History
Current Medications
Physical Examination
Hospital and Procedural Course
Focused Clinical Questions and Discussion Points
Question
Discussion
Question
Discussion
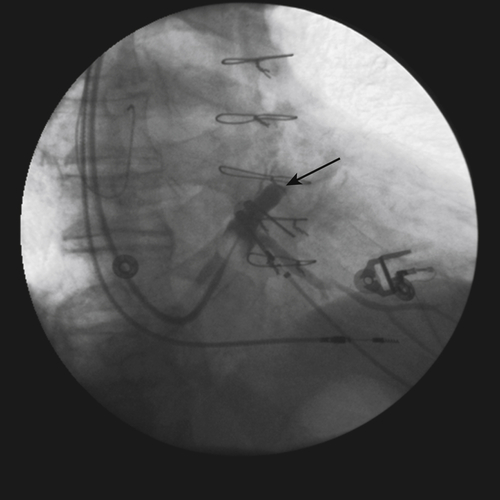
FIGURE 11-2
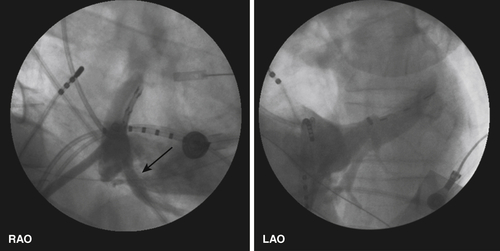
FIGURE 11-3
Question
Discussion
Intervention
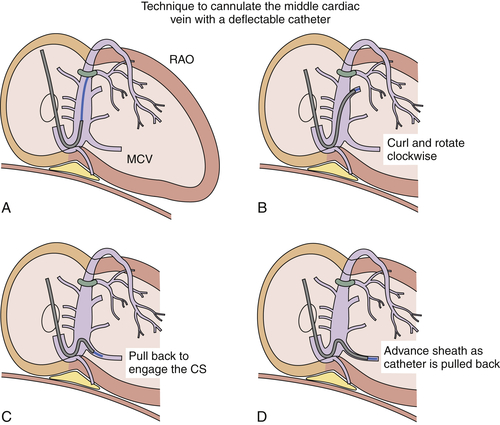
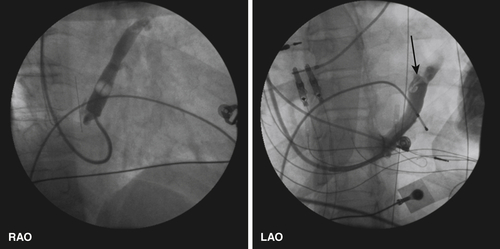
FIGURE 11-4
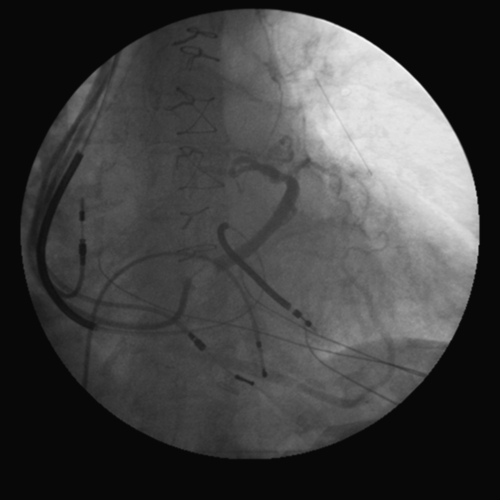
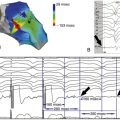
FIGURE 11-5
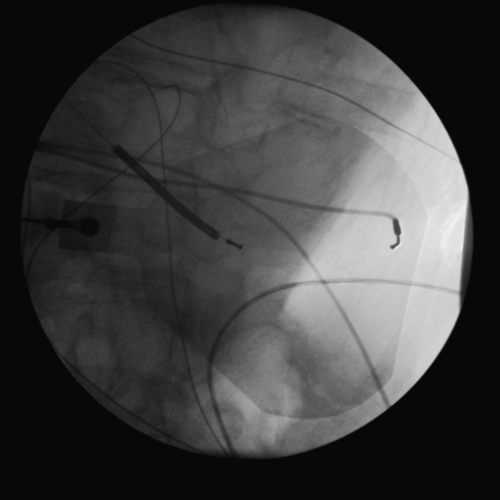
FIGURE 11-6
Focused Clinical Questions and Discussion Points
Question
Discussion
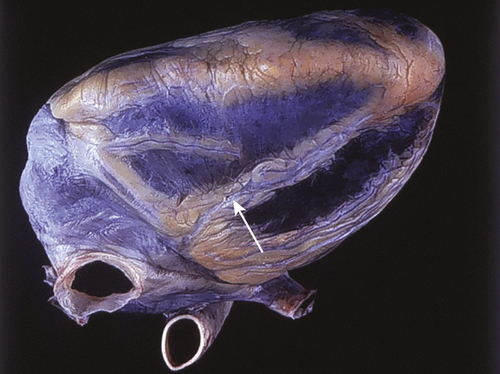
FIGURE 11-7
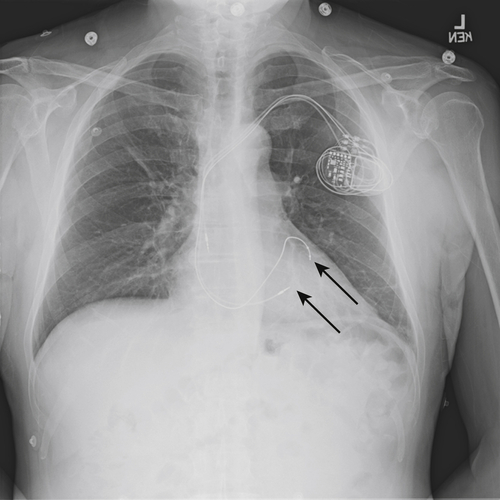
FIGURE 11-8
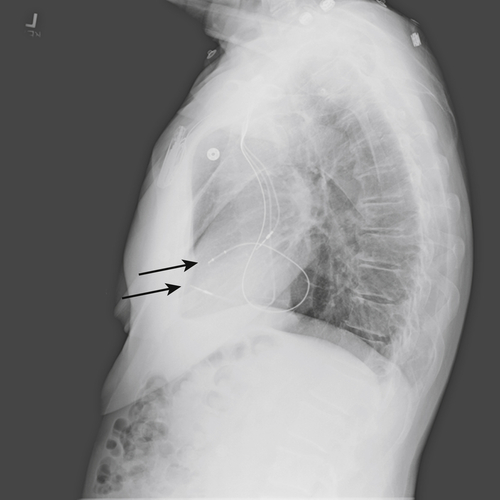
FIGURE 11-9
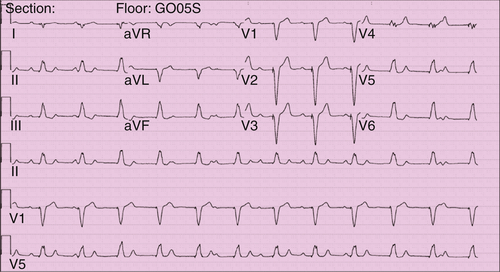
FIGURE 11-10
Outcome
Selected References
1. Anderson S.E., Lahm R., Iaizzo P.A. The coronary vascular system and associated medical devices. In: Iaizzo P.A., ed. Handbook of cardiac anatomy, physiology, and devices. Totowa, NJ: Humana; 2005:109–124.
2. Asirvatham S.J. Anatomy of the coronary sinus. In: Yu C.M., Hayes D.L., Auricchio A., eds. Cardiac resynchronization therapy. Malden: Blackwell Futura; 2008:211–238.
3. Bogaert J. Cardiac function. In: Bogaert J., Dymarkowski S., Taylor A.M., Muthurangu V., eds. Clinical cardiac MRI. Berlin: Springer-Verlag; 2012:109–166.
4. Corcoran S.J., Lawrence C., McGuire M.A. The valve of Vieussens: an important cause of difficulty in advancing catheters into the cardiac veins. J Cardiovasc Electrophysiol. 1999;10:804–808.
5. Hansky B., Lamp B., Minami K. et al. Coronary vein balloon angioplasty for left ventricular pacemaker lead implantation. J Am Coll Cardiol. 2002;40:2144–2149.
6. Hansky B., Schulte-Eistrup S., Vogt J. et al. Lead selection and implantation technique for biventricular pacing. Eur Heart J Suppl. 2004;6:D112–D116.
7. Lachman N., Syed F.F., Habib A. et al. Correlative anatomy for the electrophysiologist. II. Cardiac ganglia, phrenic nerve, coronary venous system. J Cardiovasc Electrophysiol. 2011;22:104–110.
8. Leon A.R., Delurgio D.B., Mera F. Practical approach to implanting left ventricular pacing leads for cardiac resynchronization. J Cardiovasc Electrophysiol. 2005;16:100–105.
9. Manolis A.S., Kappos K., Koulouris S. et al. Middle cardiac vein pacing avoids phrenic nerve stimulation, offers optimal resynchronization and obviates surgery for epicardial lead placement. Hosp Chron. 2007;2:44–45.