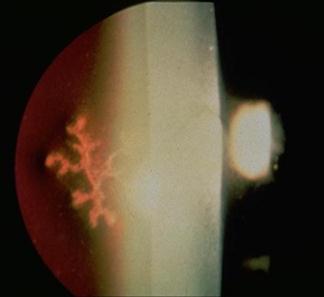
Adenoviral conjunctivitis
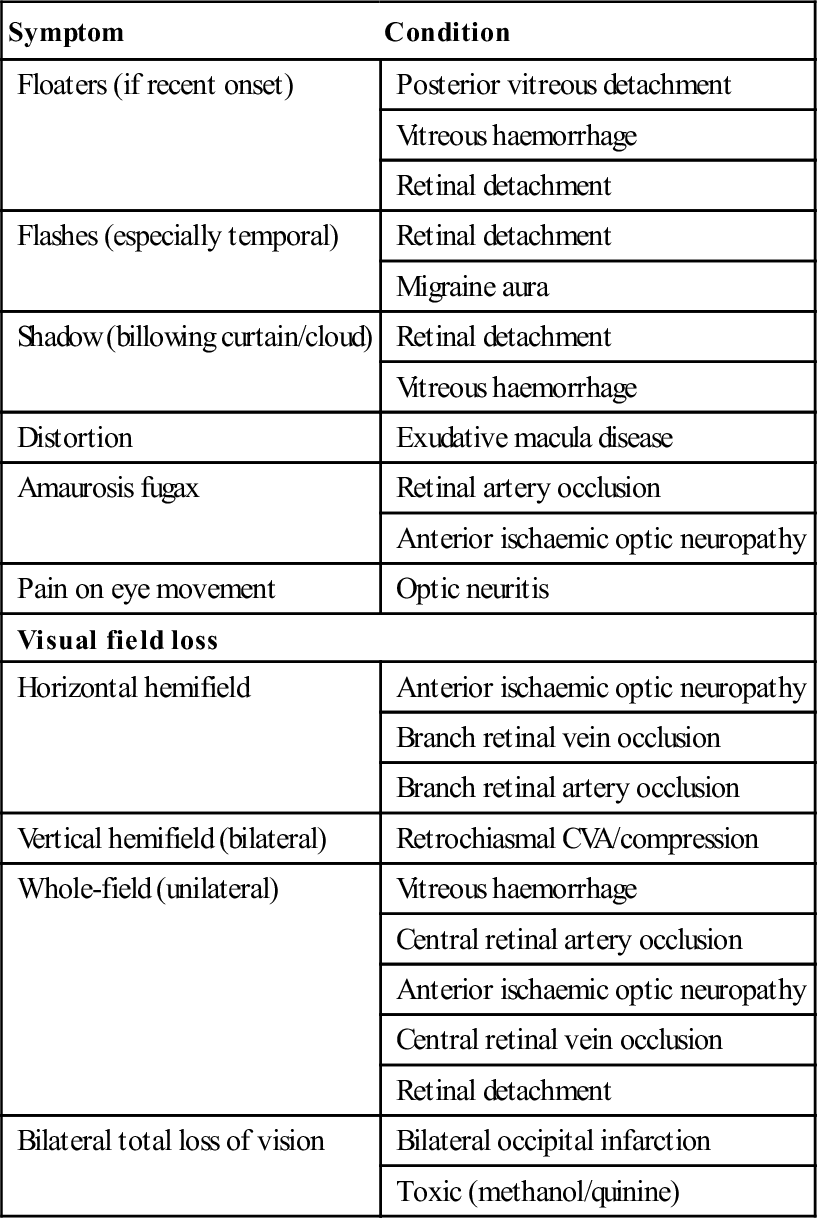
Acute visual failure
Introduction
Symptom
Condition
Floaters (if recent onset)
Posterior vitreous detachment
Vitreous haemorrhage
Retinal detachment
Flashes (especially temporal)
Retinal detachment
Migraine aura
Shadow (billowing curtain/cloud)
Retinal detachment
Vitreous haemorrhage
Distortion
Exudative macula disease
Amaurosis fugax
Retinal artery occlusion
Anterior ischaemic optic neuropathy
Pain on eye movement
Optic neuritis
Visual field loss
Horizontal hemifield
Anterior ischaemic optic neuropathy
Branch retinal vein occlusion
Branch retinal artery occlusion
Vertical hemifield (bilateral)
Retrochiasmal CVA/compression
Whole-field (unilateral)
Vitreous haemorrhage
Central retinal artery occlusion
Anterior ischaemic optic neuropathy
Central retinal vein occlusion
Retinal detachment
Bilateral total loss of vision
Bilateral occipital infarction
Toxic (methanol/quinine)
Condition
Therapy
Central (or branch) retinal artery occlusion
Acetazolamide
CO2 rebreathing
Pulsed ocular compression
Anterior chamber paracentesis
Anterior ischaemic optic neuropathy
Steroids
Exudative age-related macula degeneration
Anti-VEGF injections
Laser
Retinal detachment
Surgery
Clinical assessment
History
Examination
Bilateral vision loss
Central retinal artery occlusion (CRAO)
Central (branch) retinal vein occlusion
Anterior ischaemic optic neuropathy
Retinal detachment
Posterior vitreous detachment (PVD)
Retinal detachment
Vitreous haemorrhage
Age-related macular degeneration
Optic neuritis