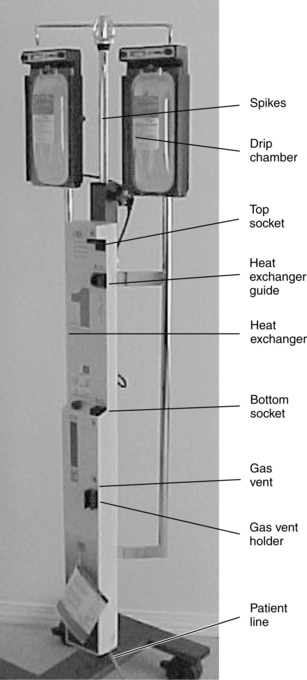
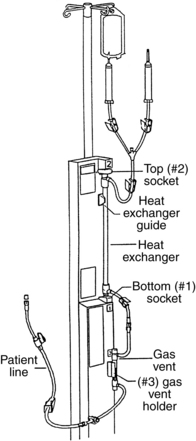
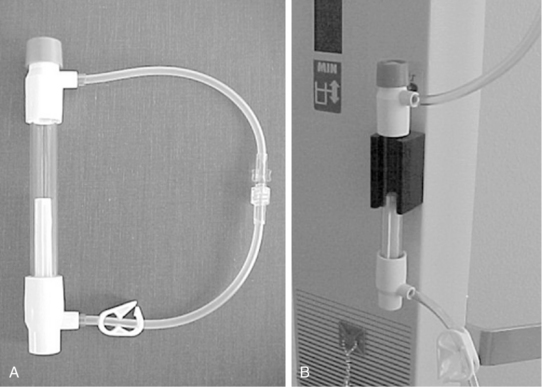
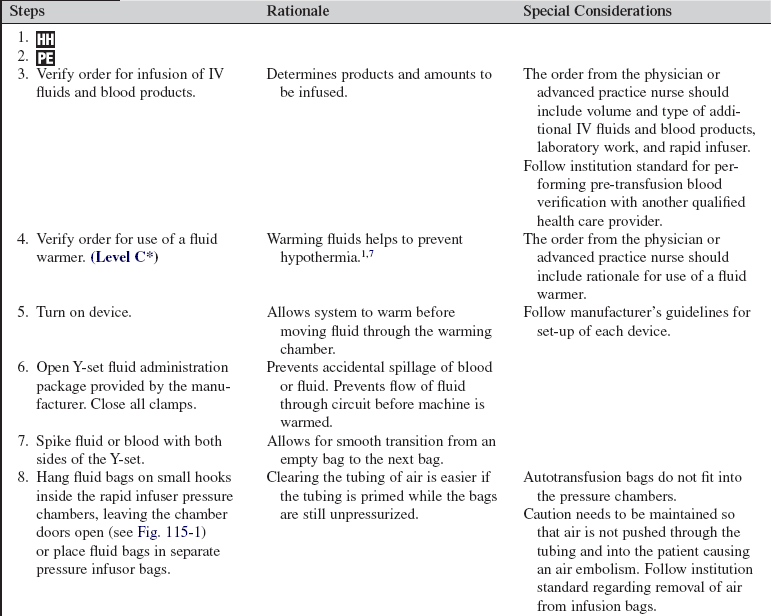
Use of a Massive Infusion Device and a Pressure Infusor Bag
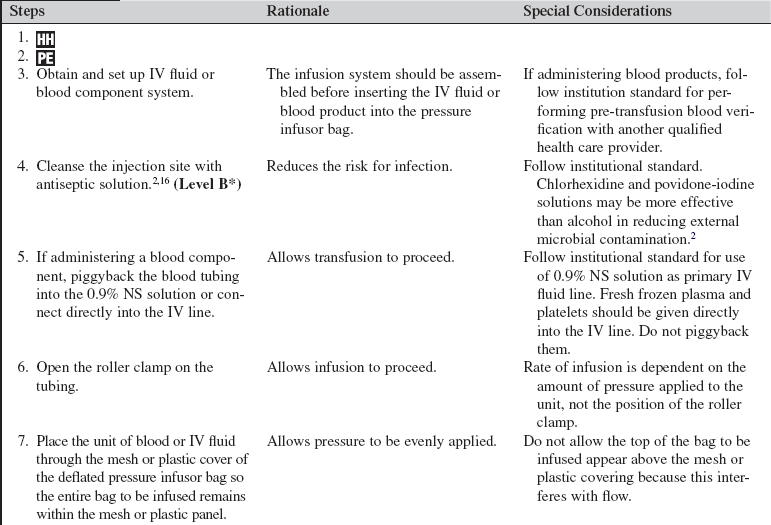
A massive transfusion is defined as 10 or more units of blood within the first 24 hours of admission.3,13 Rapid infusers are used to warm and quickly infuse multiple units of blood and intravenous fluids into patients with hemodynamically unstable conditions. Patients with severe trauma, gastrointestinal hemorrhage, postoperative hemorrhage, and intravascular losses, as occur in septic shock and burns, may need the rapid administration of large volumes of blood products and intravenous fluids to restore and maintain intravascular volumes. A pressure infusor bag is used to administer a large amount of intravenous fluid or blood products to a patient with life-threatening hemorrhage within a prescribed period of time.
PREREQUISITE NURSING KNOWLEDGE
• Knowledge of aseptic technique and principles of fluid resuscitation and blood transfusions is essential.
• A unit of fresh whole blood contains approximately 500 mL of warm blood with a hematocrit of 38% to 50%, a platelet count of 150,000 to 400,000, essentially full coagulation function, and 1500 mg of fibrinogen.1 The combination of one unit of packed red blood cells (PRBCs), one unit of platelets, one unit of fresh frozen plasma (FFP), and a 10 pack of cryoprecipitate provides 660 mL of fluid with a hematocrit of 29%, a platelet count of 87,000, coagulation activity approximating 65%, and 750 mg of fibrinigen.1
• Events from the battlefields of Afghanistan and Iraq have shown the need to rethink current practices regarding massive transfusion (MT). The Joint Theater Trauma Registry has refocused attention to rapid correction of trauma-induced coagulopathy, permissive hypotension, the early correction of hypothermia and acidosis, and the increased use of component blood transfusion therapy.1,13 Damage control resuscitation emphasizes treatment of the lethal triad of hypothermia, acidosis, and coagulopathy with surgical techniques.1,9 The use of MT occurs in 3% to 5% of all civilian trauma and 8% to 10% of all military trauma.3,10,15
• Hemorrhage is a major cause of death in the first hours after trauma.11,15
• Current strategies suggest an MT protocol with predefined blood protocols, permissive hypotension, minimizing of crystalloid products, and MT protocol of 1:1 FFP:PRBC during the first 24 hours for patients who are hypocoagulable with traumatic injuries.4,6,9 Aggressive use of FFP and platelets drastically reduces 24-hour mortality rates and early coagulopathy in patients with trauma.5
• Previously, the goal of shock resuscitation has been to support blood pressure, urine output, and reverse metabolic changes associated with ischemia and blood loss. Current civilian and combat strategies seek to immediately identify coagulopathy and simultaneously treat hypothermia and acidosis (which can impair thrombin generation rates) and achieve hemostasis to then volume load with blood components in ratios of 1:1 FFP:PRBC.9
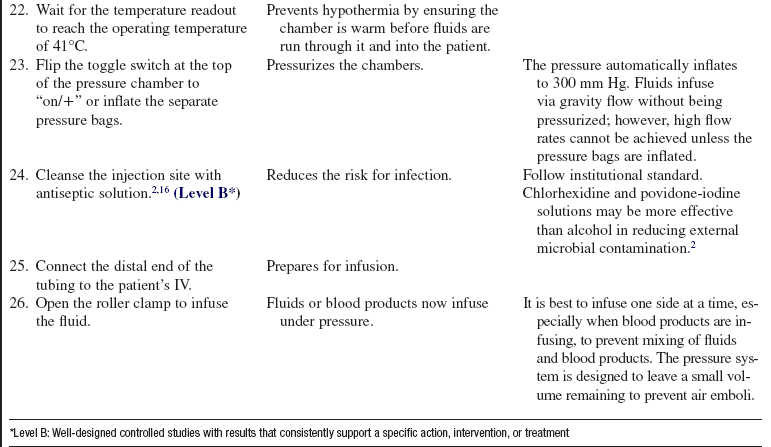
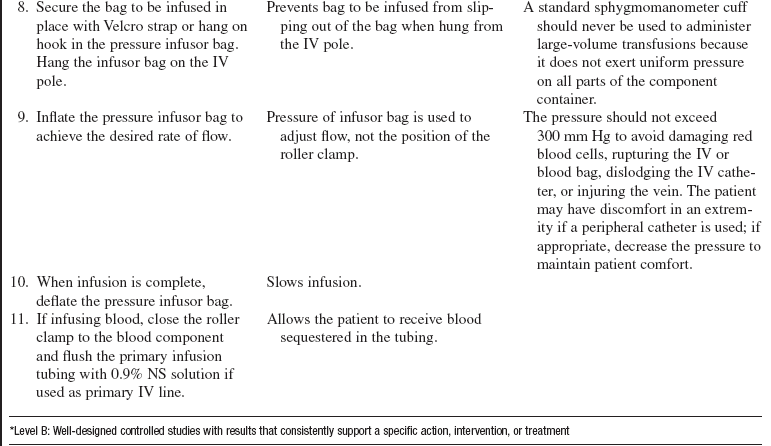
• Use of a rapid infusion device, such as the one described in this procedure (Fig. 115-1), can warm and infuse fluids at rates from 75 to 30,000 mL/hr (Level 1, Inc, Rockland, MA). The tubing is made of soft plastic that expands to allow rapid infusion of fluids under pressure. Some rapid infusers include automated pressure chambers to compress intravenous (IV) bags. They allow for fast and easy bag changes and can accommodate both 1-L IV bags and 500-mL blood product bags. Pressure is maintained at a constant 300 mm Hg and is turned on and off via a simple toggle switch at the top of each pressure chamber. Older infusers simply have an IV pole from which to hang fluids, and separate pressure infusor bags must be used.
• IV catheters for aggressive fluid resuscitation should have a large bore and short diameter to facilitate the rapid infusion of large volumes of IV fluids and blood products. Usually, multiple IVs are used, including peripheral and central sites. Venous access may also be obtained surgically via a venous cut-down of the basilic or saphenous veins when peripheral access cannot be obtained.12
• Both crystalloid and colloid IV solutions are used for resuscitating patients who are hypovolemic with hemodynamically unstable conditions. Crystalloids directly increase the intravascular volume. Colloids expand plasma volume by pulling interstitial fluid back into the vascular space via osmosis. Numerous crystalloid and colloid preparations are available in isotonic, hypotonic, and hypertonic preparations. Crystalloids most commonly used in aggressive fluid resuscitation are 0.9% normal saline (NS) and lactated Ringer’s (LR) solutions.
• The use of colloids such as albumin, dextran, and hetastarch allows the effective restoration of intravascular volume with smaller amounts of fluid; however, these colloids coat red blood cells (RBCs) and platelets, which may result in type and cross-match difficulties and clotting problems. Even slight overresuscitation with colloids increases the risk for fluid overload and pulmonary edema.12,14
• Blood and blood products are natural colloids used to replace lost blood and restore coagulation factors. In the patient with significant ongoing hemorrhage, infusion of blood and clotting factors is critical to restoring intravascular volume. Type O-negative blood is the universal donor for all patients and can be given in extreme emergencies before the completion of typing and crossmatching. PRBCs and whole blood are used to replace oxygen-carrying components; FFP, platelets, and cryoprecipitate are used to replace essential clotting factors.2
• When large volumes of IV fluids are being infused into patients, the fluids must be warmed to prevent hypothermia. (Although institutions vary in what constitutes large volumes, a good rule of thumb is to institute fluid rewarming measures when more than 2 L of fluid are required in less than 1 hour.)
• Hypothermia is a common consequence of aggressive fluid resuscitation and has serious physiologic consequences. It is correlated with mortality when the patient’s body temperature decreases below 34°C17 and significantly prolongs coagulation times at or below 35°C.7 Moderate hypothermia (32° to 34°C) reduces coagulation factor activity approximately 10% for each degree Celsius decrease in temperature while markedly affecting platelet function.8 Measures to prevent and treat hypothermia include solar blankets, heated blankets, body bags, warmed blood products and fluids, continuous arteriovenous rewarming, and cardiopulmonary bypass, used in extreme cases of hypothermia (see Procedure 94).1
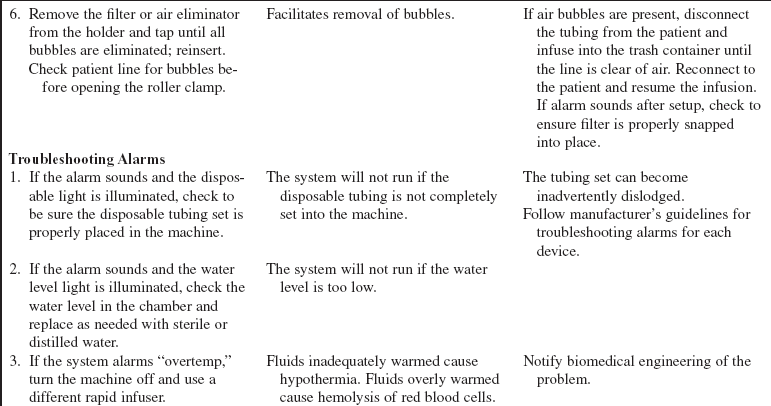
• Patients who have received multiple transfusions and aggressive fluid resuscitation are at risk for multiple complications as a result of shock and from the fluids and blood products themselves. These sequelae may include fluid overload, adult respiratory distress syndrome, acute tubular necrosis, hypothermia, hypokalemia, hypocalcemia, hemolytic and allergic reactions, and air embolism.14
EQUIPMENT
PATIENT AND FAMILY EDUCATION
• Explain the need for rapid infusion of fluids, the purpose of warming fluids, and how the equipment operates.  Rationale: Patient and family anxiety about unfamiliar equipment at the bedside is decreased.
Rationale: Patient and family anxiety about unfamiliar equipment at the bedside is decreased.
• Explain that prevention of hypothermia is among the top priorities in the resuscitation of the patient.  Rationale: The patient and family can understand the plan of care.
Rationale: The patient and family can understand the plan of care.
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess blood pressure, heart rate, respiratory rate, peripheral pulses, and level of consciousness.  Rationale: Assessment is necessary to determine the severity of the patient’s volume depletion and shock.
Rationale: Assessment is necessary to determine the severity of the patient’s volume depletion and shock.
• Assess temperature using a bladder probe or pulmonary artery catheter.  Rationale: Assessment is necessary to assess for the development of hypothermia while large volumes of fluids are infused. Core temperatures most accurately reflect true body temperature.
Rationale: Assessment is necessary to assess for the development of hypothermia while large volumes of fluids are infused. Core temperatures most accurately reflect true body temperature.
• Assess patient history, including precipitating events, surgical and medical interventions thus far, and history of cardiac problems.  Rationale: Potential or actual need for massive fluid resuscitation and risk for fluid overload are identified.
Rationale: Potential or actual need for massive fluid resuscitation and risk for fluid overload are identified.
• Assess hemodynamic parameters, including baseline central venous pressure (CVP) and, if available, pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), cardiac output (CO) and cardiac index (CI), systemic vascular resistance (SVR), and mixed venous oxygen saturation (SvO2). Assessment of right ventricular ejection fraction, oxygen delivery and consumption, and oxygen extraction ratio should also be included if the technology is available.  Rationale: Baseline information is provided about patient’s preload, afterload, and cardiac contractility.
Rationale: Baseline information is provided about patient’s preload, afterload, and cardiac contractility.
• Assess laboratory values to include arterial blood gas, serum electrolyte, serum lactate, base deficit, hemoglobin, hematocrit, and coagulation studies.  Rationale: Baseline oxygenation, presence of metabolic acidosis, severity of ongoing hemorrhage, and severity of coagulopathy are measured so that the need for intervention and the effectiveness of interventions can be determined.
Rationale: Baseline oxygenation, presence of metabolic acidosis, severity of ongoing hemorrhage, and severity of coagulopathy are measured so that the need for intervention and the effectiveness of interventions can be determined.
• Assess patency of multiple large-bore IV sites.  Rationale: Multiple sites are often necessary to infuse enough fluids and blood products to support the patient’s vital signs. Extra sites in addition to those used for rapid infusion should be kept patent in case one of the other sites becomes nonfunctional or is accidentally pulled out.
Rationale: Multiple sites are often necessary to infuse enough fluids and blood products to support the patient’s vital signs. Extra sites in addition to those used for rapid infusion should be kept patent in case one of the other sites becomes nonfunctional or is accidentally pulled out.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• Ensure that informed consent has been obtained.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
• Ensure that the patient and family understand the need and purpose for rapid infusion. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Place additional peripheral IV sites.  Rationale: Aggressive fluid resuscitation requires additional IV access besides the one site being used with the rapid infuser. Backup IV sites can be used if other sites infiltrate or become pulled out; extra sites may also be used to infuse medications, such as vasopressors, that should be kept separate from rapid infusion lines. Ideal sites for large IV catheter access are the antecubital fossae, saphenous veins, and the veins of the forearm and upper arm.
Rationale: Aggressive fluid resuscitation requires additional IV access besides the one site being used with the rapid infuser. Backup IV sites can be used if other sites infiltrate or become pulled out; extra sites may also be used to infuse medications, such as vasopressors, that should be kept separate from rapid infusion lines. Ideal sites for large IV catheter access are the antecubital fossae, saphenous veins, and the veins of the forearm and upper arm.
• Assist the physician or advanced practice nurse with placement of central venous access or pulmonary artery catheter or both.  Rationale: This placement allows for the assessment of volume status before and after infusion of fluids and blood products. It also allows for assessment of core temperature with pulmonary artery catheter thermistor and provides central venous access in the event vasoactive medications are needed.
Rationale: This placement allows for the assessment of volume status before and after infusion of fluids and blood products. It also allows for assessment of core temperature with pulmonary artery catheter thermistor and provides central venous access in the event vasoactive medications are needed.
• Place an automatic blood pressure monitor on the patient’s arm that is not being infused with the rapid infusion device. Set it to check blood pressure every 5 minutes.  Rationale: Assessment of patient’s hemodynamics and response to fluid replacement is provided. This is used temporarily until an arterial line can be placed by the physician or advanced practice nurse.
Rationale: Assessment of patient’s hemodynamics and response to fluid replacement is provided. This is used temporarily until an arterial line can be placed by the physician or advanced practice nurse.
• Assist the physician or advanced practice nurse with placement of an arterial line.  Rationale: Placement allows for continuous assessment of the blood pressure during resuscitation and provides convenient access for blood sampling.
Rationale: Placement allows for continuous assessment of the blood pressure during resuscitation and provides convenient access for blood sampling.
• Obtain a blood sample for type and cross-match. Two tubes should be sent if a large volume of blood is expected to be transfused.  Rationale: This action prepares for blood transfusion.
Rationale: This action prepares for blood transfusion.
• Obtain baseline hematocrit, chemistry panel, and coagulation studies. Repeat, as ordered, every 15 to 60 minutes until hemorrhage is controlled.  Rationale: These studies guide proper replacement of blood products and essential electrolytes.
Rationale: These studies guide proper replacement of blood products and essential electrolytes.
• Place an indwelling urinary catheter.  Rationale: Patients who need aggressive fluid resuscitation should have an indwelling urinary catheter placed to determine volume status and end-organ perfusion.
Rationale: Patients who need aggressive fluid resuscitation should have an indwelling urinary catheter placed to determine volume status and end-organ perfusion.
• Cover the patient with warm cotton blankets or a warm-air blanket. Cover the patient’s head with a warmed blanket, a towel, or an aluminum cap.  Rationale: Additional heat loss is minimized.
Rationale: Additional heat loss is minimized.
References
1. Beekley, AC, Damage control resuscitation. a sensible -approach to the exsanguinating patient. Crit Care Med 2008; 36:S267–S274.
![]() 2. Casey, AL, et al. A randomized, prospective clinical -trial to assess the potential infection risk associated with the PosiFlow needleless connector. J Hosp Infection. 2003; 54:288–293.
2. Casey, AL, et al. A randomized, prospective clinical -trial to assess the potential infection risk associated with the PosiFlow needleless connector. J Hosp Infection. 2003; 54:288–293.
![]() 3. Como, JJ, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004; 44:809–813.
3. Como, JJ, et al. Blood transfusion rates in the care of acute trauma. Transfusion. 2004; 44:809–813.
4. Cotton, BA, et al. Predefined massive transfusion protocols are associated with a reduction in organ failure and -postinjury complications. J Trauma. 2009; 66:41–49.
5. Dente, CJ, et al. Improvements in early mortality and coagulopathy are sustained better in patients with blunt trauma -after institution of a massive transfusion protocol in a civilian level 1 trauma center. J Trauma. 2009; 66:1616–1624.
6. Duchesne, JC, et al, Review of current blood transfusions strategies in a mature level 1 trauma center. were we wrong for the last 60 years. J Trauma 2008; 65:272–277.
![]() 7. Gubler, KD, et al. The impact of hypothermia on dilutional coagulopathy. J Trauma. 1994; 36:847–851.
7. Gubler, KD, et al. The impact of hypothermia on dilutional coagulopathy. J Trauma. 1994; 36:847–851.
8. Hess, JR, Lawson, JH, The coagulopathy of trauma versus disseminated intravascular coagulation. J Trauma 2006; 60 :S12–S19.
9. Holcomb, JB, et al, Damage control resuscitation . directly addressing the early coagulopathy of trauma. J Trauma 2007; 62:307–310.
10. Holcomb, JB. Damage control resuscitation. J Trauma. 2007; 62:S36–S37.
11. Kauvar, DS, Lefering, R, Wade, CE, Impact of hemorrhage on trauma outcome. an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma 2006; 60:S3–S11.
![]() 12. Koran, Z, Newberry, L. Vascular access and fluid replacement. In: Emergency Nurses Association, Newberry L, eds. Sheehy’s emergency nursing. ed 5. St Louis: Mosby; 2003:147.
12. Koran, Z, Newberry, L. Vascular access and fluid replacement. In: Emergency Nurses Association, Newberry L, eds. Sheehy’s emergency nursing. ed 5. St Louis: Mosby; 2003:147.
13. McLaughlin, DF, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008; 64:S57–S63.
![]() 14. McQuillan, KA. Initial management of traumatic shock. In: McQuillan KA, ed. Trauma nursing: from resuscitation through rehabilitation. ed 3. Philadelphia: Saunders; 2002:151.
14. McQuillan, KA. Initial management of traumatic shock. In: McQuillan KA, ed. Trauma nursing: from resuscitation through rehabilitation. ed 3. Philadelphia: Saunders; 2002:151.
15. Nunez, TC, et al, Early prediction of massive transfusion in trauma. simple as ABC (assessment of blood consumption). J Trauma 2009; 66:346–352.
![]() 16. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30(8):476–489.
16. O’Grady, NP, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2002; 30(8):476–489.
17. Shaz, BH, et al. Transfusion management of trauma patients. Anesth Analg. 2009; 108:1760–1768.
American Association of Blood Banks. Primer of blood administration. Bethesda, MD: The Association; 2002. [revised 12/02,].
Snyder, CW, et al, The relationship of blood product ration to mortality. survival benefit or survival bias. J Trauma 2009; 66:358–364.
Stammers, AH, et al. Utilization of rapid-infusor devices for massive transfusion. Perfusion. 2005; 20:65–69.