Continuous Renal Replacement Therapies
PREREQUISITE NURSING KNOWLEDGE
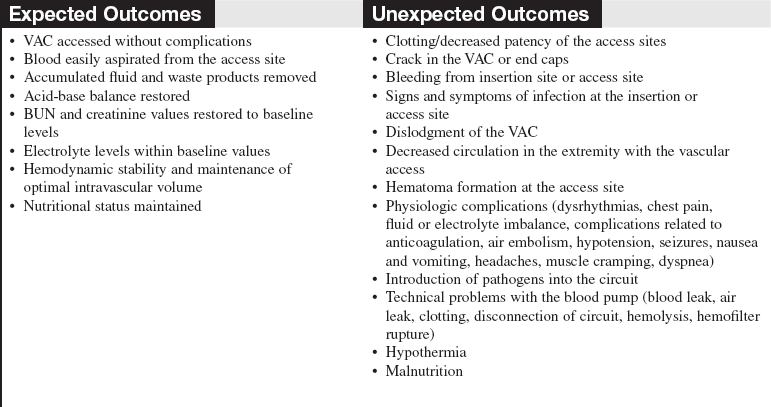
• Continuous renal replacement therapy (CRRT) is an extracorporeal blood purification therapy intended to substitute for impaired renal function over an extended period of time for, or attempted for, 24 hours per day.
• CRRT can be accomplished through a variety of methods, with either arteriovenous access or venovenous access. The venovenous access is used almost exclusively because of its less invasive nature.10,18 The following methods of CRRT are included as listed (details outlined in Table 112-1):
Table 112-1
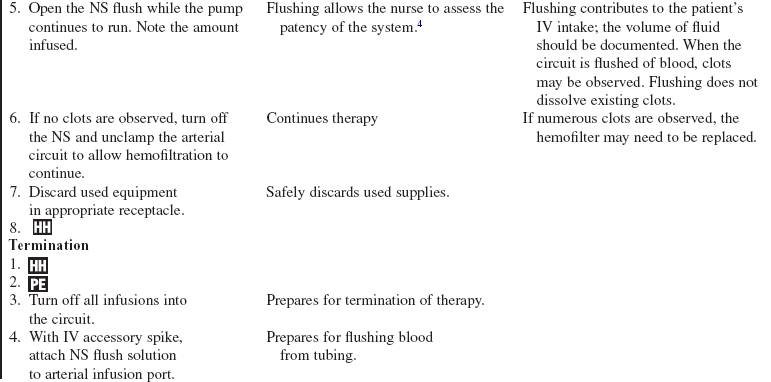
Continuous Renal Replacement Therapies
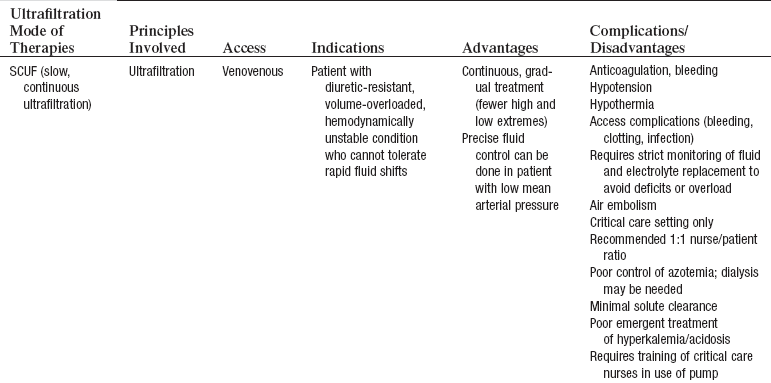
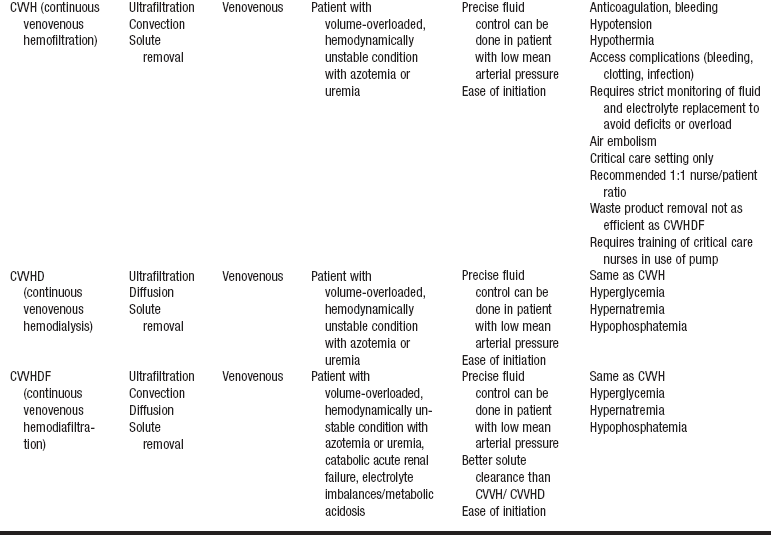
Adapted from Giuliano K, Pysznik E: Renal replacement therapy in critical care: implementation of a unit-based CVVH program, Crit Care Nurse 18:40-45, 1998.
 Slow continuous ultrafiltration (SCUF)
Slow continuous ultrafiltration (SCUF)
 Continuous venovenous hemofiltration (CVVH)
Continuous venovenous hemofiltration (CVVH)
• Basic knowledge is required of the principles of diffusion, ultrafiltration (UF), osmosis, oncotic pressure, and hydrostatic pressure and how they pertain to fluid and solute management during dialysis.
 Diffusion: The passive movement of solutes through a semipermeable membrane from an area of higher to lower concentration until equilibrium is reached.
Diffusion: The passive movement of solutes through a semipermeable membrane from an area of higher to lower concentration until equilibrium is reached.
 Convective transport: The rapid movement of fluid across a semipermeable membrane from an area of high pressure to an area of low pressure with transport of solutes. When water moves across a membrane along a pressure gradient, some solutes are carried along with the water and do not require a solute concentration gradient (also called solute drag). Convective transport is most effective for the removal of middle-molecular-weight and large-molecular-weight solutes.
Convective transport: The rapid movement of fluid across a semipermeable membrane from an area of high pressure to an area of low pressure with transport of solutes. When water moves across a membrane along a pressure gradient, some solutes are carried along with the water and do not require a solute concentration gradient (also called solute drag). Convective transport is most effective for the removal of middle-molecular-weight and large-molecular-weight solutes.
 UF: The bulk movement of solute and solvent through a semipermeable membrane in response to a pressure difference across the membrane. This movement is usually achieved with positive pressure in the blood compartment in the hemofilter and negative pressure in the dialysate compartment. Blood and dialysate run countercurrent. The size of the solute molecules as compared with the size of molecules that can move through the semipermeable membrane determines the degree of UF.
UF: The bulk movement of solute and solvent through a semipermeable membrane in response to a pressure difference across the membrane. This movement is usually achieved with positive pressure in the blood compartment in the hemofilter and negative pressure in the dialysate compartment. Blood and dialysate run countercurrent. The size of the solute molecules as compared with the size of molecules that can move through the semipermeable membrane determines the degree of UF.
 Osmosis: The passive movement of solvent through a semipermeable membrane from an area of higher to lower concentration.
Osmosis: The passive movement of solvent through a semipermeable membrane from an area of higher to lower concentration.
 Oncotic pressure: The pressure exerted by plasma proteins that favor intravascular fluid retention and movement of fluid from the extravascular to the intravascular space.
Oncotic pressure: The pressure exerted by plasma proteins that favor intravascular fluid retention and movement of fluid from the extravascular to the intravascular space.
 Hydrostatic pressure: The force exerted by arterial blood pressure that favors the movement of fluid from the intravascular to the extravascular space.
Hydrostatic pressure: The force exerted by arterial blood pressure that favors the movement of fluid from the intravascular to the extravascular space.
 Absorption: The process by which drug molecules pass through membranes and fluid barriers and into body fluids.
Absorption: The process by which drug molecules pass through membranes and fluid barriers and into body fluids.
 Adsorption: The adhesion of molecules (solutes) to the surface of the hemofilter, charcoal, or resin.
Adsorption: The adhesion of molecules (solutes) to the surface of the hemofilter, charcoal, or resin.
• CRRT uses an artificial kidney (i.e., hemofilter, dialyzer) with a semipermeable membrane to create two separate compartments: the blood compartment and the dialysis solution compartment. The semipermeable membrane allows the movement of small molecules (e.g., electrolytes) and middle-size molecules (creatinine, vasoactive substances) from the patient’s blood into the dialysis solution but is impermeable to larger molecules (red blood cells, plasma proteins).
• Each dialyzer has four ports: two end ports for blood (in one end and out the other) and two side ports for dialysis solution ultrafiltrate (in one end and out the other). In most cases, the blood and dialysate are run through the dialyzer in opposite or countercurrent directions.
• With hollow-fiber dialyzers, the blood flows through the center of hollow fibers, and the dialysis solution (dialysate) flows around the outside of the hollow fibers. The advantages of hollow-fiber filters include a low priming volume, low resistance to flow, and high amount of surface area. The major disadvantage is the potential for clotting as a result of the small fiber size.
• All dialyzers have UF coefficients; thus, the dialyzer selected varies in different clinical situations.1–5 The higher the UF coefficient, the more rapid the fluid removal. UF coefficients are determined with in vivo measurements done by each dialyzer manufacturer.
• Clearance refers to the ability of the dialyzer to remove metabolic waste products or drugs from the patient’s blood. The blood flow rate, the dialysate flow rate, and the solute concentration affect clearance. Clearance occurs by the processes of diffusion, convection, and UF.
• The dialysate (when used during CRRT) is composed of water, a buffer (i.e., lactate or bicarbonate), and various electrolytes. Most solutions also contain glucose. The buffer helps neutralize acids that are generated as a result of normal cellular metabolism and that usually are excreted by the kidney. The concentration of electrolytes is usually the normal plasma concentration, which helps to create a concentration gradient for removal of excess electrolytes. The glucose aids in increasing the oncotic pressure in the dialysate (thus aiding in fluid removal) and in caloric replacement. Although glucose comes in various concentrations, it is usually used in normal plasma concentrations to prevent hyperglycemia.
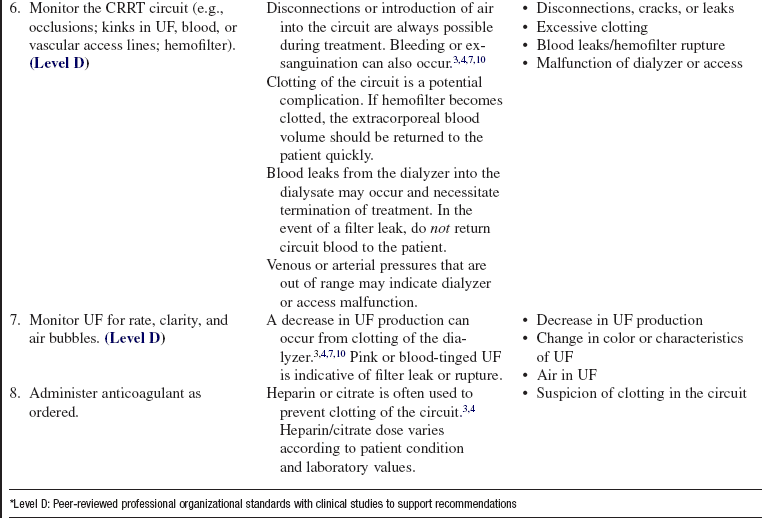
• Heparin or citrate is often used during CRRT to prevent clotting of the circuit during treatment. Saline solution flushes can be used alone or with other anticoagulants to maintain circuit patency.3–7,9
• An anticoagulant is used to maintain vascular access patency when CRRT is not in use.3,10
• If the patient is taking angiotensin-converting enzyme (ACE) inhibitors, contact with certain filters or membranes in the CRRT system can cause an anaphylactic reaction and severe hypotension as a result of increased levels of bradykinin, a potent vasodilator. ACE inhibitors are recommended to be withheld for 48 to 72 hours before treatment, if possible.
• Continuous venovenous renal replacement therapy is achieved with a pumped system.
• The patient’s volume status and serum electrolyte levels are changed gradually so that patients have fewer problems than they do with hemodialysis. Specifics of these therapies are outlined in Table 112-1.1,2,4,7,13–15,17
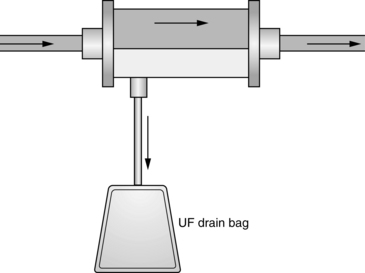
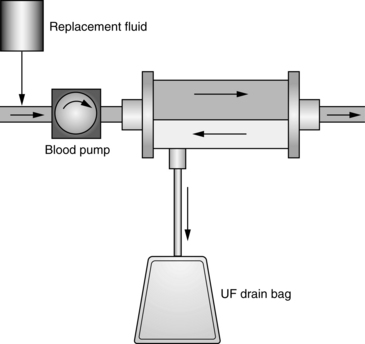
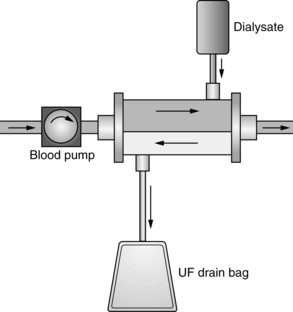
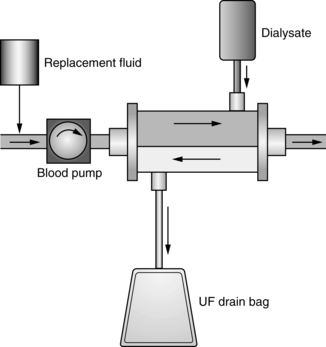
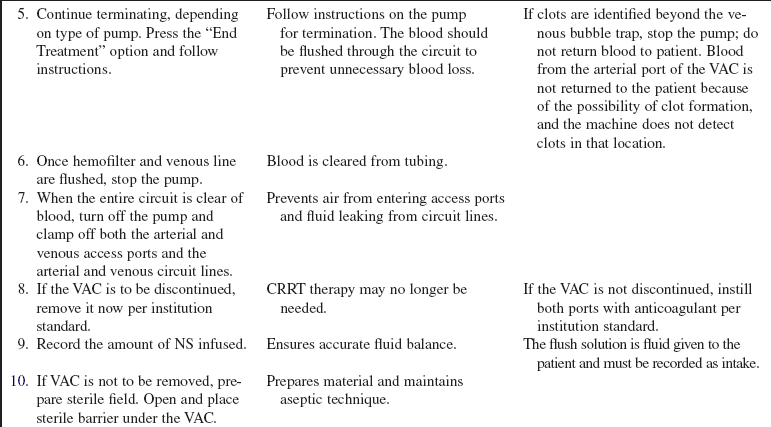
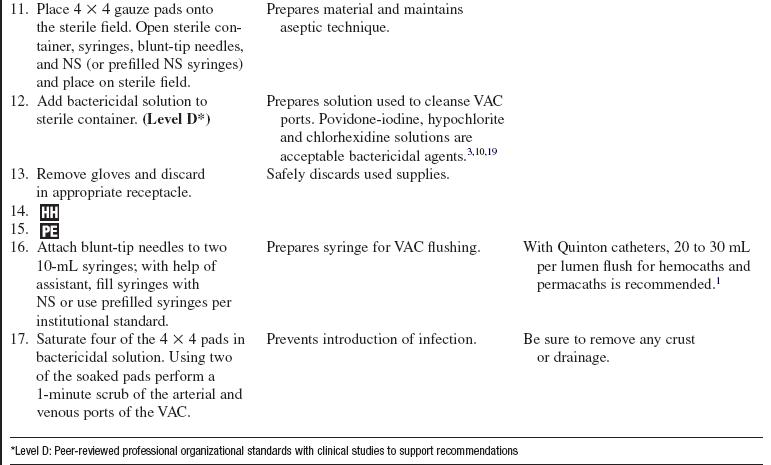
• SCUF (Fig. 112-1) is a nonpumped system, CVVH (Fig. 112-2), CVVHD (Fig. 112-3), and CVVHDF (Fig. 112-4) use pumped systems. These therapies are used to remove both plasma water and solutes and require venous access, most commonly provided with a double-lumen vascular access catheter (VAC). External arteriovenous (AV) hemodialysis shunts or surgically created AV hemodialysis anastomoses have been used in the past for CRRT; however, because of increased incidence rates of vascular injury, bleeding, and infection, they are not recommended for CRRT access.1,10 Common sites for the VAC are the internal jugular, subclavian, and femoral veins. The internal jugular approach is the preferred access. Cannulation of the subclavian vein may cause stenosis and prevent placement of upper extremity grafts or fistulas if long-term dialysis is necessary.3,10 Femoral cannulation is associated with increased infection.3,10,16,19,20
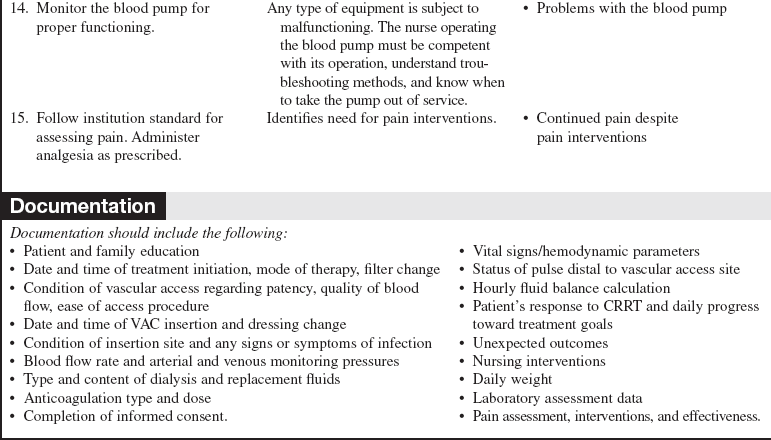
• A blood pump provides the pressure that drives the system; the blood circuit consists of blood lines, a blood pump, and various monitoring devices. The blood lines carry the blood to and from the patient. The blood pump controls the speed of the blood through the circuit. The monitoring devices include arterial and venous pressure monitors and an air detection monitor to prevent air that may have entered the circuit from being returned to the patient. Anticoagulant, dialysate, and replacement fluids can also be added to the system.
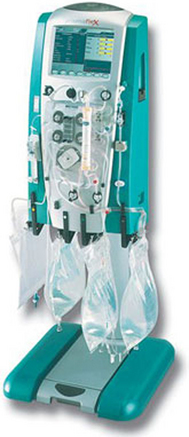
 Integrated pump systems have separate pumps for blood, dialysate, ultrafiltrate/effluent, and replacement fluids (Fig. 112-5). The pumps are controlled by a computerized control module. Blood flow rate, dialysate flow rate, anticoagulation rate, and fluid removal rates are entered by the nurse per medical guidelines. Dialysate, ultrafiltrate/effluent, and replacement fluids are measured by weight or volumetric scales on the unit. The module calculates and adjusts pump speeds to achieve the selected fluid goal. The module also records and displays treatment data.
Integrated pump systems have separate pumps for blood, dialysate, ultrafiltrate/effluent, and replacement fluids (Fig. 112-5). The pumps are controlled by a computerized control module. Blood flow rate, dialysate flow rate, anticoagulation rate, and fluid removal rates are entered by the nurse per medical guidelines. Dialysate, ultrafiltrate/effluent, and replacement fluids are measured by weight or volumetric scales on the unit. The module calculates and adjusts pump speeds to achieve the selected fluid goal. The module also records and displays treatment data.
• SCUF (see Fig. 112-1) is used primarily to remove plasma water. A hemofilter with a large surface area, high sieving coefficient, and low resistance is used to facilitate slow continuous fluid removal.
• CVVH (see Fig. 112-2) removes fluids and solutes via convection. Replacement solution is part of the setup; the replacement solution creates a solute drag effect.
• CVVHD (see Fig. 112-3) is used to remove solutes primarily via diffusion. Dialysate solution is part of the setup; flow of the dialysate is countercurrent to the blood flow.
• CVVHDF (see Fig. 112-4) removes fluids and solutes via diffusion and convection. Dialysate runs countercurrent to the blood flow. Intravenous or blood circuit replacement fluid is used continuously based on the amount of UF removed each hour and net fluid goals for the patient.
• Other extended renal replacement therapy techniques or “hybrid” techniques (sustained low-efficiency dialysis [SLED], extended daily dialysis [EDD]) generally use standard hemodialysis equipment with reduced blood flow and dialysate rates to gradually remove plasma water and solutes. They are used from 4 to 12 hours a day.
EQUIPMENT (FOR INITIATION)
• Hemofilter/pump system with blood lines
• Replacement fluid, as ordered
• Fluid shield, face masks, or goggles
• Sterile and nonsterile gloves
• Two 10-mL prefilled syringes with normal saline (NS) solution
• Two 5-mL and 10-mL syringes and blunt-tip needles (to draw up NS for injection)
• NS for injection (if prefilled NS syringes are not used)
• Dressing supplies (alcohol wipes, sterile barrier, gauze pads, transparent dressing, tape)
• Povidone-iodine, hypochlorite, or chlorhexidine bactericidal solution (follow institution standard)
• Heparin, 1000 units/mL, or citrate (for priming follow institution standard)
EQUIPMENT (FOR TERMINATION)
• Intravenous (IV) accessory spike to connect NS bag to arterial tubing
• Hemostats (extra clamps if needed)
• Dressing supplies (alcohol wipes, sterile barrier, gauze pads, transparent dressing, tape)
• Povidone-iodine, hypochlorite, or chlorhexidine bactericidal solution (follow institution standard)
• Sterile and nonsterile gloves
• Fluid shield, face mask, or goggles
• Heparin (1000 or 5000 units/mL, per institution standard), needleless caps (if VACs are used), labels for VAC (other anticoagulants may be used per institution standard)
• Prefilled syringes filled with NS
• 10-mL syringes and blunt-tip needles (if prefilled syringes are not used)
PATIENT AND FAMILY EDUCATION
• Explain the purpose of CRRT, specifically why the treatment is performed, and the expected clinical outcomes.  Rationale: The patient and family should understand that CRRT is necessary to perform the physiologic functions of the kidneys when fluid overload or renal failure is present (or other specific purpose for the therapy).
Rationale: The patient and family should understand that CRRT is necessary to perform the physiologic functions of the kidneys when fluid overload or renal failure is present (or other specific purpose for the therapy).
• Explain the procedure, including risks, anticipated length of treatment, and patient positioning, and review any questions the patient may have.  Rationale: Explanation provides information and may decrease patient anxiety.
Rationale: Explanation provides information and may decrease patient anxiety.
• Explain the need for careful sterile technique for the duration of treatment.  Rationale: The patient and family must know the importance of sterile technique to decrease the likelihood of systemic infection.
Rationale: The patient and family must know the importance of sterile technique to decrease the likelihood of systemic infection.
• Explain the need for careful monitoring of the patient during the treatment, particularly for fluid and electrolyte imbalance.  Rationale: The patient and family should understand that careful monitoring is a routine part of CRRT.
Rationale: The patient and family should understand that careful monitoring is a routine part of CRRT.
• Explain the signs and symptoms of possible complications during CRRT.  Rationale: Patients and family should be fully prepared if complications occur (e.g., hypotension, hemorrhage, manifestations of fluid/electrolyte/acid-base imbalance).
Rationale: Patients and family should be fully prepared if complications occur (e.g., hypotension, hemorrhage, manifestations of fluid/electrolyte/acid-base imbalance).
• Explain the CRRT circuit setup to the patient and family.  Rationale: The patient and family must know that blood will be removed from the patient’s body and will be visible during the CRRT treatment.
Rationale: The patient and family must know that blood will be removed from the patient’s body and will be visible during the CRRT treatment.
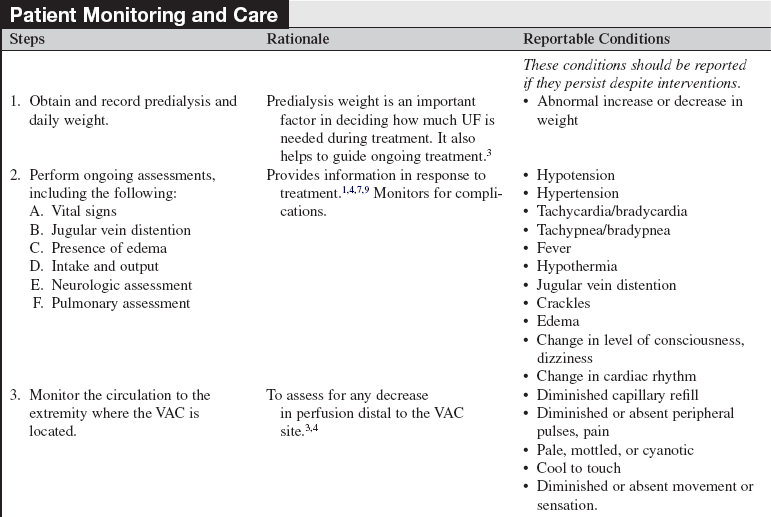
PATIENT ASSESSMENT AND PREPARATION
Patient Assessment
• Assess baseline vital signs, including hemodynamic parameters, weight, laboratory values (blood urea nitrogen (BUN), creatinine, electrolytes, hemoglobin, hematocrit), and neurologic status.  Rationale: Patients in renal failure often have altered baseline assessment results, both in physical assessment and in laboratory values. Knowledge of this information before treatments are started is helpful so that interventions, including net fluid balance and dialysate fluid, can be individualized. Alterations during treatment are common because of the rapid removal of fluid and solutes.
Rationale: Patients in renal failure often have altered baseline assessment results, both in physical assessment and in laboratory values. Knowledge of this information before treatments are started is helpful so that interventions, including net fluid balance and dialysate fluid, can be individualized. Alterations during treatment are common because of the rapid removal of fluid and solutes.
• Assess the insertion site for signs and symptoms of infection.  Rationale: Insertion sites provide a portal of entry for infection, which may result in septicemia if unrecognized or untreated. If the insertion site appears to be infected, further interventions (e.g., site change, culture, antibiotic treatment) may be necessary.
Rationale: Insertion sites provide a portal of entry for infection, which may result in septicemia if unrecognized or untreated. If the insertion site appears to be infected, further interventions (e.g., site change, culture, antibiotic treatment) may be necessary.
• Assess patency of VAC and the ability to easily aspirate blood from both ports.  Rationale: Adequate blood flow is necessary during a treatment to facilitate optimal fluid and solute removal. Patent catheter ports are necessary for adequate blood flow.
Rationale: Adequate blood flow is necessary during a treatment to facilitate optimal fluid and solute removal. Patent catheter ports are necessary for adequate blood flow.
• Assess adequate circulation to the distal parts of the access limb.  Rationale: The placement of vascular access may compromise circulation.
Rationale: The placement of vascular access may compromise circulation.
Patient Preparation
• Verify correct patient with two identifiers.  Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
Rationale: Prior to performing a procedure, the nurse should ensure the correct identification of the patient for the intended intervention.
• When CRRT is initiated, ensure that informed consent has been obtained.  Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
Rationale: Informed consent protects the rights of the patient and makes a competent decision possible for the patient.
• Ensure that patient understands preprocedural teachings. Answer questions as they arise, and reinforce information as needed.  Rationale: Understanding of previously taught information is evaluated and reinforced.
Rationale: Understanding of previously taught information is evaluated and reinforced.
• Position the patient in a comfortable position (that also facilitates optimal blood flow through the vascular access).  Rationale: The patient and family must understand that movement during the procedure may affect the blood flow through the system and that a comfortable position before the initiation of therapy is important. They should also understand that different access sites may require different patient positions to facilitate optimal blood flow. Choose a position that allows for setup of the sterile field. The nurse who sets up the sterile field and initiates therapy must be able to easily reach all the necessary supplies.
Rationale: The patient and family must understand that movement during the procedure may affect the blood flow through the system and that a comfortable position before the initiation of therapy is important. They should also understand that different access sites may require different patient positions to facilitate optimal blood flow. Choose a position that allows for setup of the sterile field. The nurse who sets up the sterile field and initiates therapy must be able to easily reach all the necessary supplies.
References
![]() 1. Bellomo, R, Ronco, C, Mehta, R. Nomenclature for continuous renal replacement therapies. Am J Kidney Dis. 1996; 28:S2–S7.
1. Bellomo, R, Ronco, C, Mehta, R. Nomenclature for continuous renal replacement therapies. Am J Kidney Dis. 1996; 28:S2–S7.
2. Bouman, CS, High-volume hemofiltration as adjunctive therapy for sepsis and systemic inflammatory response syndrome. background, definition and a descriptive analysis of animal and human studies. Adv Sepsis 2007; 6:57–74.
3. Craig, M. Slow extended daily dialysis (SLEDD) and continuous renal replacement therapies (CRRT). In: Counts C, ed. Core curriculum for nephrology nursing. ed 5. Pitman, NJ: American Nephrology Nurses’ Association; 2008:132–229.
4. Dirkes, S, Hodge, K, Continuous renal replacement therapy in the adult intensive care unit. history and current trends. Crit Care Nurse 2007; 27:61–81.
5. Fiore, GB, Ronco, C. Principles and practice of internal hemodiafiltration. Contrib Nephrol. 2007; 158:177–184.
6. Gibney, N, et al. Volume management by renal replacement therapy in acute kidney injury. Int J Artif Organs. 2008; 31:145–155.
![]() 7. Giuliano, K, Pysznik, E, Renal replacement therapy in critical care. implementation of a unit-based CVVH program. Crit Care Nurse 1998; 18:40–45.
7. Giuliano, K, Pysznik, E, Renal replacement therapy in critical care. implementation of a unit-based CVVH program. Crit Care Nurse 1998; 18:40–45.
8. Goldstein-Fuchs J. Nutrition and chronic kidney disease. In: Molzahn A, Butera E, eds. Contemporary nephrology nursing: principles and practice. ed 2. Pitman, NJ: American Nephrology Nurses’ Association; 2006:371–391.
![]() 9. Jones, S. Heat loss and continuous renal replacement therapy. AACN Clin Issues. 2004; 15:223–230.
9. Jones, S. Heat loss and continuous renal replacement therapy. AACN Clin Issues. 2004; 15:223–230.
10. National Kidney Foundation (NKF), KDOQI clinical practice guidelines for vascular access. update 2006. Am J Kidney Dis 2006; 48:S176–S307.
11. Ricci, Z, Ronco, C, Dose and efficiency of renal replacement therapy. continuous replacement therapy versus intermittent hemodialysis versus extended daily dialysis. Crit Care Med 2008; 36:S229–S237.
![]() 12. Rickard, C, et al, Preventing hypothermia during continuous veno-venous haemodiafiltration. a randomized controlled study. J Adv Nurs 2004; 47:393–400.
12. Rickard, C, et al, Preventing hypothermia during continuous veno-venous haemodiafiltration. a randomized controlled study. J Adv Nurs 2004; 47:393–400.
13. Ronco, C, et al. Potential interventions in sepsis-related acute kidney injury. Clin J Am Soc Nephrol. 2008; 3:531–544.
14. Ronco, C, et al, Outcome comparisons of intermittent and continuous therapy in acute kidney injury. what do they mean. Int J Artif Organs 2008; 31:213–220.
15. Pannu, N, et al. Renal replacement therapy in patients with acute renal failure. JAMA. 2008; 299:793–805.
16. Pronovost, P, et al. An intervention to decrease catheter-related blood stream infections in the ICU. N Engl J Med. 2006; 355:2725–2734.
17. Tolwani, AJ, et al. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol. 2008; 19:1233–1238.
18. Uchino, S, et al, Continuous renal replacement therapy . a worldwide practice survey. Intensive Care Med 2007; 33 :1563–1570.
19. White, RB, Vascular access for hemodialysisMolzahn A, Butera E, eds.. Contemporary nephrology nursing: principles and practice. ed 2. 2006 . American Nephrology Nurses’ Association: Pitman, NJ:559–578.
![]() 20. Young, E, et al. Incidence and influencing factors associated with exit site infections in temporary catheters for hemodialysis and apheresis. Nephrol Nurs J. 2005; 32:41–50.
20. Young, E, et al. Incidence and influencing factors associated with exit site infections in temporary catheters for hemodialysis and apheresis. Nephrol Nurs J. 2005; 32:41–50.
Clark, WR, et al. Recent clinical advances in the management of critically ill patients with acute renal failure. Blood -Purification. 2006; 24:487–498.
Oudemans-van Straaten HM, et al, Anticoagulation strategies in continuous renal replacement therapy. can the choice be evidence based. Intensive Care Med 2006; 32:188–202.
Palsson, R, et al. Choice of replacement solution and -anticoagulation in continuous venovenous hemofiltration. Clin Nephrol. 2006; 65:34–42.