CHAPTER 12
Clinical biochemistry of the gastrointestinal tract
Ingvar T. Bjarnason; Roy A. Sherwood
CHAPTER OUTLINE
Diagnosis of H. pylori infection
SMALL BOWEL BACTERIAL OVERGROWTH
The normal intestinal microflora
Definition, causes and symptoms of small bowel bacterial overgrowth
Diagnosis of small bowel bacterial overgrowth
MALDIGESTION AND MALABSORPTION
FAECAL TESTS OF INTESTINAL INFLAMMATION
Neuroendocrine tumours of the gastrointestinal tract and pancreas (NETs)
INTRODUCTION
Gastrointestinal complaints continue to account for a sizeable proportion of medical consultations. This is best illustrated by the fact that irritable bowel syndrome (IBS), the most commonly encountered gastrointestinal disorder, is estimated to have a prevalence of 20% in the UK. About 30% of these people seek medical attention, which in turn accounts for as many as 20–50% of patients attending general gastroenterology outpatient clinics in the UK. Furthermore, the prevalence of reflux oesophagitis and non-specific dyspepsia is increasing, and with the advent of safe and effective drugs to inhibit gastric acid secretion, it is estimated that the cost of treatment for these disorders may grow to account for as much as 20% of drug expenditure in primary care.
The development of endoscopic techniques has revolutionized the investigation of gastrointestinal disorders. The combination of upper gastrointestinal endoscopy, colonoscopy and optic and/or wireless capsule enteroscopy now allows visualization of the whole of the gastrointestinal tract and biopsies can be obtained from all parts of the bowel. This has resulted in loss of demand for many of the classic biochemical investigations (such as gastric acid secretion) and, as some of these methods are now obsolete, we have omitted them from this chapter. Endoscopy and biochemical investigations, however, provide different kinds of information that are, in many respects, complementary. Endoscopy provides a static morphological picture that by itself, or with biopsy, has the potential to provide a diagnosis that automatically translates to treatment. Biochemical methods, on the other hand, provide a wider range of information. These include the following.
• Clinical screening tests prior to invasive investigation. Examples of these kinds of investigations are serum transglutaminase antibody measurements and tests of intestinal permeability, which, if normal, may avoid the need for more invasive tests and, if positive, are an indication for jejunal or duodenal biopsy.
• The detection and assessment of the severity of intestinal dysfunction. These tests are called upon in order to provide an explanation for clinical signs (such as weight loss), for monitoring response to therapy and for confirmation of diagnosis (e.g. measurement of intestinal permeability following gluten withdrawal and challenge in coeliac disease, although this is rarely necessary in practice).
• Providing prognostic information. Biochemical tests are uniquely suited to assess functional changes that may herald a drastic change in the activity of the disease. This is best exemplified by increased concentrations of inflammatory markers in the faeces of patients with clinically quiescent inflammatory bowel disease (IBD, i.e. ulcerative colitis and Crohn disease), as these predict imminent clinical relapse.
• Investigating the impact of non-intestinal factors on intestinal function, whether biochemical or physiological. These may be exogenous, for example related to radiotherapy, drugs, alcohol, dietary or environmental factors, or endogenous, for example due to malnutrition, reduced blood flow, anaemia etc. Non-steroidal anti-inflammatory drug (NSAID)-induced enteropathy is an example of gastrointestinal disease caused by an exogenous factor.
Our intention in this chapter is to review new and established laboratory-based investigations of the gastrointestinal tract that are useful clinically or for research purposes. We will not always provide the reference ranges for test results, as these may differ depending on environmental, geographical and racial factors, as well as differences in methodology.
MOUTH AND OESOPHAGUS
Clinical biochemistry has not had a major impact in the investigation of oral or oesophageal diseases. The reason is obvious in that the main functions of these organs are physical, namely grinding of food and transport to the stomach. Nevertheless, the buccal mucosa is a common site for obtaining material for genetic analysis and saliva can be assayed for a number of antibodies (although this is rarely done in clinical practice). Although the salivary glands and parotid secretions contain amylase (and initiate digestion of complex carbohydrates), peptides and growth factors (that may confer a degree of protection to the stomach and accelerate healing of gastric lesions), measurements of these are not helpful clinically and are used only in research.
The common oesophageal disturbances such as reflux and dysmotility (oesophageal spasms, neuromuscular incoordination, achalasia etc.) are investigated and diagnosed by imaging techniques (endoscopy, radiology), high-resolution manometry and pH recording, rather than by biochemical methods. Oesophageal cancer, infections (cytomegalovirus, Candida albicans and herpes simplex) and drug- and radiation-induced diseases are similarly diagnosed largely from the clinical history and by endoscopy and biopsy.
STOMACH
The stomach acts as a reservoir for ingested food, where it is mixed with acid, mucus and pepsin and then released at a controlled rate into the duodenum. The mucosal surface of the stomach is lined with mucus-secreting columnar epithelial cells, interrupted by gastric pits containing acid-secreting parietal (or oxyntic) cells and pepsinogen-secreting chief cells. The secreted hydrochloric acid kills many ingested bacteria, provides the acid pH necessary for pepsin (from pepsinogen) to begin digesting proteins and stimulates bile secretion. Mucus secretion is necessary to protect the mucosal surface of the stomach wall from its acidic contents. The stomach also secretes intrinsic factor, which binds vitamin B12 and allows it to be absorbed in the terminal ileum. The muscular contractions of the stomach wall have the mechanical effect of macerating food.
Helicobacter pylori
Helicobacter pylori is now accepted to be the main cause of gastric and duodenal ulcers; other causes include NSAIDs and, very rarely, the Zollinger–Ellison syndrome. Helicobacter pylori may play an important role in gastric cancers (adenocarcinomas and mucosal–associated lymphoid tissue lymphomas) and is undoubtedly the main cause of chronic gastritis, though in most patients this is asymptomatic. Many experts also believe H. pylori to be a major cause of non ulcer-related dyspepsia, and recent recommendations suggest that anyone who so wishes should be tested for H. pylori and treated if found to be positive.
The mode of transmission of H. pylori is uncertain. It is thought to be an infection acquired in childhood, presumably by the faeco–oral route, which would explain the higher prevalence in developing countries and the progressive decline in prevalence in developed countries, with better hygiene, preservation of food and smaller family sizes. Whatever the mode of transmission, H. pylori elicits an inflammatory response that is usually asymptomatic. This takes the form of chronic gastritis, with an acute inflammatory cell infiltrate of variable severity. Nine out of ten people infected by H. pylori do not develop ulcers. The variable development of clinically significant disease relates to the site of infection, virulence factors (e.g. phospholipases, vacuolating cytotoxins (VAC), CagA protein) and poorly defined host factors that include blood flow, mucus secretion and pepsinogen stimulation. There are three recognized patterns of infection by H. pylori in the stomach. The most common type is that of low-grade inflammation of the mid-body of the stomach. This occurs in people with a high threshold for immune response and H. pylori that has a low expression of CagA and VAC. Gastric acid secretion is reduced and there are usually no significant clinical consequences. If the microbe is predominantly in the antrum, the inflammation leads to dysfunction of the antral G-cells that become hyper-reactive and secrete disproportionate amounts of gastrin in response to food and gastric distension. Gastrin has a trophic effect on the gastric parietal cells, increasing their number and stimulating them directly, via cell surface receptors, to produce more acid, resulting in the hyper-acidic state characteristic of patients with duodenal ulceration.
The third pattern, a pangastric infection with H. pylori, is characteristic of those who develop gastric ulcers and those at risk of developing gastric cancer. These individuals tend to have normal or reduced gastric acid secretion. In theory, it would be possible to perform an endoscopy on every patient with indigestion and document the pattern of infection. However, this would be prohibitively expensive owing to the large number of symptomatic patients. A less expensive option would be to assess a panel of hormones that are secreted from defined anatomical locations in the stomach (for instance pepsinogen 1 and 2 and gastrin-17), but further work is required before this approach becomes widely accepted. A more pragmatic approach has, therefore, been adopted in adult patients under 50–55 years of age, namely to test and treat for H. pylori infection, irrespective of the precise diagnosis (ulcer, gastritis etc.), unless ‘red flag’ indicators suggestive of malignancy (e.g. weight loss, dysphagia) are present.
Having established the presence of H. pylori (see below), there are a number of eradication regimens available, and the eradication failure rate, after trying at least three different regimens, should be < 5–10%. If H. pylori is successfully eradicated, reinfection rates are extremely low (< 1%).
Diagnosis of H. pylori infection
There are various methods for the diagnosis of H. pylori infection. At endoscopy, it is possible to take biopsies from which the organism can be visualized histologically or cultured, the latter being performed only if there are repeated treatment failures. Alternatively, the biopsy can be tested directly using commercially available kits incorporating a gel containing urea and an indicator that changes colour at an alkaline pH. In the presence of H. pylori (which contains urease), the urea is broken down to carbon dioxide and ammonia. The latter increases the pH of the gel and a colour change takes place. Less invasive methods involve measurement of H. pylori-specific antibodies (IgG or IgA). These are eminently suitable for screening (sensitivity 92%, specificity 83%), but cannot be used to document the success of treatment as high antibody titres can persist despite successful eradication.
The H. pylori breath test is currently the most widely used method for non-invasive diagnosis. It uses the same principle as the biopsy-based test and comes at a fraction of the cost of endoscopy and biopsy. Patients are given isotopically labelled urea (13C or 14C) to drink. If there is no urease present, the urea is absorbed intact and excreted in the urine. If H. pylori is present, labelled carbon dioxide is absorbed into the circulation and exhaled in the breath. Breath samples are obtained before, and 45–60 min after, drinking the labelled urea. The detection of labelled carbon is most commonly performed by mass spectrophotometry. The breath test is widely used and can also be used to assess the success of the treatment (sensitivity 95%, specificity 96%). Lastly, there are commercial kits that use the polymerase chain reaction to amplify nuclear sequences specific for H. pylori from saliva or faeces (sensitivity 95%, specificity 94%). The stool tests are increasingly being used for the detection and confirmation of successful eradication of H. pylori rather than the breath test.
Gastric acid secretion
Before the discovery of the role of H. pylori in peptic ulcer disease, it was common practice to investigate gastric acid secretion in patients with duodenal ulcers, who tend to be hyper-secretors, and those with gastric ulcers, who tend to have normal or low secretion rates. Gastric cancers are often associated with hypochlorhydria, and achlorhydria is common in pernicious anaemia and in gastric cancer.
Acid secretion tests are now only rarely used in the research setting and are no longer available in the vast majority of clinical biochemistry departments: they have become obsolete as the test results do not alter clinical practice or management.
Gastrin
In < 0.5% of patients with gastroduodenal ulcers, the cause is unregulated gastrin release from an endocrine tumour termed a gastrinoma, which can lead to the Zollinger–Ellison syndrome characterized by multiple gastroduodenal ulcers. The persistently high plasma gastrin concentrations not only lead to marked hypersecretion of gastric acid, but also, because the hormone is trophic for parietal cells, to increased parietal cell mass. Patients may present with symptoms that are indistinguishable from H. pylori-associated peptic ulcer disease. However, coexisting diarrhoea (due to acidic inactivation of pancreatic enzymes), multiple ulcers involving the second part of the duodenum, recurrent ulceration and ulcers refractory to conventional treatment should always arouse suspicion. Gastrinomas are either sporadic (the more common form) or associated with multiple endocrine neoplasia type 1 (MEN 1, Wermer syndrome), a syndrome characterized by the presence of two or more of pituitary, pancreatic islet and parathyroid tumours. Multiple endocrine neoplasia type 1 is also associated with an increased prevalence of carcinoid, adrenocortical and thyroid tumours.
Gastrinomas are commonly situated in the pancreas, but are increasingly recorded arising from the duodenum, stomach and bones. Some 60% are clearly malignant, with multiple metastases at diagnosis. Given a suspicion of the Zollinger–Ellison syndrome, the first step is to measure fasting serum gastrin, as virtually all cases are associated with high concentrations. The differential diagnosis of a mildly elevated gastrin concentration includes hypochlorhydria, long-term proton pump inhibitor drug therapy, pernicious anaemia and antral G-cell hyperplasia. Given a strong suggestion of a gastrinoma, the next step is to localize the tumour, which is best done at a specialist centre. Techniques for localization of the tumour include endoscopic ultrasound, octreotide scanning, magnetic resonance imaging (MRI), positron emission tomography (PET) and computerized tomography (CT) (the latter being particularly useful to detect metastases). Definitive diagnosis is histological. The treatment of gastrinomas usually involves a combination of surgery, chemotherapy and acid suppression for symptomatic relief.
Intrinsic factor
Intrinsic factor is a glycoprotein principally secreted by the parietal cells of the stomach. Its secretion is governed by the same biochemical processes that regulate acid secretion and its action is to assist in the absorption of vitamin B12. Vitamin B12 is released from dietary proteins by the action of pepsin and then combines with R-binders (haptocorrins), glycoproteins secreted by the stomach, that assist in its protection against acid degradation. It is subsequently cleaved from R-binder complexes by pancreatic proteolysis in the duodenum and then binds to intrinsic factor to form a resistant complex that is taken up by ileal cell receptors (cubulin).
The commonest cause of intrinsic factor deficiency is autoimmunity; the reason for requesting intrinsic factor antibody measurement is usually the discovery of a low plasma vitamin B12 concentration – a cause of macrocytic anaemia and various neurological disorders. The diagnosis of pernicious anaemia is based on the finding of a low plasma B12 concentration together with the presence of antiparietal cell antibodies and/or intrinsic factor antibodies (see Chapter 27). Current automated vitamin B12 assays suffer, in differing amounts, from interference from intrinsic factor antibodies; alternative strategies for assessment of B12 status include measurement of serum homocysteine, methylmalonic acid and holotranscobalamin concentrations. Other causes of intrinsic factor deficiency leading to low plasma B12 include gastrectomy, achlorhydria and congenital absence of intrinsic factor, which is rare. Vitamin B12 deficiency can also occur because of deficient intake (e.g. in vegans, starvation or reduced food intake of any cause) or in small bowel disease involving the terminal ileum. Gastric biopsies showing histopathological features of atrophic gastritis may be helpful and there are some indications that patients should undergo surveillance endoscopies once a diagnosis is made because of an increased incidence of gastric cancer. In patients in whom small bowel disease is suspected, the choice is that of wireless capsule enteroscopy or CT/MRI enteroclysis (specialized techniques in which a contrast medium is infused into the small intestine) for diagnosis. Previously, on finding a low vitamin B12 concentration, it was customary to request a Schilling test, which had the potential to help in the differential diagnosis, but this test has become obsolete as imaging techniques have improved. Although proton pump inhibitors induce a state of gastric acid hyposecretion and are widely used, it is exceptionally rare to see vitamin B12 deficiency in these patients.
PANCREAS
The exocrine function of the pancreas includes the production of bicarbonate and enzymes, including amylase, lipase, trypsin, chymotrypsin, esterases and carboxypeptidases. The differential diagnosis of exocrine pancreatic disease in neonates and children is predominantly between cystic fibrosis and pancreatic acinar cell aplasia (Shwachman–Diamond syndrome). The major diseases affecting the pancreas in adults are acute pancreatitis, chronic pancreatitis leading to pancreatic insufficiency and carcinoma of the pancreas. Chronic pancreatitis results in progressive loss of both islet cells and acinar tissue. Presentation is typically with recurrent upper abdominal pain radiating to the back, although malabsorption, for example steatorrhoea, may be the presenting feature. Approximately 90% of the pancreatic acinar tissue must be lost before features of malabsorption become apparent and clinically significant reduction in endocrine function generally occurs late in the disease process.
Pancreatic function tests
Tests of pancreatic function are usually divided into the direct (invasive) tests and indirect tests on blood, urine or faecal samples.
Direct or invasive function tests
The ‘gold standard’ test of pancreatic function is the secretin–pancreozymin test. This test assesses exocrine function by measuring bicarbonate and pancreatic enzyme secretion (amylase and trypsin) in aspirates from a tube sited in the duodenum, usually under fluoroscopic control. Secretin is given to induce fluid secretion, while pancreozymin or its analogue caerulein (secretin–caerulein test), is given to induce enzyme production. This test requires meticulous attention to technique in positioning the tube correctly and maintaining its patency, and is uncomfortable for the patient. This test is now rarely used in routine practice. However, it remains the test that has the highest sensitivity and specificity for the differential diagnosis of pancreatic insufficiency.
Non-invasive pancreatic function testing
Serum enzymes
The measurement of pancreatic enzymes in serum is standard practice in acute pancreatitis, but is seldom useful in the investigation of chronic pancreatitis.
Amylase is the most commonly measured enzyme owing to the availability of cheap, easily automated methods. A disadvantage is the lack of specificity for the pancreas, as amylase in the circulation is derived from both pancreatic and non-pancreatic (mostly salivary) sources in approximately equal amounts. Measurement of the specific pancreatic amylase can be achieved using immunosubtraction techniques. Consideration of the ethnicity of the patient is important when interpreting amylase results, as subjects of African origin have a higher reference range for non-pancreatic amylase. The mechanism for this difference is unknown. An increase in both the pancreatic and non-pancreatic fractions may indicate the presence of macroamylasaemia – immunoglobulin–amylase complexes that have a prolonged half-life in the circulation owing to a reduction in clearance. Non-pancreatic causes of an increased serum amylase are given in Table 12.1. Lipase has greater specificity for pancreatic disease than amylase, but methods available tend to be more complex and expensive. The majority of the lipase in blood is the pancreatic form, although a sublingual lipase is also present. Lipase is not affected by ethnicity. Trypsin is 100% specific to the pancreas and would therefore, theoretically, be the best of the three enzymes to measure. However, there are physiological obstacles that mean that trypsin itself is seldom measured in blood samples. Trypsin is produced and stored in the pancreas as its inactive zymogen form (trypsinogen), and is activated after secretion into the intestinal tract. Active trypsin entering the circulation is bound immediately to the protease inhibitors α2-macroglobulin and α1-antitrypsin. Once bound, trypsin is not measurable using standard techniques, but any trypsinogen entering the circulation can be measured as ‘immunoreactive trypsinogen’ (IRT). Immunoreactive trypsinogen can be used as a first-line test for screening for cystic fibrosis using the blood spots taken on Guthrie cards at 7–10 days of age. A national programme of cystic fibrosis screening started in 2006–2007 in the UK; IRT is used for initial screening with subsequent genetic testing for confirmation.
TABLE 12.1
Non-pancreatic causes of an elevated plasma amylase activity
| Condition | Reason for hyperamylasaemia |
| Perforated peptic ulcer | Increased entry of pancreatic enzymes into the circulation |
| Small bowel obstruction or perforation | Increased entry of pancreatic enzymes into the circulation |
| Ruptured ectopic (tubal) pregnancy and salpingitis | Release of Fallopian tube amylase |
| Salivary gland inflammation, e.g. with calculi or mumps | Release of salivary gland amylase |
| Opiate administration | Contraction of the sphincter of Oddi |
| Renal failure | Impaired renal clearance of amylase |
| Macroamylasaemia | Amylase becomes combined with a plasma protein (in some cases, an immunoglobulin), and the increased size of the complex results in impaired renal clearance. Thus, hyperamylasaemia may be detected in the absence of disease |
| Certain tumours of the lung and ovary | Production of amylase by the tumour |
| Diabetic ketoacidosis | Impaired renal clearance |
| Subjects of African origin | Higher circulating amylase of unknown cause |
Faecal tests
The measurement of faecal fat excretion as a test of fat malabsorption (and thus indirectly of pancreatic exocrine function), is now regarded as obsolete by most clinical biochemists and gastroenterologists.
Pancreatic enzymes that have been measured in faeces include chymotrypsin and elastase. Stool chymotrypsin measurements have suffered from a lack of standardization in the techniques used, making it difficult to compare results obtained from the various groups who have used the test. Measurement of faecal pancreatic elastase-1 is now recommended as the marker of choice for detecting pancreatic insufficiency. Elastase is an endopeptidase and sterol binding protein. As with chymotrypsin, elastase is not degraded during transit through the intestinal tract and is stable in faecal samples in vitro. Two commercially available enzyme linked immunosorbent assays (ELISAs) using antibodies specific for pancreatic elastase have been studied in patients with pancreatic insufficiency. Sensitivities of 60–100% for moderate to severe pancreatic disease, using a cut-off of 200 μg/g wet weight, have been reported for faecal elastase. Discrimination between diarrhoea of pancreatic and non-pancreatic origins has been reported to be good, with better specificity than chymotrypsin. Faecal elastase measurements may also be useful in determining the amount of pancreatic enzyme replacement therapy required in patients with cystic fibrosis or chronic pancreatic insufficiency (chymotrypsin cannot be used for this purpose). While biochemical tests attempt to give a functional diagnosis in chronic pancreatitis, imaging techniques provide further information that may disclose the cause. Plain abdominal radiography, endoscopic ultrasound, CT and magnetic resonance cholangiopancreatography (MRCP) are all useful. Endoscopic retrograde cholangiopancreatography (ERCP) with, or more commonly without, secretin stimulation is widely used, but carries a 10% risk of significant complications.
SMALL BOWEL BACTERIAL OVERGROWTH
With the discovery of microbes in the 19th century, theories were formulated linking the intestine with the development of systemic disease as a result of an unfavourable interaction between intestinal luminal microbes and the body. These ideas are equally evident today in the mass media, whereby producers of live yoghurts (containing viable bacteria) seek to equate ingestion of their products (probiotics) with health, vitality and happiness.
The normal intestinal microflora
Despite the perceived importance of intestinal bacteria, the reality is that we are still at the descriptive stages of assessing the normal intestinal flora and do not fully understand its function and impact on our well-being. Though the bulk of intestinal microbes inhabit the lower bowel, even the stomach is not usually sterile with bacterial population counts (predominantly Gram-positive aerobes) of up to 103 per gram of luminal content. The jejunum has a similarly Gram-positive flora, with a higher population of up to 104 per gram of luminal contents. The ileum contains a more varied flora that includes both aerobes and anaerobes, with populations in the region of 105–108 per gram. In the large intestine, Gram-negative anaerobes become the predominant species, overall bacterial populations rising significantly to 1010–1012 per gram of caecal content. Similar counts are present in the distal colon where the bacterial flora corresponds to that seen on faecal analyses.
The above data have been obtained using invasive techniques not commonly used in clinical practice. However, intestinal bacterial populations and species differ between individuals and relate in a complex way to age, diet, geographical and racial factors, antimicrobial treatment and intrinsic gut diseases.
Definition, causes and symptoms of small bowel bacterial overgrowth
The relatively small numbers of bacteria normally present in the small intestine are probably of little significance. However, problems can arise when the bacterial population of the small intestine is increased – bacterial overgrowth. An essential consideration of this definition is that bacterial overgrowth is based on quantitative and qualitative estimates of coliform bacteria in small bowel aspirates, something which is not possible in routine clinical practice. There are a number of diseases and conditions associated with small bowel bacterial overgrowth, a combination of factors predisposing to overgrowth in any given condition. Gastric hypochlorhydria of any cause may contribute to small bowel bacterial overgrowth. Other factors include: alterations in systemic immunology (e.g. isolated immunoglobin A deficiency, hypogammaglobulinaemia, combined immune deficiency, infection with human immunodeficiency virus); impaired motility (e.g. advanced age, autonomic neuropathy in diabetes, fibrosis in scleroderma); pancreatic insufficiency; oral antibiotics; intrinsic small bowel disease (e.g. jejunal diverticulosis, coeliac disease, small bowel Crohn disease), and surgery (removal of the ileocaecal junction, surgically induced blind loops and, increasingly, bariatric surgery).
Small bowel bacterial overgrowth can be asymptomatic or be associated with non-specific symptoms, such as abdominal distension (bloating), eructation, flatulence, borborygmi and diarrhoea. The classic clinical presentation includes vitamin B12 deficiency (intestinal bacteria compete with enterocytes for this vitamin), high blood folate concentrations (bacteria are a source of this vitamin) and fat malabsorption, and is rarely seen today. Rather, the condition is thought of when patients who are predisposed to overgrowth present with intestinal symptoms, for example diabetic patients with diarrhoea, weight loss and anaemia. An interesting suggestion is that small bowel bacterial overgrowth (intestinal dysbiosis) may be responsible for the symptoms in a proportion of patients diagnosed with IBS.
Diagnosis of small bowel bacterial overgrowth
Because of geographical and/or racial differences in small bowel bacterial flora, it is advisable that each laboratory independently establishes its own reference ranges for investigatory tests. The gold standard diagnostic procedure is microbiological examination (quantitative and qualitative bacteriological cultures) of small bowel contents, but this is impractical for routine clinical practice. More convenient, non-invasive biochemical procedures, based on oral administration of substances that are metabolized by bacteria to yield products that can be detected in breath, have now been introduced, but their reliability remains controversial. The substances employed are either not absorbed from the small intestine (e.g. lactulose), therefore becoming available for metabolism by bacteria, or normally absorbed from the small bowel (e.g. glucose and D-xylose) and, therefore, only subject to metabolism by bacteria when these are present in the small bowel. Lactulose and similar test substances have the theoretical advantage of assessing the whole of the small bowel while the readily absorbed ones may be more selective for the proximal small bowel. The test results are also dependent upon the rate of intestinal transit. When transit is very rapid, the ingested load may be delivered to the caecum and give a false positive result. Furthermore, the tests vary in their sensitivity, presumably due to the influence of other ill-defined factors.
The 14C-glycocholate breath test was one of the first to be used for the non-invasive diagnosis of small bowel bacterial overgrowth, but it is obsolete because false negative results are common. Small bowel bacterial breath tests now in routine use include those involving test substances labelled with 13C (less commonly 14C), labelled CO2 and breath hydrogen tests. Hydrogen breath tests depend on the fact that mammalian cells do not produce hydrogen. A breath hydrogen > 20 ppm in expired gas after administration of the test substance (50 g glucose or 10 g lactulose) indicates bacterial overgrowth. Breath hydrogen is relatively simple to measure as a near-patient test. However, there are inherent limitations in the detection of breath hydrogen, for example with smoking and because of hydrogen production by oral flora. Carbohydrate may also be retained within the gut from an earlier meal. Abnormalities in gut transit and concomitant antibiotic therapy can also influence the result. Curiously, some individuals fail to produce breath hydrogen when challenged with a non-absorbed carbohydrate such as lactulose. There are a number of different protocols available for the performance of such breath tests and their value continues to be debated.
MALDIGESTION AND MALABSORPTION
It is conventional to distinguish between impaired uptake of nutrients due to maldigestion on the one hand and malabsorption on the other. The symptoms produced by the two conditions share many clinical features and there is a very broad spectrum of underlying causes. However, as the two processes are integrated and so inextricably linked, they are often lumped together as ‘malabsorption’. A very clear understanding of both the normal processes of digestion and absorption and the ways in which they can be disturbed is crucial to identifying the role that clinical biochemistry plays, both in making the clinical diagnosis and assessing prognosis.
Clinical features
Malabsorption can involve single or multiple nutrients. Accordingly, some patients may present with a single clinical feature; others may have several. Clinical features can relate to nutrient deficiency (or deficiencies), the retention of nutrients in the gut (leading, e.g. to increased bacterial fermentation) or both.
Genetically determined intestinal lactase deficiency (see below) is a paradigm for maldigestion. The symptoms range from none, to those of abdominal bloating, excessive flatulence and watery diarrhoea (reminiscent of IBS), largely depending on the degree of the lactase deficiency, intake of lactose and intestinal transit time. Coeliac disease is an example of a condition causing malabsorption. Early recognition of the disease is now common but, in the past, the classic presenting features included: frequent loose stools, steatorrhoea, anaemia (iron or folic acid deficiency), wasting or impaired growth, osteomalacia (malabsorption of vitamin D and calcium), tetany (hypocalcaemia) and, occasionally, easy bruising (vitamin K deficiency). Exposure to gluten fractions present in wheat and several other cereals is an essential predisposing factor for coeliac disease. These fractions are thought to undergo an enzymatic reaction by transglutaminase (which catalyses the deamidation of specific residues of glutamine to glutamate). In patients with the disease (and mostly in those with the characteristic human leukocyte antigen (HLA) alleles DQ2 and DQ8), the peptide produced has an affinity for a groove in the encoded HLA molecules. This in turn evokes an abnormal intestinal mucosal T cell response, resulting in an inflammatory response that causes the characteristic changes of coeliac disease. The antibody response evoked can be useful in diagnosis. Anti-tissue transglutaminase antibodies are not a substitute for the definitive diagnostic test (the demonstration of total villous atrophy in a small bowel biopsy), but are useful as a screening procedure prior to endoscopy and in follow-up, as they usually become normal with successful treatment. Examples of ways in which malabsorption can present are shown in Table 12.2. Some of the basic laboratory investigations that should be carried out in patients in whom a diagnosis of malabsorption is suspected, are shown in Table 12.3. If all of these tests are normal, the diagnosis is unlikely; if they are abnormal, the next step is to look for a specific cause, guided by the history, clinical findings and laboratory results. Anaemia is a particularly common feature of gastric and small bowel disease. The physiology and biochemistry of the vitamins and minerals involved in blood formation is discussed in detail in Chapter 27. Metabolic bone disease is discussed in detail in Chapter 31. In patients with malabsorption, much emphasis has hitherto been placed on malabsorption of calcium and vitamin D leading to osteomalacia. The advent of dual energy X-ray absorptiometry (DEXA) scans with reliable, sensitive and reproducible quantitation of bone mineral density has made it clear that there is a high prevalence of reduced bone mineral density in patients with coeliac disease and Crohn disease, even with successful treatment. Bone mineral density is frequently reduced in both disorders and osteoporosis may be seen in up to 25% of patients. Bone disease in these conditions predominantly affects the hips, with vertebrae and the forearm bones somewhat less severely affected. An important point is that reduced bone mineral density is often present in IBD at diagnosis and is independent of corticosteroid treatment (which is itself a cause of osteoporosis). Reduced bone mineral density in patients with IBD is associated with cytokine spillover from the gut into the circulation, leading to impairment of osteoblastic activity with no concomitant decrease in osteoclastic activity.
TABLE 12.2
Examples of the way in which malabsorption can present
| Presentation | Cause |
| Passage of pale, bulky, offensive stools | Fat malabsorption or maldigestion |
| Abdominal distension, borborygmi, watery diarrhoea and excessive flatus | Malabsorption of carbohydrate |
| Vague malaise and tiredness, sometimes with weight loss (adults); growth retardation (children) | Generalized deficiency of nutrients |
| Anaemia | Deficiency of iron, folate or vitamin B12 |
| Easy bruising or bleeding | Deficiency of vitamin K |
| Failure to thrive (infants) | Generalized deficiency of nutrients |
Carbohydrate absorption
Dietary carbohydrates
In most human diets, carbohydrates are the principal source of energy. The major form in the diet is starch, which accounts for about two-thirds of digestible dietary carbohydrate, the remaining third being made up of sucrose, lactose and their constituent monosaccharides. Starch is a polymer of glucose consisting of two forms: amylose and amylopectin. Amylose consists of long, unbranched chains of glucose molecules joined by α-1,4 links. Amylopectin consists of chains of glucose molecules with branching points for side chains every 12–25 glucose units. At each branching point, there is an α-1,6 link. Sucrose and lactose are disaccharides, sucrose consisting of a dimer of glucose and fructose, while lactose is a dimer of glucose and galactose.
Digestion of carbohydrates
Luminal events in carbohydrate digestion
The initial step in starch absorption is enzymatic digestion of α-1,4 links by salivary amylase to release maltose (dimer of two glucose molecules) and maltotriose (trimer of three glucose molecules). The salivary enzyme is rapidly inactivated by gastric acid, but hydrolysis is continued by pancreatic amylase, the end-products being maltose, maltotriose, short-branched oligosaccharides and α-limit dextrins, the latter two being residual branched segments resulting from incomplete digestion of amylopectin. No free glucose is produced.
Enterocyte events in carbohydrate digestion
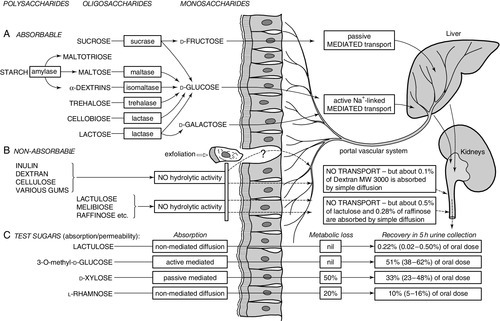
The final step of carbohydrate digestion, the conversion of oligosaccharides to monosaccharides, is performed by the disaccharidases of the small intestinal enterocytes. The main disaccharidases are maltase, sucrase-isomaltase and lactase (see Fig. 12.1). These enzymes are synthesized on the endoplasmic reticulum, transported to the Golgi apparatus and then to the brush border. They are distributed throughout the length of the small intestine, but sucrase and lactase are in highest concentrations in the jejunum. They are normally present in considerable excess, so that some reduction in activity does not normally result in symptoms.
FIGURE 12.1 Digestion and absorption of saccharides. (A) Ingested starch is broken down to the various disaccharides by salivary and pancreatic amylase. The disaccharides are cleaved by the various brush border disaccharidases to yield D-fructose, D-glucose and D-galactose. These in turn are transported across the brush border and enterocytes, and into the circulation by specific transport systems. (B) Non-hydrolysable polysaccharides, disaccharides and trisaccharides are thought to be excluded from permeation across the brush border. The small quantities that do permeate the intestine appear to pass by a paracellular route or through areas of cell extrusion (apoptosis). (C) Four commonly used sugars, used in a combined test of intestinal absorptive capacity and intestinal permeability, use different transport routes across the intestine, which allows assessment of the various intestinal functions. This increases the discriminatory value of the tests when assessing intestinal disease.
The three main monosaccharides derived from the diet (glucose, galactose and fructose) are absorbed by saturable carrier-mediated transport systems in the enterocyte brush border. Secondary active transport of glucose and galactose occurs through a sodium co-transport system, driven by a sodium gradient dependent on Na+,K+-ATPase on the basolateral surface of the cell. Fructose absorption is not active, but occurs through carrier-mediated (facilitated) diffusion.
Both the digestion and absorption of carbohydrates are highly efficient, but the process is incomplete, particularly for starch, with up to one-fifth of dietary starch failing to be absorbed. The efficiency of starch absorption varies, depending on the foodstuff from which it is derived. For example, rice starch is highly absorbed, but between 10 and 20% of starch in wheat and certain beans enters the colon, where bacteria produce short chain fatty acids (which may be an important energy source for colonocytes), methane and hydrogen.
Clinical aspects of carbohydrate absorption
Although inherited disorders of sucrase-isomaltase and of transport proteins causing glucose-galactose malabsorption and fructose malabsorption are recognized, they are very uncommon. Adult lactase deficiency, however, is common in humans and may be regarded as a normal finding in many ethnic groups. Malabsorption of carbohydrates predisposes to osmotic diarrhoea with excessive flatus, abdominal distension and discomfort (‘griping’ pains).
Lactase deficiency
Lactase activity is highest in the earliest months of life, but levels of the enzyme usually decline in all races after weaning. The prevalence of adult lactase deficiency is highly variable between races; so, for example, 5–15% of northern Europeans have demonstrable hypolactasia or alactasia, but > 70% of Africans, Asians and especially Inuits have deficiency of the enzyme. However, the vast majority of affected individuals are asymptomatic, because overall completeness of disaccharide hydrolysis depends on the amount ingested, intestinal dilution and transit times, as well as the state of the enzyme (reduced or absent enzyme activity).
In practice, the diagnosis is often made by a therapeutic trial of a diet low in lactose, but metabolic tests are available (see below) and the diagnosis can be confirmed by assaying lactase activity in small bowel biopsy samples (though this is rarely required). Congenital lactase deficiency (i.e. lactase deficiency that is present at birth) has been described, but is extremely rare. The form of enzyme deficiency discussed above is usually known as primary or ‘genetic’ lactase deficiency. However, lactase deficiency may also complicate mucosal diseases of the small intestine and is then referred to as ‘secondary’. This secondary lactase deficiency is a regular feature of coeliac disease, and is also seen in tropical sprue, IBD, radiation enteritis, chronic alcoholism with malnutrition and the enteropathy associated with acquired immune deficiency syndrome (AIDS). It may also accompany infections such as acute gastroenteritis and giardiasis, but usually resolves following resolution or successful treatment of the illness.
Investigation of carbohydrate absorption
The glucose tolerance test is of no value in the detection of malabsorption. The small intestinal reserve capacity for absorption of D-glucose is so great that absorption is usually well maintained, even when mucosal disease is severe. Furthermore, the rise in blood glucose concentration following oral administration is influenced by additional factors such as gastric emptying and endocrine status, which complicate interpretation.
Xylose absorption test
D-Xylose, a pentose sugar of plant origin, has been the basis of an absorption test in routine clinical use since the 1930s. In the human, D-xylose is absorbed mainly from the jejunum, by a passive carrier-mediated system distinct from that utilized by D-glucose. Unlike D-glucose, there is no reserve capacity for the intestinal uptake of D-xylose, which made this sugar a potentially good indicator, responsive to minor changes in jejunal absorption. However, the concentration of xylose measured after an oral dose is affected by other factors including renal, hepatic and cardiac disease and the test is no longer widely available.
Lactose tolerance test
This test may be used in suspected lactase deficiency. In essence, serial blood samples for glucose estimation are taken after oral ingestion of 50 g of lactose. A rise in venous plasma glucose of < 1.1 mmol/L is indicative of impaired lactose hydrolysis. The occurrence of typical symptoms following ingestion of lactose provides further evidence for the diagnosis.
Differential tests of intestinal disaccharide hydrolysis
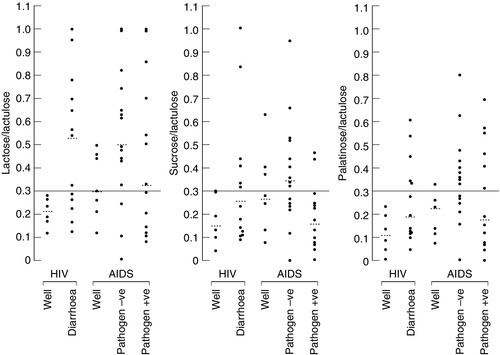
Small amounts of non-hydrolysable disaccharides are absorbed by passive diffusion and appear in the urine: when hydrolysable disaccharides are not hydrolysed for some reason, the same thing happens. The intestinal uptake and urinary excretion of intact disaccharides in urine are, therefore, influenced by the extent of small intestinal disaccharidase activity: the greater the rate of hydrolysis, the less the excretion of intact disaccharide in urine. Excretion ratios of hydrolysable:non-hydrolysable disaccharides following ingestion of a mixture are, therefore inversely proportional to the efficacy of intestinal hydrolysis. Accurate, non-invasive assessment of intestinal lactase, sucrase or isomaltase can be made, using measurements of lactose/lactulose, sucrose/lactulose or palatinose/lactulose excretion ratios respectively, either separately or in combination. Ratios of lactose, sucrose or palatinose divided by lactulose (% molar dose excreted) may indicate isolated (genetically determined or primary) deficiency of lactase, sucrase (rare, a cause for severe diarrhoea in children) or palatinase (for isomaltase, not clinically relevant). However, if the absorption of two or more disaccharides is impaired, this suggests a secondary deficiency, for example mucosal diseases such as coeliac disease, infection or immune deficiency. Representative results using this test in patients with human immunodeficiency virus (HIV) infection are shown in Figure 12.2.
FIGURE 12.2 Quantitative estimation of the rate of intestinal hydrolysis of ingested disaccharides in patients with HIV and AIDS. The test is carried out after an overnight fast and a preceding 12–18 h dietary exclusion of lactose- and sucrose-containing foods. Subjects ingest a solution containing lactulose 5 g, lactose 10 g, sucrose 10 g and palatinose 10 g (with or without L-rhamnose, which allows the integrity of intestinal permeability to be assessed at the same time), followed by a 10 h urinary collection with measurement of the disaccharides (% dose excreted) and calculation of the ratios. Well subjects have molar lactose/lactulose, sucrose/lactulose and palatinose/lactulose urine excretion ratios < 0.3, indicative of normal intestinal lactase, sucrase and palatinase activities. Increased ratios in patients with the various stages of HIV are indicative of impaired hydrolysis of each of these sugars. The fact that the hydrolysis of all three sugars is impaired shows that this is secondary disaccharidase deficiency.
This is undoubtedly the test of choice for the accurate and reliable assessment of whole bowel disaccharidase activity. It is, however, also more labour intensive and demanding on the patient than the other procedures mentioned above.
Protein absorption
The average Western diet contains some 80–100 g of protein per day, which provides not only the amino acids necessary for protein synthesis, but also some (10–15%) of the energy content of the diet. In addition, the gut digests and absorbs substantial amounts (perhaps 60 g) of endogenous protein derived from intestinal secretions, mucus and the shedding of mucosal cells.
Digestion of proteins
Protein digestion takes place throughout the length of the small intestine and is efficient, with only 5% of the protein entering the gut lumen being lost in the stools each day.
Protein digestion begins in the stomach with the grinding and mixing action of gastric peristalsis and the food being exposed to gastric acid and pepsin. Pepsin is a protease that cleaves internal peptide bonds adjacent to hydrophobic amino acids. Pepsin is inactivated at a pH > 4.0 and its action is, therefore, confined to the stomach. Digestion of proteins proceeds further through the action of the pancreatic proteases trypsin, chymotrypsin, elastase and carboxypeptidases A and B in the proximal jejunum. These are secreted as inactive proenzymes. Trypsin is released from trypsinogen by the brush border-derived enterokinase and activates the other proenzymes. Trypsin, chymotrypsin and elastase are endopeptidases, hydrolysing peptide bonds adjacent to certain specific amino acids, while carboxypeptidases A and B are exopeptidases, removing amino acids one at a time from the C-terminal ends of the peptides. The proteases reduce chyme to a mixture of free amino acids and oligopeptides (of between two and six amino acid residues). These are then further digested by brush border peptidases. There are at least eight mucosal peptidases that are synthesized and transported in a similar manner to the disaccharidases. However, unlike carbohydrates, the peptides are not completely digested to their constituent units, brush border enzyme action leaving a mixture of amino acids, di- and tripeptides. There are specific transport systems that effectively absorb di- and tripeptides and, indeed, do so at a faster rate than for free amino acids; this discovery has had implications for the development of enteral feeding regimens. Peptide uptake is by sodium-linked active transport. Once inside the mucosa, peptidases within the enterocyte cleave small peptides to their constituent amino acids. A number of transport systems exist for the uptake of luminal amino acids and there are some rare inherited defects of amino acid absorption, including Hartnup disease (p. 171) and cystinuria (p. 170).
Clinical aspects of protein absorption
The most common cause of protein malnutrition worldwide is inadequate intake, often combined with poor absorption due to tropical enteropathy and frequent episodes of infective enteritis. The clinical syndrome is best illustrated by children from famine areas of the third world with their pot bellies (ascites because of hypoalbuminaemia). There are no specific syndromes of protein malabsorption, although protein deficiency can contribute to the wasting and nutritional disturbance of severe malabsorption syndrome. In several diseases, for example Crohn disease, ulcerative colitis and Whipple disease, excessive protein loss into the gut produces the syndrome of protein-losing enteropathy. This process is not one of malabsorption, although there may be associated malabsorption.
Investigation of protein absorption
There is no clinically useful non-invasive method of assessing protein digestion and absorption. Furthermore, there is little clinical need for such tests as mucosal diseases causing amino acid malabsorption are so rare. Protein-losing enteropathy can, however, be detected easily, most accurately by measuring the faecal excretion of intravenously administered albumin labelled with 51Cr and somewhat less reliably by measurement of faecal α1-antitrypsin and α1-antichymotrypsin.
Fat absorption
Although the average fat content of the diet has been falling in the Western world over the last 20 years in response to medical advice, the average fat intake is still of the order of 90–100 g/day. There has also been a relative decline in the proportion of saturated fat in the diet with a concomitant increase in polyunsaturated fatty acids. The principal long chain polyunsaturated fatty acids are linoleic (C18:2) and linolenic (C18:3) acids. The other dominant fatty acids are oleic (C18:1) and palmitic (C16:0) acids. In food, these are mainly present as esters with glycerol (triacylglycerols or triglycerides). Additional dietary fat consists of phospholipid, cholesterol and fat-soluble vitamins. Because fats are insoluble in water, the mechanisms for their digestion and absorption are complex, but an understanding of the mechanism of fat digestion and absorption is crucial to an understanding of the wide variety of diseases that cause malabsorption of fat and are consequently associated with steatorrhoea.
Digestion of triacylglycerols
Luminal digestion
The first stage in the digestion of triacylglycerols is the process of peristaltic emulsification. Physical grinding of food, first by chewing and then in the stomach, produces an initial, relatively unstable emulsion that enters the duodenum. The emulsion is stabilized as the fat droplets are coated with phospholipid, provided exogenously in the diet and endogenously from bile. Further stabilization is achieved by a coating of bile salts and with small amounts of monoacylglycerols and fatty acid ions.
The main digestion of the emulsion occurs through the action of pancreatic lipase, but a minor initial component of digestion may be provided through the actions of lingual and gastric lipases, which have pH optima in the acid range. This component is important, however, in providing partial digestion of triglycerides to enhance and contribute to the stability of the emulsion. Pancreatic lipase acts in conjunction with a cofactor, pancreatic colipase, which is secreted in an inactive form (procolipase) and subsequently activated by tryptic digestion. Pancreatic lipase activity is greatest at near neutral pH and is therefore dependent on adequate bicarbonate secretion from the pancreas, under the influence of secretin. The combined action of lipase and colipase hydrolyses triacylglycerols to release fatty acids from the 1 and 3 positions, so that each triacylglycerol molecule is hydrolysed to a monoacylglycerol (with a fatty acid attached at the 2 position) and two free fatty acids. Bile salts are amphipathic, that is, they contain both water-soluble and lipid-soluble components. This property allows them to aggregate in micelles such that the hydrophobic components line up adjacent to one another on the inside of the micelle with the hydrophilic aspects facing outwards into the aqueous phase. The water-insoluble fatty acids, monoglycerides and cholesterol are, therefore, held ‘inside’ the micelle to form a highly stable particulate emulsion. While triacylglycerol absorption is fairly efficient in the absence of bile salts, by contrast the absorption of cholesterol and fat-soluble vitamins is severely compromised unless the bile salt concentration in the duodenum is above a critical micellar concentration.
Absorption of triacylglycerols
The transport of lipids across the surface of enterocytes is by passive diffusion, that is, the lipids partition across lipid components in the brush border membrane. The microclimate at the enterocyte surface is slightly more acidic than in the jejunal lumen, which encourages the liberation of fatty acids from micelles. There is some suggestion, however, that a component of fat absorption for certain fatty acids, particularly linoleic and oleic acids, may occur by facilitated diffusion. Triacylglycerols are resynthesized from monoacylglycerols and fatty acids in enterocytes and incorporated into chylomicrons. The carrier protein apolipoprotein B48 is essential for chylomicron synthesis, and absence of this protein, as in congenital abetalipoproteinaemia, produces severe fat malabsorption. While longer chain triacylglycerols (above C10) pass into the lymphatics, medium chain triacylglycerols (C6–10) are at least partly absorbed intact, probably by passive diffusion, to reach the liver by the portal circulation.
Digestion and absorption of other fats
Phospholipids are hydrolysed to fatty acids and lysophosphatidylcholine by pancreatic phospholipase A2, while cholesteryl esters are hydrolysed by a pancreatic esterase. The fatty acids form micelles with bile acids, and thus both lipids are ultimately transported to enterocytes in a similar way to the digestion products of triacylglycerols. Phospholipids are resynthesized in the endoplasmic reticulum of mucosal cells and cholesterol is esterified by acyl-CoA cholesterol acyltransferase before being incorporated into chylomicrons. The efficiency of fat absorption is considerable so that, in health, the process is virtually complete. Most fat absorption occurs within the proximal jejunum.
Clinical aspects of fat malabsorption
Fat absorption is a complex, multistage process. Therefore, disease at several levels in the alimentary system can result in fat malabsorption, which, when severe, is characterized by the presence of steatorrhoea. Interference with delivery of bile salts into the duodenum, pancreatic exocrine deficiency, failure to achieve near-neutral duodenal pH either from hypersecretion of gastric acid or hyposecretion of pancreatic bicarbonate, and mucosal disease due to villous atrophy, lymphangiectasia, abetalipoproteinaemia etc, can all result in steatorrhoea. Tests of fat absorption have been used in the past as screening methods to detect malabsorption. In modern clinical practice, it is increasingly evident that confirmation of steatorrhoea (rather than quantification) by microscopy of faeces for fat after suitable staining is sufficient to initiate appropriate investigation of the relevant organs that may play a role in fat digestion and absorption.
Investigation of fat absorption
Faecal fat excretion
Quantitative measurement of faecal fat excretion is the most sensitive method of detecting fat malabsorption. However, this investigation is unpleasant both for patients and laboratory staff, and few laboratories now include it in their repertoires. There have been a number of efforts to find alternative measures of fat absorption. The 14C-triolein (or 13C) breath test is probably the best of these, but, nevertheless, is not widely used.
13/14C-triolein breath test
Following an overnight fast and collection of a basal sample of expired air, the patient is given 13C- or 14C-triolein in a 60 g fat meal. Hourly breath samples are then collected for 7 h for measurement of labelled carbon expiration. If triolein is effectively absorbed, large amounts of labelled CO2 will appear in the expired breath, fat malabsorption being indicated by smaller amounts being exhaled. However, when steatorrhoea is macroscopically obvious, confirmation by measurement is not required, and investigation should be directed towards diagnosing the cause.
INTESTINAL PERMEABILITY
An alternative approach to the measurement of absorptive function in the assessment of small bowel disease is to assess barrier function – that is the ability of the intestine to exclude compounds from being absorbed. Increased intestinal permeability is not characteristic of any single disease, but is seen in a variety of gastrointestinal pathologies.
Previously, tests using the differential permeability of combinations of sugars were used to assess intestinal permeability, but these are now largely obsolete, because of capsule enteroscopy. For those interested in these tests, details are provided in Further reading.
FAECAL TESTS OF INTESTINAL INFLAMMATION
The intestinal tract is a most hospitable environment for bacteria. The intestine has developed a very effective and complicated way of accommodating them and protecting the host, but it is clear that the defence system is somewhat less than 100% efficient. If there is a breach in mucosal integrity of any sort, the microbes gain an advantage and elicit a significant inflammatory reaction. Hence, almost all colonic and most small bowel disorders are associated with inflammation.
The first reliable quantitative non-invasive test for assessing intestinal inflammation was the 111indium white cell technique, which involved abdominal scintigraphy (for localization of disease) and a four-day faecal collection (for quantitative estimation of the inflammatory activity). The test is uniquely sensitive for the detection of inflammation, but is not disease-specific. Disadvantages are its cost, the requirement for special labelling facilities, a high dose of radiation and the inconvenience of a complete four-day faecal collection. Simpler methods were needed and subsequently developed. The qualities of a marker for assessing intestinal inflammation are that it should be stable, resistant to degradation by intestinal bacteria, easily extractable from faeces, easily assayed and, preferably, specific for a particular type of inflammatory cell. Two such markers have undergone rigorous testing, namely calprotectin and lactoferrin with other proteins, including M2PK and S100A12, entering the field more recently. Calprotectin acts as a representative for this group of proteins and its use will, therefore, be described in detail.
Calprotectin
Calprotectin accounts for about 60% of total soluble proteins in the cytosolic fraction of neutrophil granulocytes. It is a member of the S100 family of calcium and zinc binding proteins (a heterodimer of S100A8/A9) and is found in neutrophils, monocytes and some squamous epithelial cells. It is released by activation of leukocytes as a consequence of inflammatory disease. Calprotectin has antimicrobial properties and, in addition, it can inhibit the proliferation of both normal and malignant cells, probably by sequestration of zinc, which is a critical element for many enzymes. In particular, the metalloproteinases MMP-2, MMP-3, MMP-7, MMP-8, MMP-9 and MMP-13 are inhibited in vitro by calprotectin at biologically relevant concentrations, suggesting that it may have important regulatory functions.
In the presence of calcium, calprotectin is remarkably resistant to proteolytic degradation and it is this fact that underlies its stability in faeces. Commercially available kits can be obtained for the extraction of calprotectin from faeces and quantitation by ELISA and require no more than a 200 mg sample. Reference ranges should be established by individual laboratories, as there are almost certainly racial and geographical variations. In the UK, the upper limit of the reference range for faecal calprotectin is 50–60 μg/g. African Caribbeans resident in the UK may have values up to 200 μg/g without disease. In general, when used as a screening procedure for intestinal disease, increased values between 50 and 200 μg/g rarely disclose significant disease. In these cases, we recommend repeating the test before further investigation. Calprotectin concentrations between 200 and 500 μg/g are commonly seen in patients with diverticulitis, colon malignancies and polyps, and those on NSAIDs, whilst those with values > 500 μg/g almost invariably have IBD or intestinal infections.
Calprotectin in disease
Inflammatory bowel disease
Studies from different groups have shown that faecal calprotectin concentrations are almost invariably increased above healthy controls in patients with active ulcerative colitis and Crohn disease. The clinical implication is that faecal calprotectin clearly has potential as a screening test to distinguish patients with IBS, who will have normal concentrations, from those with IBD, whose concentrations are characteristically increased by at least ten-fold in active disease.
Patients with clinically inactive IBD may have a normal faecal calprotectin (depending on treatment), but often have an increased concentration. The considerable overlap between the values in clinically active and inactive disease is such that it is inadvisable to make predictions of a patient’s clinical status based solely on faecal calprotectin concentrations.
Faecal calprotectin is particularly useful in patients with inactive ulcerative colitis and Crohn disease, as values five times above the upper limit of normal have 90% sensitivity and 83% specificity in predicting clinical relapse within six months, allowing the opportunity for early treatment.
Colorectal cancer
Colorectal cancer (adenocarcinoma) is the second most common cause of death from malignancy in the Western world. Survival rates are closely related to the stage of cancer at the time of diagnosis, and the most promising approach to reducing mortality rates is the early detection of precancerous or cancerous lesions. The most widely accepted non-invasive method for detecting colorectal cancer is faecal occult blood (FOB) testing. Screening in asymptomatic populations has, at best, reduced mortality rates by 15–33%; a national bowel cancer screening programme using faecal occult blood testing was initiated in the UK in 2006.
Various studies have consistently demonstrated that faecal calprotectin concentrations are increased in patients with colorectal cancer. Sensitivity is of the order of 80–90%, whereas that for FOB is only 40–58%. Furthermore, faecal calprotectin is increased in 50% of patients with colorectal polyps (some of which may be precancerous). However, the test is not recommended for cancer screening purposes because of its lack of specificity. Numerous faecal molecular marker probes, usually polymerase chain reaction (PCR) based, are currently being tested for the detection of colorectal cancer. To-date, they lack sensitivity, but are very specific.
Irritable bowel syndrome
Given that faecal calprotectin has a sensitivity of almost 100% in detecting patients with active IBD, this raises the possibility of using it as a screening test to distinguish between these patients attending outpatient clinics and the more common patients with IBS who do not warrant invasive investigation. The main value of the test in patients with IBS is that a normal test result practically rules out chronic inflammatory bowel disease and thereby reduces the need for invasive investigation. Of patients with IBS, 15–20% have raised faecal calprotectin, typically to 2-3 times normal.
Neuroendocrine tumours of the gastrointestinal tract and pancreas (NETs)
Neuroendocrine tumours of the gastrointestinal tract are relatively rare, but easily diagnosed when they produce clinically distinct and specific syndromes. However, many of the tumours do not secrete substances that lead to the specific syndromes, and are only detected because of the physical effects of local or metastatic lesions. A proportion of these tumours have a genetic predisposition due to a loss of tumour suppressor genes, namely multiple endocrine neoplasia (MEN) type 1 and 2 (see Chapter 41).
As a group, the neuroendocrine tumours are characterized histologically by sheets of small round cells that are derived from intestinal neuroendocrine cells in the submucosa, with very infrequent mitosis. It is, therefore, not usually possible to distinguish between a benign and malignant tumour histologically, unless there is evidence of metastasis. This contrasts very sharply with the common gastrointestinal cancers (oesophageal, gastric and colonic) that originate from the epithelial cells (squamous cell cancer and adenocarcinomas), where histopathological diagnosis is usually straightforward. From the clinical biochemistry viewpoint, it is most logical to discuss the different tumours according to the clinical syndromes that they cause.
Intestinal carcinoid tumours and the carcinoid syndrome
The carcinoid syndrome
The cardinal presenting symptoms of the carcinoid syndrome are persistent diarrhoea (40–80%), flushing (30–70%) and nondescript abdominal pain (10%). The diarrhoea is watery and the need to defecate often coincides with the flushing periods. The flushing is intermittent, of sudden onset, brief (lasting for < 15 min), associated with a feeling of warmth and can be precipitated by alcohol ingestion, exercise or by certain foods, especially cheese. The flushing is mainly on the neck and face and is otherwise not distinctively different from menopausal flushing or drug-induced flushing (chlorpropamide and calcium channel blockers). Moreover, there are other well-recognized, albeit less common, manifestations of the carcinoid syndrome. Cardiac valvular involvement (10% of patients) present as right sided heart failure. A further 10% of patients develop asthma-like symptoms, and those with metabolically active tumours that utilize much of the ingested tryptophan may have pellagra-like skin lesions due to nicotinic acid (niacin) deficiency.
The carcinoid syndrome is a manifestation of a carcinoid tumour, a tumour of argentaffin cells that can occur anywhere in tissue derived from the embryonic gut, but most frequently is found in the ileum. Its pathophysiology, diagnosis and management are discussed in detail in Chapter 41. Measurement of urine 5-HIAA and/or serum chromogranin A is used in the diagnosis and monitoring of carcinoid syndrome patients.
Pancreatic endocrine tumours
Pancreatic endocrine tumours have been variously called ‘islet cell tumours’ or APUDomas (amine precursor uptake and decarboxylation) and are of similar origin to carcinoids. They share certain features, consisting of homogeneous sheets of small cells with very few mitoses. Malignant potential may be indicated by local invasion or metastasis, and each tumour is usually associated with symptoms attributable to a single hormone/peptide, although the tumour may release a number of hormones. Unlike carcinoids, pancreatic endocrine tumours are mostly functional, that is, they are associated with symptoms that are attributable to the hormone that they release. Their prominence in textbooks is disproportionate to their clinical importance, and the vast majority of patients are managed and treated within specialist units. As a group of tumours, they are exceptionally rare with an annual incidence of 1–2/millon population. Insulinomas and gastrinomas are the most common, with vasoactive intestinal peptide (VIPomas), glucagonomas and somatostatinomas being ten times rarer, while others are confined to single case reports. Pancreatic endocrine tumours are not infrequently discovered because of their association with MEN-1 and 2 (see Chapter 41) or the phacomatoses (tuberous sclerosis, von Hippel–Lindau and von Recklinghausen disease).
Insulinomas
Tumours of the β cells of the pancreas secrete insulin without the normal physiological feedback action of circulating blood glucose. Fifty per cent of insulinomas are < 1 cm in diameter at diagnosis, most are solitary (except when associated with MEN) and 90% are benign. Insulinomas typically present with symptoms of hypoglycaemia, especially after fasting or exercise. The most common symptoms are loss of consciousness (sometimes presenting as coma), dizziness, diplopia, blurred vision, confusion, abnormal behaviour, excessive sweating, palpitations and weight gain (because of the increased food intake that relieves some of the symptoms). The diagnosis is based on the presence of ‘Whipple’s triad’, namely symptoms of hypoglycaemia, documented hypoglycaemia and relief of symptoms when glucose is administered to restore normal blood glucose concentrations.
The first step in the diagnosis of insulinoma is the documentation of fasting hypoglycaemia, which can best be differentiated from postprandial hypoglycaemia from the clinical history. A low plasma glucose concentration in the presence of inappropriately high insulin and C-peptide concentrations is strongly suggestive of an insulinoma. Insulin and C-peptide are formed in equimolar amounts from the enzymatic cleavage of proinsulin. A high insulin concentration in the presence of a low C-peptide concentration is indicative of the administration of exogenous insulin. Hypoglycaemia associated with misuse of sulfonylureas may need to be excluded from the differential diagnosis by measurement of serum sulfonylurea concentrations. Other causes of hypoglycaemia, including alcohol misuse, Addison disease and liver failure, should also be excluded. Hypoglycaemia can be life-threatening and only surgery can offer curative treatment. Techniques such as CT and MRI may localize tumours larger than 1 cm, but at least 50% of tumours are smaller than this. Angiography has a high detection rate for smaller insulinomas, as these are highly vascular, and endoscopic ultrasound is increasingly used for their detection. In the most difficult cases, it may be necessary to sample venous blood selectively from the pancreas in order to detect a differential increase in C-peptide concentrations following arterial injection of calcium.
Glucagonoma
The clinical hallmark of a glucagonoma is the symptom complex of migratory necrolytic erythema, weight loss and anaemia. Glucose concentrations may be high due to the antagonist action of glucagon to insulin. The skin lesions are the most distinct component of glucagonoma, and the difficulty recognizing them may account for the fact that they have often been a feature of the disease for an average of six years prior to diagnosis.
Unlike insulinomas, glucagonomas are usually large (> 5 cm) at the time of diagnosis and > 50% are malignant. Localization by conventional imaging techniques is, therefore, not a problem. The diagnosis can be confirmed by finding raised plasma glucagon concentrations: these are usually elevated five-fold or more. Minor elevations of glucagon can be found in chronic renal failure, acute pancreatitis and severe trauma, especially with burns and sepsis, but these conditions are easily identified clinically. Surgery should always be considered despite the malignant nature of many of the tumours. The medical treatment is largely directed at control of diabetes; octreotide is sometimes useful.
VIPoma
VIPomas are rare tumours, characterized by copious watery diarrhoea with dehydration and hypokalaemia. The severity of the diarrhoea is comparable to that seen in cholera with excretions in excess of 2 L/24 h. The diagnostic feature of the diarrhoea is that it continues despite prolonged fasting. Plasma VIP concentrations are elevated 4–5-fold; the same imaging techniques are used to localize the tumour as in glucagonomas. VIPomas have the same propensity as glucagonomas for malignancy. While surgery is usually considered, their medical management remains important with rehydration and correction of electrolyte disturbances in severe cases, and use of antidiarrhoeals (e.g. loperamide, codeine phosphate and somatostatin analogues).
Somatostatinoma
Excessive secretion of somatostatin (the somatostatin syndrome) is characterized by diabetes (somatostatin inhibits release of insulin); diarrhoea (because of inhibition of exocrine pancreatic function); weight loss and gallstones (a result of impaired gallbladder contraction). However, as pancreatic surgery for tumours becomes more common, it is evident that only 10% of somatostatinomas are functional, that is, associated with the somatostatin syndrome. Somatostatinomas tend to be large (2–10 cm at diagnosis), and the majority are malignant with evidence of metastasis being present at the time of diagnosis. A significant number are detected during cholecystectomy or following pancreatic tumour work-up. Occasionally, symptomatic patients with the somatostatin syndrome are diagnosed on the basis of increased plasma somatostatin concentrations. Treatment options include surgery and chemotherapy.
THE ACUTE ABDOMEN
Introduction
The term acute abdomen is used to indicate the rapid onset of severe symptoms that may indicate potentially life-threatening intra-abdominal pathology requiring urgent surgical intervention. Its clinical importance is not in doubt. Many of the diseases that present in this way have a high mortality if not treated surgically; conversely, others are associated with high mortality and morbidity if surgery is performed. The vast majority of patients with an acute abdomen present initially to an accident and emergency department (at least in the UK) and are then transferred to the care of surgeons.
The clinical diagnosis is based principally on the nature of the pain. Common causes of the acute abdomen are shown in Box 12.1. Additionally, there are diseases such as measles causing ileitis and perforation that have a higher incidence in the developing world. Non-abdominal pathologies may simulate an acute abdomen.
History and examination often yield a likely diagnosis, but investigations are helpful in providing confirmation, e.g. testing for pregnancy in women with lower abdominal pain, measurement of amylase when acute pancreatitis is suspected. In addition, a full blood count may reveal an increase in neutrophil count suggestive of infection or anaemia secondary to blood loss, and it is important to have knowledge of renal function and electrolytes prior to undertaking surgery.
There follows a brief account of the disorders where clinical biochemistry may play an important role in confirming the diagnosis of diseases that present as an acute abdomen, namely acute pancreatitis, ectopic pregnancy and porphyria. It should, however, be appreciated that other diagnostic modalities can also provide important information, for example, ultrasound or CT examination in suspected acute pancreatitis.
Acute pancreatitis
Acute pancreatitis is an acute inflammation of the pancreas. Some cases may be self-limiting, but others may be severe and associated with organ failure including pulmonary insufficiency and acute kidney injury and with local complications such as pancreatic necrosis, abscess or pseudocyst formation.
Table 12.4 shows the factors that are recognized to be important in its aetiology, of which gallstones and alcohol account for > 80% of cases. The precise pathogenesis is often uncertain, but the most widely accepted hypotheses suggest that insults to the pancreas cause activation of zymogens (inactive pro-enzymes) within the gland and consequent autolytic destruction and production of inflammatory cytokines and chemokines. How the initial insult brings about these changes is disputed, although ischaemia may promote both.
TABLE 12.4
Causes of acute pancreatitis
| Factors | Comment |
| Gallstones | The most frequent, and best established, aetiological factor. Migrating small gallstones may block the pancreatic duct (some people believe that reflux of bile into the pancreas is also important) and thereby initiate the damage |
| Alcohol misuse | The acute effects of ethanol on the pancreas are complex. Alcohol alters pancreatic blood flow and, coupled with the generation of free radicals from ethanol metabolism, this may cause free radical damage. Alcoholic stimulation of pancreatic secretion coupled with spasm of the sphincter of Oddi could lead to obstructive injury. Ethanol may also sensitize the pancreas to the effects of other agents that lead to zymogen activation and the generation of harmful cytokines. The overall contribution of these factors to the development of alcoholic pancreatitis is unclear |
| Drugs | Thiazide diuretics, oral contraceptives, corticosteroids, 5-aminosalicylic acids (used in the treatment of inflammatory bowel disease), azathioprine, 6-mercaptopurine, asparaginase, didanosine, valproate, statins and other drugs have all been implicated as aetiological agents, but only rarely. The mechanisms are unknown |
| Hypercalcaemia | Often quoted, but the majority of patients with hypercalcaemia do not develop pancreatitis |
| Hypertriglyceridaemia | Severe hypertriglyceridaemia is important in its own right, but excessive alcohol ingestion is a confounding factor in many cases |
| Trauma | Blunt trauma to the abdomen may occasionally cause acute pancreatitis; the trauma may also be iatrogenic, e.g. following endoscopic retrograde cholangiopancreatography (ECRP) |
| Infectious causes | Coxsackievirus, Ascaris lumbricoides, Candida, Salmonella, HIV |
| Rare causes | Pancreatic tumours, hereditary pancreatitis, autoimmune pancreatitis, scorpion toxins, idiopathic |
Almost all patients with acute pancreatitis have abdominal pain. This is severe epigastric pain, usually of sudden onset, often with radiation to the back. In more severe cases, there is nausea and vomiting, fever, hypotension, shock and multiorgan failure that may lead to death. Biochemical features of acute pancreatitis include: uraemia (compromised renal function); hypoalbuminaemia (extravasation); hypocalcaemia (partly related to hypoalbuminaemia and partly because fatty acids released in and around the inflamed pancreas by pancreatic lipase form insoluble calcium salts); hyperglycaemia (increased sympathetic activity and destruction of the islets of Langerhans); metabolic acidosis; hypoxaemia, and raised plasma activities of liver enzymes. None of these is invariably present and none is diagnostic for pancreatitis. In acute necrotizing pancreatitis, methaemalbuminaemia may be detectable. The main use of these tests is to provide information about the prognosis, and they may also influence management. Biochemical tests that are used for their diagnostic potential in acute pancreatitis include amylase and lipase.
Amylase
In acute pancreatitis, amylase activity rises within 2–12 h of the onset of symptoms, usually returning to normal within 3–5 days. Serum amylase above five times the upper reference limit is very suggestive of acute pancreatitis. Smaller increases may have other explanations (see Table 12.1).
In exceptionally rare cases, pancreatitis severe enough to cause death may occur without a rise in serum amylase. A persistently high amylase following an attack of acute pancreatitis suggests the formation of a pancreatic pseudocyst, a cyst containing pancreatic enzymes, which needs to be differentiated from an abscess, which is infective in nature. Since amylase is excreted in urine, the diagnostic efficacy of urinary amylase measurement in acute pancreatitis has also been investigated. Diagnostic performance can be improved by the simultaneous measurement of amylase and creatinine in paired serum and urine samples. The normal ratio of amylase clearance to creatinine clearance is 2.2–4.2%, rising to 6.3–13.3% in acute pancreatitis. This approach is less convenient than simply measuring serum amylase. In general, the main use of urinary amylase is in establishing a diagnosis of macroamylasaemia. This is a condition where serum amylase is consistently raised (characteristically 2–3-fold) owing to the formation of complexes of amylase from non-pancreatic and pancreatic origin with immunoglobulins, usually IgG. When this is the case, a raised serum activity is not accompanied by a correspondingly increased urinary amylase. The isoenzyme raised in the plasma in illness depends on the source of the amylase, for example in pancreatitis, the P enzyme is raised, whereas in ruptured tubal pregnancy, the S isoenzyme is increased, and in renal failure, both S and P are raised. An increase in both isoenzyme fractions of amylase with a normal lipase is also suggestive of macroamylasaemia.
Lipase
The rise in plasma lipase activity approximately parallels that of amylase. Measurement of both enzymes may improve diagnostic accuracy, at least in part because the fall in lipase is slower, so that it remains raised for longer following an acute attack. Lipase activity increases in any condition where hyperamylasaemia is due to pancreatic pathology, but not when the amylase is of non-pancreatic origin. In particular, serum lipase activity is normal in macroamylasaemia. While lipase measurements overcome some of the non-specificity of amylase estimation in acute pancreatitis, lipase may still be raised in non-pancreatic disease. This, together with the fact that analytical methods for lipase are more complex and expensive than those for amylase means that lipase measurements are not widely used in the UK. Nevertheless, national guidelines recommend the measurement of lipase for diagnosis.
Choice of test for pancreatitis
Of the approaches discussed above, the measurement of serum amylase activity is the most frequently used biochemical test for pancreatitis, mainly because it is easy to perform. In areas where there is a large population of African Caribbeans (who have a higher reference range), the measurement of lipase may occasionally be useful to establish whether a raised amylase is of pancreatic origin. A number of other markers of acute pancreatitis have been investigated (e.g. elastase and phospholipase A2), but as yet these have not become established in routine use.
Ectopic pregnancy
A fertilized ovum occasionally may implant outside the uterus, where it gives rise to an ectopic pregnancy. The most common site is in one of the Fallopian tubes. Although this is not a common cause of an acute abdomen, even in young women, it is important because if a tubal pregnancy ruptures, the resulting massive intra-abdominal haemorrhage is life-threatening.
Serum progesterone is lower in absolute concentration and human chorionic gonadotrophin (hCG), which normally doubles in concentration every two days after implantation, tends to increase somewhat less rapidly in ectopic than in normal pregnancy. However, while such measures can be useful, they are not applicable in an acute situation. Rapid, sensitive urinary hCG assays are now available as near patient tests and will usually indicate whether or not the patient is pregnant (although occasionally hCG is undetectable in both maternal urine and serum in ectopic pregnancy). Quantitation of serum hCG is valuable, since, in a normal pregnancy, a gestational sac should be detectable in the uterus on transabdominal ultrasonography once the hCG is > 1000 IU/L. The clinical diagnosis of ectopic pregnancy can be difficult, and the results of biochemical tests have to be considered in light of the clinical assessment and the results of other techniques, such as transabdominal and transvaginal ultrasonography.
Acute porphyria
Acute porphyria is an exceptionally rare cause of an acute abdomen. The diagnosis may be suggested by the history (e.g. the association of the abdominal pain with the ingestion of a drug known to provoke acute attacks in susceptible subjects), or occasionally by someone noticing that the patient’s urine is dark red, or that it darkens on standing.
The porphyrias are discussed in detail in Chapter 28, but in a patient with acute abdominal pain, a negative screening test for urinary porphobilinogen (PBG) effectively excludes porphyria as the cause of the pain. (δ-Aminolaevulinic acid dehydratase deficiency remains a theoretical possibility, but only isolated cases of this condition have been reported.) If the test for urinary PBG is positive, then one of the acute porphyrias is likely.